Abstract
The health benefits of resistance exercises are well established; however, the effects of resistance training on cognition are not as well understood. The purpose of this meta-analysis was to evaluate the evidence of resistance exercise’s effects on cognition. A systematic search identified 24 studies that were included in the analyses. These articles ranged in the protocols utilized and in how they studied the effects of resistance training on cognition. Four primary analyses were carried out to assess the effects of resistance exercise on cognitive outcomes: (1) composite cognitive scores, (2) screening measures of cognitive impairment, (3) measures of executive functions, and (4) measures of working memory. Results revealed positive effects of resistance training on composite cognitive scores (SMD 0.71, 95% CI 0.30–1.12), screening measures of cognitive impairment (SMD 1.28, 95% CI 0.39–2.18), and executive functions (SMD 0.39, 95% CI 0.04–0.74), but no effect on measures of working memory (SMD 0.151, 95% CI − 0.21 to 0.51). High heterogeneity was observed in all analyses. Resistance training appears to have positive effects on cognition; however, future research will need to determine why the effects are so variable.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The health and physical benefits of exercise are well established, including increased cardiorespiratory fitness, increased muscular strength, improved body composition, and even decreased risk of certain diseases (Hillman et al., 2008; Janssen and Leblanc, 2010; Nelson et al., 2007; Ortega et al., 2008; Penedo and Dahn, 2005; Radak et al., 2008; Voss et al., 2011; Warburton et al., 2006). In contrast, the effects of exercise on our emotional and cognitive processes are not as clear. For example, although exercise has been shown to improve mood and symptoms associated with depression and anxiety (Penedo and Dahn, 2005; Singh et al., 1997), and in general has been shown to improve the cognitive capacity of individuals (Erickson et al., 2015; Hillman et al., 2008), the mechanisms responsible and the nature of these changes (i.e., changes in cognitive processing due to exercise) are not as well understood.
Studies of the effects of exercise on cognition have varied the forms of exercise (i.e., aerobic, strength training and multimodal forms of exercise), the duration of exercise (i.e., acute and long-term), the cognitive domains tested (i.e., executive function, memory, global cognition, etc.) and the age groups of participants (i.e., children to older adults) (Altug, 2014; Colcombe and Kramer, 2003; Hillman et al., 2008; Sibley and Etnier, 2003; Verburgh et al., 2014). Reviews of this literature have generally revealed positive effects of exercise on cognition. However, the vast majority of current research on this topic has investigated the effects of aerobic exercise on broad domains of cognition such as executive functions and memory (Baker and Frank, 2012; Cassilhas et al., 2016; Colcombe and Kramer, 2003; Hopkins et al., 2012; Hötting et al., 2012; Skriver et al., 2014; Smith et al., 2010; Stroth et al., 2009; Vasques et al., 2011; Voss et al., 2011) while ignoring the effects that isolated resistance exercise may have on cognition. Hence, the purpose of this meta-analysis was to assess the effects and potential benefits of resistance exercise on human cognitive abilities.
Resistance training, exemplified by activities such as weight lifting, is associated with numerous health benefits in both younger and older populations (Cavani et al., 2002; Hillman et al., 2008; Latham et al., 2004) and is engaged in by millions of people daily as a primary form of physical activity (“Physical Activity”, 2016). It also serves as an alternative form of exercise for those who suffer from cardiorespiratory problems (e.g., asthma) or individuals who are limited physically (e.g., lower limb restrictions) and cannot perform other forms of exercise such as jogging or cycling (Ouellette et al., 2004; Yerokhin et al., 2012). This is especially true in older populations, in which cardiorespiratory and physical limitations are more prevalent. Resistance exercise can also help prevent decreases in strength and muscular size that have been correlated with aging and which make it more difficult to perform crucial everyday tasks, such as walking, getting up after falling, and lifting objects (Borst, 2004; Frontera et al., 2000; Hughes et al., 2001; Kim et al., 2012; Kimura et al., 2010).
Similarly, aging has also been associated with neurological changes (e.g., decreased white and gray matter) and cognitive changes (e.g., decreased processing speeds) that make it more difficult to complete everyday tasks, such as driving or remembering to take medications (Anstey et al., 2005; Insel et al., 2006). Prior studies and reviews have found that increased levels of fitness can aid in the prevention of neural and cognitive declines associated with aging (Kennedy et al., 2017; Middleton et al., 2011; Voss et al., 2011). For example, Colcombe and colleagues found that fitness training could benefit the cognitive abilities of sedentary older adults (Colcombe and Kramer, 2003) and in another study found that an aerobic training intervention lead to increases in both gray and white matter volume in older adults (Colcombe et al., 2006). These results have been echoed by numerous other studies and reviews have found that increased fitness levels (i.e., increased aerobic capacity and/or strength) are associated with a decreased risk and prevention of Alzheimer’s disease and other forms cognitive decline (Carvalho et al., 2014; Kirk-Sanchez and McGough, 2014; Paillard, 2015).
In sum, although the physical benefits of resistance training have been established, there has not been as much attention given to how isolated resistance training impacts cognition. Reviews specifically examining how resistance exercise affects cognition have in general found positive effects (Chang et al., 2012; Gates et al., 2013; Heyn et al., 2004; Kelly et al., 2014; Li et al., 2018). However, these reviews were either qualitative in nature (e.g., Chang et al., 2012; Li et al., 2018) or were restrictive in their approach (i.e., Kelly et al., 2014 excluded studies with cognitively impaired participants and Gates et al., was exclusive to studies of participants with MCI). Therefore, the purpose of this meta-analysis was to take a broader and more inclusive approach in evaluating the effects of resistance exercise on cognition and to determine if this relationship is moderated by factors such as age and mental health.
Methods
The meta-analysis was carried out in accordance with the Cochrane Review Guidelines, which provides authors with a set of recommendations for carrying out systematic reviews and meta-analyses (e.g., only including randomized control trials and rules for assessing bias). For more information, see Higgins and Green, 2011.
Search strategy
Literature searches were conducted in the following databases: Web of Science,Footnote 1 PsycInfo, SportsDiscus and PubMed. The final search was carried out by the first author in October, 2018 and included all years prior to the search dates. The search string was broken into three components: a component for exercise modality, a portion for cognitive terms, and an age group term. The final search string used was:
(“Anaerobic Exercise” OR “Resistance Training” OR “Resistance Exercise” OR “Strength Training” OR “Strength Exercise” OR “Weight Lifting”)
AND
(“Cognition” OR “Memory” OR “Attention” OR “Executive Function”)
AND
(“Adults”)
This string was designed to catch as many papers as possible and included multiple terms for resistance exercise as researchers describe it in various ways. All search result references were downloaded and imported into an EndNote library for screening. An initial total of 783 articles were found, however after removing duplicate references there was a final total of 547 articles, which were screened for inclusion in the review.
Screening
Papers were screened by two of the authors for inclusion. The initial screening was based on the abstracts and titles of the articles, then on their full text. To be included in the review, articles needed to meet the following criteria:
-
1.
Define resistance exercise as exercise that forces skeletal muscles to contract due to external force and whose intervention protocols called for the use of resistance bands, machines or free weights to perform their exercises. Studies that used balance training as their resistance intervention were excluded because some studies used balance training as an active control.
-
2.
Participants in the study were aged 18 or older. Studies of children were excluded due to the variability caused by development. In addition, there has been very little (if any) research on the exclusive effects of resistance exercise in children. Thus, comparisons between adult and youth populations were inappropriate.
-
3.
Directly measured the effect of resistance exercises on cognition (i.e., an individual’s mental capacity to process and understand information) using performance-based cognitive measures (e.g., Stroop, Erickson Flanker, etc.).
-
4.
Was a long-term intervention (i.e., minimum of 4 weeks) comparing a resistance training group to an active or passive control group.
Studies were excluded from the review for the following reasons:
-
1.
Review articles that did not provide novel evidence, but only summarized prior findings including some of the individual articles identified.
-
2.
Studies that mixed exercise modalities (i.e., both aerobic and resistance) or added other factors to their intervention (i.e., diet protocols). This was done to examine the effects of resistance training programs on cognition as exclusively as possible.
-
3.
Studies that did not explicitly state the contents of their exercise intervention.
-
4.
Investigations that did not measure cognitive performance directly. For example, those that used neural measures such as EEG, and made indirect inferences about cognition.
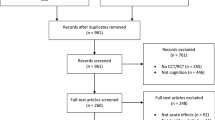
Two of the authors performed the screening of abstracts and full text articles for inclusion, and 28 articles were included (κ = 91%, any disagreements were settled by a third author). However, when a study produced multiple publications they were considered one study within the analysis (i.e., Fiatarone Singh et al., 2014, Mavros et al., 2017 and Suo et al., 2016; Iuliano et al. (2015) and Iuliano et al. (2017); Nagamatsu et al., 2012 and ten Brinke et al., 2015), resulting in 24 studies for the analysis. See Fig. 1 for a diagram of the search and screening process. In cases where studies included multiple resistance training groups that varied in intensity or frequency, only the higher intensity or more frequent training groups were included in the analysis (i.e., Cassilhas et al., 2007; Liu-Ambrose et al., 2012; Yoon et al., 2016). These higher intensity/frequency conditions better matched the exercise intensity/frequency of the other studies, thus producing a more consistent meta-analysis without biasing the results by over-sampling studies with multiple intervention groups.
Data pooling
Within studies, outcomes were recorded as mean differences (changes in cognitive scores) before and after exercise and control interventions. All mean differences were transformed so that positive values indicate cognitive enhancement due to resistance training (expected direction) and negative values indicate cognitive decrement after resistance exercise (unexpected direction) after controlling for practice effects. For most cases, standard deviation of differences and paired correlations were unreported. Therefore, imputation was required to calculate effect size and previous literature has shown valid results using imputation (Furukawa et al., 2006). The imputation method for this investigation utilized the formula suggested by Borenstein et al. (2010). Next, a standard mean difference for cognitive domain(s) was calculated for each study. This value is equivalent to the Cohen’s d measure of effect size and represents the magnitude of cognitive change from resistance training intervention compared to control conditions.
In the case of articles that reported multiple measures within a single domain, a single effect size and standard error were calculated to meet the assumption of independence in our statistical analyses. This was accomplished by pooling the effects and standard errors across the measures. The pooled outcome was calculated by taking the mean of the effect sizes and the root mean squared standard errors across the included measures. See Table 1 for citations of the included studies and general study characteristics and Table 2 for the effect sizes included in analysis.
Analysis
Meta-analyses were conducted to determine the impact of resistance training interventions on cognition. However, the studies varied very broadly both in the number and type of cognitive measures, from screening tests of cognitive impairment such as the MMSE to tests of specific domains of cognitive function such as the Stroop task. Therefore, four separate sets of analyses were carried out: (1) an analysis of the composite cognitive scores, which included all cognitive-behavioral measures, (2) an analysis on screening measures of cognitive impairment (e.g., MMSE, MoCA which are used as clinical measures to assess the cognitive impairment of individuals), (3) tests primarily examining executive functions (e.g., Stroop), and (4) tests of working memory (e.g., Digit Span). For each of these primary analyses, the following potential moderators of the relationship between resistance training and cognition were tested: (a) cognitive health status (i.e., healthy: individuals with no reported cognitive impairments vs. impaired: participants were reported to have a cognitive impairment e.g., MCI), (b) duration (i.e., studies were split into groups based on a median split in duration in weeks, Mdn = 16.00), (c) age (i.e., studies were split into groups based on a median split of the mean age of the participants, Mdn = 70.1), and (d) control group type (i.e., active: control group participants who performed activities beyond their normal daily routines e.g., stretching and balance exercises vs. passive: control groups participants who did not deviate from their normal daily routines and/or incorporate other activities). For the age moderator analyses, one study (Goekint et al. 2010) was excluded because it was a clear outlier in regards to the mean age of participants (M = 20.10) as compared to the other studies (M = 68.84). For the analysis of working memory, the type of working memory measure was included as a moderator: verbal versus visuospatial working memory. Random-effects meta-analysis (Borenstein et al., 2010) was used to provide a pooled effect size for each cognitive domain.
All analyses were conducted in R version 3.4.0 using functions provided by the metafor package (Viechtbauer, 2010). All tests included random-effects models using the maximum-likelihood estimator. Random-effects analyses calculate average standard mean differences without assuming that all studies come from the same population. This aligned with the review’s goal to summarize the impact of resistance training without assuming one true effect across interventions and populations. Furthermore, random effect models yielded measures of heterogeneity known as I2, which specifies the percent of variability in the effect size across studies. In addition to the pooled standard mean differences, 95% confidence intervals, I2 estimates, and forest plots for each grouping of cognitive outcomes were produced.
Risk of bias assessment
Risk of bias was assessed according to the Cochrane guidelines. The risk of bias was classified as being low, uncertain or high across the following domains: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting and other forms of bias. In accordance with Cochrane guidelines if a study did not report on a method it was deemed as uncertain risk.
Results
Of the identified studies, 23 out of the 24 studies had a mean participant age of 50 years old or above [mean age of participants in Goekint et al. (2010) was 20.1]. Ten of the studies investigated the effects of resistance exercise in cognitively impaired populations (note that the impairments varied: probable MCI, MCI, Parkinson’s disease, chronic stroke, subjective memory complaints, cognitive impairments due to depression, sleep disorders). The duration in weeks varied across studies ranging from 4 to 96 weeks. Finally, 15 studies compared the intervention to a passive control group and the majority of interventions were carried out twice weekly.
Composite cognitive scores
This analysis included measures from all cognitive domains. Twenty-four studies were included in this analysis, totaling 868 participants assigned to intervention groups and 774 control participants. Composite scores were computed for 11 out of the 24 studies. Seven of the studies solely used screening measures of cognitive impairment, three studies used a single measure of executive functions and one study solely used a measure of working memory. Meta-analysis revealed that resistance training had a positive effect on measures of cognition (SMD 0.71, 95% CI 0.30–1.12, p < 0.001, see Fig. 2 for forest plot) though there was high heterogeneity across studies (I2 = 93.42%, p < 0.001).
Screening measures of cognitive impairment
In total, eight studies used brief screening measures for cognitive impairment (nexp = 369, nctl = 330). These included the MMSE (k = 4), the MoCA (k = 2), NIH toolbox composite (k = 1) and the ADAS-Cog (k = 1). The results of the analysis revealed that resistance training had a strong positive effect on cognitive screening measures (SMD 1.28, 95% CI 0.39–2.18, p = 0.005) though there was high heterogeneity across studies (I2 = 94.06%, p < 0.001). See Fig. 3 for accompanying forest plot of effect sizes.
Executive functions
A total of 16 studies were included in this analysis. 608 participants were assigned to the intervention conditions and 546 participants were assigned to control groups. Tests in this analysis included letter digit substitution (k = 1), Stroop tasks (k = 6), trail making tests (k = 4), Toulouse-Pieron cancellation numbers (k = 1), tests of immediate recall and recognition (k = 6), the Brief Test of Attention (k = 1), WAIS-Matrices (k = 1), WAIS-Similarities (k = 1), symbol digit tests (k = 2), peripheral visuomotor reaction time (k = 1), neurotracker threshold speed spatial awareness (k = 1), Ravens Test (correct answers) (k = 1), Attentive Matrices (time) (k = 1), flanker interference score (k = 1), Conner’s Continuous Performance test (reaction time) (k = 1), Cognitrone psychomotor test (k = 1), Frontal Assessment Battery, (k = 1), Walking Response and Inhibition Test (k = 1), and a choice reaction time test (k = 1) (when a study used multiple measures, the SMD scores were pooled into a single effect, see Sect. 2.3 for details). Resistance training had a positive effect on measures of executive function (SMD 0.39, 95% CI 0.04–0.74, p = 0.029), and compared to the prior analyses relatively less heterogeneity (I2 = 86.45%, p < 0.001) (Fig. 4).
Working memory
In total, 11 studies investigated the effects of resistance training (n = 318) on working memory as compared to a control group (n = 324). Two of the studies (Cassilhas et al., 2007 and Fernandez-Gonzalo et al., 2016) included measures of both verbal and visuospatial working memory. Rather than pooling these measures together, only the visuospatial measures were included from these studies, as the majority of studies only used measures of verbal working memory and this exclusion enabled the subsequent moderator analysis of verbal versus visuospatial working memory. The final set of measures included in this analysis was digit span tasks (forwards and backwards) (k = 11), Rey auditory verbal learning task (k = 4), Corsi block tapping task (forward and backward) (k = 2), spatial span (forward and backward) (k = 2), spatial memory task (k = 1), Prose-Immediate Recall (k = 1), and the list learning memory task from ADAS-Cog (k = 1). There was no statistically significant effect of resistance training on measures of working memory (SMD 0.151, 95% CI − 0.21 to 0.51, p = 0.408), with relatively lower heterogeneity across studies (I2 = 79.55%, p < 0.001). See Fig. 5 for the accompanying forest plot.
Moderator analyses
Effects on screening measures of cognitive impairment were the only ones to exhibit statistically significant effects of moderator variables. The effect of resistance training on screening measures was significantly moderated by cognitive health (SMD − 1.57, 95% CI − 3.01 to − 0.13, p = 0.03, accounting for 41.53% of the heterogeneity), intervention duration (SMD − 1.57, 95% CI − 2.96 to − 0.18, p = 0.03, accounting for 45.21% of the heterogeneity), and control group type (SMD − 2.73, 95% CI − 5.19 to − 0.27, p = 0.03, accounting for 43.15% of the heterogeneity). More specifically, participants suffering from cognitive impairments tended to improve more than healthy participants, studies falling below the median duration (Mdn duration = 16.00 weeks) showed larger effects then those with durations above the median duration and studies that compared the effects of resistance training against active control groups (stretching and balance exercises) showed larger effects than those with passive control groups. Note the moderation of control type and duration is most likely being driven by the unusually large effect in the Yoon et al., 2016 study (SMD 3.84), which had a below-median duration and compared the intervention group to an active control group. None of the other moderator analyses revealed any statistically significant moderators of the relationship between resistance training and cognition. See Table 3 for percent heterogeneity explained for each analysis by each moderator.
Including type of working memory (i.e., verbal k = 9 or visuospatial k = 3) as a moderator had a significant effect (SMD − 0.75, 95% CI − 1.46 to − 0.04, p = 0.039), accounting for 37.88% of the heterogeneity. The negative direction of the effect suggests that there was slightly more improvement on tests of visuospatial working memory as opposed to tests of verbal working memory.
Risk of bias
Most of the studies reported using a random sequence to assign participants and only a small subset of studies reported on concealment methods. All studies were deemed as having uncertain risk in regards to the blinding of participants and personnel to the intervention because it is impossible to blind participants in the exercise groups, and it is unclear how this would affect performance on the outcome measures. This is a limitation inherent in research on exercise interventions and behavioral interventions more generally. A majority of the studies did not report on the blinding of participants to the outcomes, and were therefore deemed as uncertain risk in accordance with the Cochrane guidelines.
It was also unclear whether the studies reported all of the measures that they collected, so a majority of the studies were deemed as having uncertain risk. For the full summary, see Fig. 6. To determine if there was publication bias, funnel plots were created for each of the primary analyses (Fig. 7), which reiterate the high degree of variability among study results and suggest that publication bias was present in each of those analyses. The publication bias appeared to be less severe in the analysis of resistance exercises effects on executive functions as compared to the other analyses (note that the executive functions analysis also had substantially lower heterogeneity).
Discussion
Summary and interpretation
Researchers have taken a keen interest in the effects that exercise may have on an individual’s cognitive abilities. However, the majority of that research has focused on aerobic exercise while the role of isolated resistance exercise has been somewhat overlooked. Therefore, the purpose of this meta-analysis was to take a broad look at the available evidence and evaluate the effects of resistance training on cognition, in isolation from other forms of exercise. In all, 24 studies were included based on: (1) strength training interventions that used resistance bands, machines, or free weights, (2) included study populations that were 18 years of age or older, (3) used direct behavioral measures of cognition, and (4) were long term interventions comparing an exercise group and a control group. Although this is a relatively small number of studies compared to other fields of psychological research, it represents a useful point to take stock of what we know and to identify promising future directions and challenges that will need to be overcome. In line with prior reviews on the topic, it appears that resistance exercise has beneficial effects on cognition (Chang et al., 2012; Heyn et al., 2004; Kelly et al., 2014; Li et al., 2018). Specifically, analyses revealed positive effects of resistance training on composite cognitive scores, on screening measures of cognitive impairment, and on executive functions. The effect on measures of working memory was not statistically significant. Only the analysis of screening measures revealed significant moderator effects of the mean age of the participants, duration of intervention, and control group type.
Although these results show some promise for the use of resistance exercise to improve cognitive abilities, there was a high amount of heterogeneity. Nevertheless, all included effects in the analyses of composite cognitive scores and executive functions were positive (see Figs. 2, 4), only one of the seven effects in the analysis of screening measuring of cognitive impairment was zero (Fig. 3), and effects ranged from slightly negative to greater then two in the analysis of working memory measures (Fig. 5). The moderators included in the analyses only accounted for a small amount of the heterogeneity (i.e., only two of the 16 moderator analyses were statistically significant). Moving beyond the included moderators, another possible factor contributing to the heterogeneity is the differences in the measures that were used. The strongest effects were observed for screening measures of cognitive impairment (e.g., MMSE, MoCA), which are specifically designed to measure changes in cognition that indicate clinically meaningful levels of cognitive impairment or dementia. In contrast, laboratory measures of specific aspects of executive functions (e.g., the Stroop task) and measures of working memory (e.g., the digit span task) often yield small and noisy effects, and therefore, when results are pooled together, the effects may be washed out.
It could also be that resistance exercise selectively enhances aspects of cognition due to differential cognitive demands. More specifically, resistance training may selectively improve the cognitive abilities that are more heavily engaged during the exercises. For example, while weight lifting individuals need to constantly attend to what they are doing so that they do not harm themselves or the individuals around them. These bouts of vigilance may act as a form of attention training and explain why there was improved performance on tests of executive functions, as many of these tasks measure an individual’s ability to attend to specific stimuli. Conversely, weight lifting does not engage working memory as much and the meta-analysis found no effect on measures of working memory, though this was moderated by working memory type, with visuospatial working memory showing larger effects. Note that visuospatial working memory may be engaged during resistance training for visualizing and recalling body and weight positions. On this view, consistent long-term resistance training may act as a form of cognitive training, similar to computer programs and games that aim to improve or sustain various cognitive abilities. Although there is some controversy about the effectiveness and generalizability of computerized cognitive training (Au et al., 2015; Simons et al., 2016), the effects tend to be stronger for tasks that are similar to those that were performed during the training program (Lustig et al., 2009; Sala and Gobet, 2017). Thus, if resistance exercise is another form of cognitive training, then it may similarly lead to enhanced neural and cognitive efficiency specifically in the domains that are most engaged during the exercises. This suggestion aligns with prior reviews on the topic, which have also found differential effects of resistance training depending on the cognitive outcome examined (e.g., Chang et al., 2012; Kelly et al., 2014). Specifically, Kelly et al., 2014 suggested that resistance exercise could have greater effects on specific tasks of executive functions. Differential effects of resistance exercise on cognition could also aid in explaining the observed high level of heterogeneity. Specifically, combining measures of different aspects of cognition in single analyses could equate to more variance in the observed effects (e.g., the effects on measures of executive functions were generally positive and the heterogeneity was much less then the heterogeneity of the composite scores which combined measures from multiple domains). This is just one possibility to keep in mind for future research, not a conclusion that can be drawn from the currently available evidence.
There is also the possibility that the effects of resistance exercise on cognition may be mediated by neurobiological mechanisms that are unrelated to the specific cognitive demands of exercise. Such mechanisms include increases in neurotropic factors such as brain derived neurotropic factor (BDNF) (Bramham and Messaoudi, 2005), increases in proteins such as insulin-like growth factor 1 (IGF-1) (see Cotman, Berchtold, and Christie, 2007 for review), changes in hormone levels, increases in cerebral blood flow, and others (Babaei et al., 2014; Cassilhas et al., 2007; Fragala et al., 2014; Hillman et al., 2008; Kraemer and Ratamess, 2005; Moreau et al., 2015; Timinkul et al., 2008). These molecular level changes are believed to lead to structural changes, such as increased white and gray matter volume (Colcombe et al., 2006), which could then lead to cognitive changes as well (for further discussion see Cassilhas et al., 2016). Neurobiological and cognitive mechanisms may also work synergistically. For example, the neurobiological mechanisms may increase neuroplasticity, which then enhances the cognitive training effects for the cognitive functions that are most strongly and consistently engaged during resistance exercises. However, clear connections have yet to be demonstrated between exercise, neurobiological mechanisms, and cognitive changes.
Open questions and future directions
Much of the literature investigating the effects of resistance exercise on cognition was motivated by the important possibility that resistance training may help to stave off cognitive declines associated with aging and neurological impairments. Although the inclusion of cognitive health status and age as moderating variables accounted for a large amount of variance in the effects on screening measures of cognitive impairment, these factors were not significant moderators on any of the other outcomes. Further, the age distribution was largely skewed towards older adults (i.e., 65+) and there was a mix of cognitive impairments (i.e., self reported impairment, diagnosed mild cognitive impairment, cognitive impairments attributed to depression, and others). The limited age distribution and mixed set of impairments could have limited the ability to detect interactions between age and health. For example, if resistance training is particularly effective for individuals diagnosed with mild cognitive impairment but not for individuals with depression, then combining them into one “impaired” group would reduce the ability to detect moderation by cognitive health status. Unfortunately, at this point, there are not enough published studies with any one form of cognitive impairment to conduct a more precise analysis of the effects of resistance training. Therefore, although the observed benefits of resistance training show some promise for its use in staving off cognitive decline, it remains unclear how resistance training interacts with age and varying disease progressions, both at cognitive and neurobiological levels.
Further, and possibly most important for the public interest, more ecologically valid tests need to be used. For example, although there were benefits found on measures of executive functions, there is no real way to quantify how much this will translate into benefits in everyday living. Using tasks or follow-up measures that relate better to everyday life would help to evaluate the real-life impacts of possible cognitive benefits of resistance training. Along these lines, exercise in older populations, resistance exercise in particular (LaStayo et al., 2003), has been associated with both reduced risk and fear of falling, leading to increased levels of daily activity (Barnett et al., 2003; Chou et al., 2012; Li et al., 2003; Pluijm et al., 2006). Increased levels of daily activity (performing chores, etc.) have been associated with benefits to cognitive functions (Kramer and Erickson, 2007) and decreased risk of dementia and Alzheimer’s disease (Rovio et al., 2005). Therefore, there may be an interactive effect: exercise leads to increased amounts of daily activity, which further enhances cognitive functions and helps to stave off cognitive declines. As such, it would be beneficial for future studies to examine this relationship more closely (i.e., the relationship between exercise, daily activity, and cognition) to determine how the potential cognitive and functional gains translate to everyday life and to determine whether the gains are worth the costs (e.g., gym memberships and personal trainer costs).
Another area that needs to be investigated more thoroughly is the role of exercise duration, frequency, and intensity. Although several of the studies (Cassilhas et al., 2007; Liu-Ambrose et al., 2012; Yoon et al., 2016) investigated how differences in these factors contribute to the effects of resistance training on cognition, the number of studies was too small and their designs too heterogeneous to perform a formal meta-analysis. Therefore, it is important for future studies to investigate these factors with multiple intervention groups to determine the optimal levels of duration, frequency, and intensity of exercise in relation to cognitive performance.
Finally, long-term interventions need to carefully consider the variety of the exercises utilized throughout the duration of the interventions. A lack of exercise variation and/or intensity could lead to periods of physical adaptation, which could hinder cognitive benefits (Baker and Newton, 2011; Fleck, 1999; Peterson et al., 2005; Rhea et al., 2003; Sale, 1988). If a person becomes overly accustomed to the exercises, then those exercises will not be as physically taxing, which will decrease the physical benefits and could lead to decreased neurobiological and cognitive demands, hindering any potential cognitive and neural benefits. One way to test this claim would be to utilize a stable long-term intervention (i.e., not varying exercises used throughout the intervention) and to perform multiple physical and cognitive tests throughout the protocol period (rather than just pre- and post-intervention). This would allow testing for periods of physical adaptation (i.e., periods where physical benefits plateau) and whether such periods correspond with plateaus of cognitive performance. If this is the case, then simply varying the exercises could enhance both the physical and the cognitive benefits.
Limitations
There were a number of limitations in carrying out this meta-analysis. One of the primary limitations was that there was a large amount of heterogeneity in the observed effects. This heterogeneity, may have affected the results of the analysis by skewing the effects away from the true effect. Further, the observed results may have been subject to publication bias: studies that found significant effects may have been more likely to be published than studies with non-significant effects, thus skewing the results in the literature. Note, however, that many of the studies included multiple measures of cognition [e.g., Anderson-Hanley et al., (2010) who included digit span, Trail Making task, and Stroop] and while some effects were significant, others were not, possibly reducing the risk of publication bias (i.e., some non-significant effects were published because they were included with significant effects on other measures).
Conclusion
The results of this meta-analysis revealed an overall effect of resistance training on cognition, on screening measures of cognitive impairment, and on executive functions, but no effects were found on measures of working memory. This shows promise for the use of resistance exercise as a way to improve cognition and/or stave off cognitive decline. However, the reported effects were highly variable and more investigation is needed, especially in regards to the precise mechanisms that drive these improvements, before any firm recommendations can be made.
Supplementary materials
The data used in this meta-analysis (i.e., Tables 1 and 2) can be found here: https://osf.io/8shn5/?view_only=f91e072d18884ad38ba7253101297e11.
Notes
The search performed in the Web of Sciences database was limited to the following categories: neuroscience, sport sciences, pediatrics, psychology, rehabilitation, clinical neurology, psychology experimental, public environmental occupational health, psychology developmental, behavioral sciences, psychiatry, psychology multidisciplinary, geriatrics gerontology, physiology, psychology biological, multidisciplinary sciences, gerontology, psychology applied, education educational research, psychology clinical, psychology educational and medicine research experimental.
References
Altug, Z. (2014). Resistance exercise to improve cognitive function. Strength and Conditioning Journal, 36, 46–50.
Ansai, J. H., & Rebelatto, J. R. (2015). Effect of two physical exercise protocols on cognition and depressive symptoms in oldest-old people: A randomized controlled trial. Geriatrics and Gerontology International, 15(9), 1127–1134. https://doi.org/10.1111/ggi.12411.
Anstey, K. J., Wood, J., Lord, S., & Walker, J. G. (2005). Cognitive, sensory and physical factors enabling driving safety in older adults. Clinical Psychology Review, 25, 45–65. https://doi.org/10.1016/j.cpr.2004.07.008.
Anderson-Hanley, C., Nimon, J. P., & Westen, S. C. (2010). Cognitive health benefits of strengthening exercise for community-dwelling older adults. Journal of Clinical and Experimental Neuropsychology, 32(9), 996–1001. https://doi.org/10.1080/13803391003662702.
Au, J., Sheehan, E., Tsai, N., Duncan, G. J., Buschkuehl, M., & Jaeggi, S. M. (2015). Meta-analysis, improving fluid intelligence with training on working memory. Psychonomic Bulletin and Review, 22, 366–377. https://doi.org/10.1016/j.cognition.2008.05.007.
Babaei, P., Damirchi, A., Mehdipoor, M., & Tehrani, B. S. (2014). Long term habitual exercise is associated with lower resting level of serum BDNF. Neuroscience Letters, 566, 304–308. https://doi.org/10.1016/j.neulet.2014.02.011.
Baker, D. G., & Newton, R. U. (2011). Adaptations in upper-body maximal strength and power output resulting from long-term resistance training in experienced strength-power athletes. Journal of Strength and Conditioning Research, 26, 1098–1103.
Baker, L. D. L., & Frank, L. (2012). Effects of aerobic exercise on mild cognitive impairment: A controlled trial. Archives of Neurology, 67, 71–79. https://doi.org/10.1001/archneurol.2009.307.Effects.
Barnett, A., Smith, B., Lord, S. R., Williams, M., & Baumand, A. (2003). Community based group exercise improves balance and reduces falls in at risk older people: A randomised controlled trial. Age and Ageing, 32, 407–414. https://doi.org/10.1093/ageing/32.4.407.
Best, J. R., Chiu, B. K., Liang Hsu, C., Nagamatsu, L. S., & Liu-Ambrose, T. (2015). Long-term effects of resistance exercise training on cognition and brain volume in older women: Results from a randomized controlled trial. Journal of the International Neuropsychological Society, 21(10), 745–756. https://doi.org/10.1017/S1355617715000673.
Borenstein, M., Hedges, L. V., Higgins, J., & Rothstein, H. R. (2010). A basic introduction to fixed-effect and random-effects models for meta-analysis. Research synthesis methods, 1, 97–111.
Borst, S. E. (2004). Interventions for sarcopenia and muscle weakness in older people. Age and Ageing, 33, 548–555. https://doi.org/10.1093/ageing/afh201.
Bramham, C., & Messaoudi, E. 2005. BDNF function in adult synaptic plasticity: The synaptic consolidation hypothesis. Progress in Neurobiology. https://doi.org/10.1016/j.pneurobio.2005.06.003.
Carvalho, A., Rea, I. M., Parimon, T., & Cusack, B. J. (2014). Physical activity and cognitive function in individuals over 60 years of age: A systematic review. Clinical Interventions in Aging, 9, 661–682. https://doi.org/10.2147/CIA.S55520.
Cassilhas, R. C., Tufik, S., & Mello, M. T. (2016). Physical exercise, neuroplasticity, spatial learning and memory. Cellular and Molecular Life Sciences, 73, 975–983. https://doi.org/10.1007/s00018-015-2102-0.
Cassilhas, R. C., Viana, VaR., Grassmann, V., Santos, R. T., Santos, R. F., Tufik, S., & Mello, M. T. (2007). The impact of resistance exercise on the cognitive function of the elderly. Medicine and Science in Sports and Exercise, 39, 1401–1407. https://doi.org/10.1249/mss.0b013e318060111f.
Cavani, V., Mier, C. M., Musto, A. a., & Tummers, N. (2002). Effects of a 6-week resistance-training program on functional fitness of older adults. Journal of Aging and Physical Activity, 10, 443–452.
Chang, Y. K., Pan, C. Y., Chen, F. T., Tsai, C. L., & Huang, C. C. (2012). Effect of resistance-exercise training on cognitive function in healthy older adults: A review. Journal of Aging and Physical Activity, 20, 497–517.
Cherup, N., Roberson, K., Potiaumpai, M., Widdowson, K., Jaghab, A., Chowdhari, S., Armitage, C., Seeley, A., & Signorile, J. (2018). Improvements in cognition and associations with measures of aerobic fitness and muscular power following structured exercise. Experimental Gerontology, 112, 76–87. https://doi.org/10.1016/j.exger.2018.09.007.
Chou, C. H., Hwang, C. L., & Wu, Y. T. (2012). Effect of exercise on physical function, daily living activities, and quality of life in the frail older adults: A meta-analysis. Archives of Physical Medicine and Rehabilitation, 93, 237–244. https://doi.org/10.1016/j.apmr.2011.08.042.
Chupel, M. U., Direito, F., Furtado, G. E., Minuzzi, L. G., Pedrosa, F. M., Colado, J. C., et al. (2017). Strength training decreases inflammation and increases cognition and physical fitness in older women with cognitive impairment. Frontiers in Physiology, 8, 1–13. https://doi.org/10.3389/fphys.2017.00377.
Colcombe, S., Erickson, K., Scalf, P., Kim, J., Prakash, R., McAuley, E., Elavsky, S., Marquez, D., Hu, L., & Kramer, A. (2006). Aerobic exercise training increases brain volume in aging humans. The Journals of Gerontology Series A Biological Sciences and Medical Sciences, 61A, 1166–1170.
Colcombe, S., & Kramer, A. F. (2003). Fitness effects on the cognitive function of older adults: A meta-analytic study. Psychological Science, 14, 125–130.
Cotman, C. W., Berchtold, N. C., & Christie, L. A. (2007). Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends in Neurosciences, 30, 464–472. https://doi.org/10.1016/j.tins.2007.06.011.
Erickson, K. I., Hillman, C. H., & Kramer, A. F. (2015). Physical activity, brain, and cognition. Current Opinion in Behavioral Sciences, 4, 27–32. https://doi.org/10.1016/j.cobeha.2015.01.005.
Davis, J. C., Bryan, S., Marra, C. A., Sharma, D., Chan, A., Beattie, B. L., et al. (2013). An economic evaluation of resistance training and aerobic training versus balance and toning exercises in older adults with mild cognitive impairment. PloS One, 8(5), e63031. https://doi.org/10.1371/journal.pone.0063031.
David, F. J., Robichaud, J. A., Leurgans, S. E., Poon, C., Kohrt, W. M., Goldman, J. G., et al. (2015). Exercise improves cognition in Parkinson’s disease: The PRET-PD randomized, clinical trial. Movement Disorders, 30(12), 1657–1663. https://doi.org/10.1002/mds.26291.
Fallah, N., Hsu, C. L., Bolandzadeh, N., Davis, J., Beattie, B. L., Graf, P., et al. (2013). Amultistate model of cognitive dynamics in relation to resistance training: the contribution of baseline function. Annals of Epidemiology, 23(8), 463–468. https://doi.org/10.1016/j.annepidem.2013.05.008.
Fernandez-Gonzalo, R., Fernandez-Gonzalo, S., Turon, M., Prieto, C., Tesch, P. A., & García-Carreira, M. D. C. (2016). Muscle, functional and cognitive adaptations after flywheel resistance training in stroke patients: A pilot randomized controlled trial. Journal of NeuroEngineering and Rehabilitation, 13(1), 1–11. https://doi.org/10.1186/s12984-016-0144-7.
Fiatarone Singh, M., Gates, N., Saigal, N., Wilson, G. C., Meiklejohn, J., Brodaty, H., Wen, W., Singh, N., Baune, B. T., Suo, C., Baker, M. K., Foroughi, N., Wang, Y., Sachdev, P. S., & Valenzuela, M. (2014). The Study of Mental and Resistance Training (SMART) study—resistance training and/or cognitive training in mild cognitive impairment: A randomized, double-blind, double-sham controlled trial. Journal of the American Medical Directors Association, 15, 873–880. https://doi.org/10.1016/j.jamda.2014.09.010.
Fleck, S.J., 1999. Periodized strength training: A critical review. The Journal of Strength and Conditioning Research, 13, 82–89. https://doi.org/10.1519/1533-4287(1999)013%3C0082:PSTACR%3E2.0.CO;2
Fragala, M. S., Beyer, K. S., Jajtner, A. R., Townsend, J. R., Pruna, G. J., Boone, C. H., Bohner, J. D., Fukuda, D. H., Stout, J. R., & Hoffman, J. R. (2014). Resistance exercise may improve spatial awareness and visual reaction in older adults. The Journal of Strength and Conditioning Research, 28, 2079–2087.
Frontera, W. R., Hughes, V., Fielding, R. A., Fiatarone Singh, M., Evans, W. J., & Roubenoff, R. (2000). Aging of skeletal muscle: A 12-yr longitudinal study. Journal of Applied Physiology, 88, 1321–1326.
Furukawa, T. A., Barbui, C., Cipriani, A., Brambilla, P., & Watanabe, N. (2006). Imputing missing standard deviations in meta-analyses can provide accurate results. Journal of Clinical Epidemiology, 69(1), 7–10.
Goekint, M., De Pauw, K., Roelands, B., Njemini, R., Bautmans, I., Mets, T., et al. (2010). Strength training does not influence serum brain-derived neurotrophic factor. European Journal of AppliedPhysiology, 110(2), 285–293. https://doi.org/10.1007/s00421-010-1461-3.
Gates, N., Fiatarone Singh, M., Sachdev, P. S., & Valenzuela, M. (2013). The effect of exercise training on cognitive function in older adults with mild cognitive impairment: A meta-analysis of randomized controlled trials. The American Journal of Geriatric Psychiatry, 21, 1086–1097. https://doi.org/10.1016/j.jagp.2013.02.018.
Heyn, P., Abreu, B. C., Ottenbacher, K. J. et al. (2004). The effects of exercise training on elderly persons with cognitive impairment and dementia: A meta-analysis. Archives of Physical Medicine and Rehabilitation, 85, 1694–1704. https://doi.org/10.1016/j.apmr.2004.03.019.
Higgins, J. P., & Green, S. (2011). Cochrane handbook for systematic reviews of interventions. Hoboken: Wiley.
Hillman, C. H., Erickson, K. I., & Kramer, A. F. (2008). Be smart, exercise your heart: exercise effects on brain and cognition. Nature Reviews Neuroscience 9, 58
Hopkins, M. E., Davis, F. C., VanTieghem, M. R., Whalen, P. J., & Bucci, D. J. (2012). Differential effects of acute and regular physical exercise on cognition and affect. Neuroscience, 215, 59–68. https://doi.org/10.1037/a0030561.Striving.
Hötting, K., Schauenburg, G., & Röder, B. (2012). Long-term effects of physical exercise on verbal learning and memory in middle-aged adults: Results of a one-year follow-up study. Brain Sciences, 2, 332–346. https://doi.org/10.3390/brainsci2030332.
Hughes, V., Frontera, W. R., Wood, M., Evans, W. J., Dallal, G. E., Roubenoff, R., & Fiatarone Singh, M. (2001). Longitudinal muscle strength changes in older adults: Influence of muscle mass, physical activity, and health. The Journals of Gerontology Series A Biological Sciences and Medical Sciences, 56, B209–B217. https://doi.org/10.1093/gerona/56.5.B209.
Insel, K., Morrow, D., Brewer, B., & Figueredo, A. (2006). Executive function, working memory, and medication adherence among older adults. The Journals of Gerontology Series B Psychological Sciences and Social Sciences, 61, P102–P107. https://doi.org/10.1093/geronb/61.2.P102.
Irandoust, K., & Taheri, M. 2018. The effect of strength training on quality of sleep and psychomotor performance in elderly males. Sleep and Hypnosis 20, 160–165.
Iuliano, E., di Cagno, A., Aquino, G., Fiorilli, G., Mignogna, P., Calcagno, G., & Di Costanzo, A. (2015). Effects of different types of physical activity on the cognitive functions and attention in older people: A randomized controlled study. Experimental Gerontology, 70, 105–110. https://doi.org/10.1016/j.exger.2015.07.008.
Iuliano, E., Fiorilli, G., Aquino, G., Di Costanzo, A., Calcagno, G., & Di Cagno, A. (2017). Twelve-week exercise influences memory complaint but not memory performance in older adults: A randomized controlled study. Journal of Aging and Physical Activity 25, 612–620.
Janssen, I., & Leblanc, A. G. (2010). Systematic review of the health benefits of physical activity and fitness in school-aged children and youth. International Journal of Behavioral Nutrition and Physical Activity, 7, 40. https://doi.org/10.1186/1479-5868-7-40.
Kelly, M. E., Loughrey, D., Lawlor, B. A., Robertson, I. H., Walsh, C., & Brennan, S. (2014). The impact of exercise on the cognitive functioning of healthy older adults: A systematic review and meta-analysis. Ageing Research Reviews, 16, 12–31. https://doi.org/10.1016/j.arr.2014.05.002.
Kennedy, G., Hardman, R. J., Macpherson, H., Scholey, A. B., & Pipingas, A. (2017). How does exercise reduce the rate of age-associated cognitive decline? A review of potential mechanisms. Journal of Alzheimer’s Disease, 55, 1–18. https://doi.org/10.3233/JAD-160665.
Kim, K.-E., Jang, S.-N., Lim, S., Park, Y. J., Paik, N.-J., Kim, K. W., Jang, H. C., & Lim, J.-Y. (2012). Relationship between muscle mass and physical performance: Is it the same in older adults with weak muscle strength? Age and Ageing, 41, 799–803. https://doi.org/10.1093/ageing/afs115.
Kimura, K., Obuchi, S., Arai, T., Nagasawa, H., Shiba, Y., Watanabe, S., & Kojima, M. (2010). The influence of short-term strength training on health-related quality of life and executive cognitive function. Journal of Physiological Anthropology, 29, 95–101. https://doi.org/10.2114/jpa2.29.95.
Kirk-Sanchez, N., & McGough, E. L. (2014). Physical exercise and cognitive performance in the elderly: Current perspectives. Clinical Interventions in Aging, 9, 51–62.
Kraemer, W. J., & Ratamess, N. A. (2005). Hormonal responses and adaptations to resistance exercise and training. Sports Medicine, 35, 339–361.
Kramer, A. F., & Erickson, K. I. (2007). Capitalizing on cortical plasticity: Influence of physical activity on cognition and brain function. Trends in Cognitive Science, 11, 342–348. https://doi.org/10.1016/j.tics.2007.06.009.
Komulainen, P., Kivipelto, M., Lakka, T. A., Savonen, K., Hassinen, M., Kiviniemi, V., et al. (2010). Exercise, fitness and cognition—A randomised controlled trial in older individuals: The DR’s EXTRA study. European Geriatric Medicine, 1(5), 266–272. https://doi.org/10.1016/j.eurger.2010.08.001.
LaStayo, P. C., Ewy, G. A., Pierotti, D. D., Johns, R. K., & Lindstedt, S. (2003). The positive effects of negative work: Increased muscle strength and decreased fall risk in a frail elderly population. The Journals of Gerontology Series A Biological Sciences and Medical Sciences, 58, 419–424. https://doi.org/10.1093/gerona/58.5.M419.
Lachman, M. E., Neupert, S. D., Bertrand, R., & Jette, A. M. (2006). The effects of strength training on memory in older adults. Journal of aging and physical activity, 14, 59–73.
Latham, N. K., Bennett, D. A., Stretton, C. M., & Anderson, C. S. (2004). Systematic review of progressive resistance strength training in older adults. The Journals of Gerontology Series B Psychological Sciences and Social Sciences, 59, 48–61.
Li, F., Fisher, K. J., Harmer, P., McAuley, E., & Wilson, N. L. (2003). Fear of falling in elderly persons: Association with falls, functional ability, and quality of life. The Journals of Gerontology Series B Psychological Sciences and Social Sciences, 58, P283–P290. https://doi.org/10.1093/geronb/58.5.P283.
Li, Z., Peng, X., Xiang, W., Han, J., & Li, K. (2018). The effect of resistance training on cognitive function in the older adults: A systematic review of randomized clinical trials. Aging Clinical and Experimental Research, 30, 1259–1273. https://doi.org/10.1007/s40520-018-0998-6.
Liu-Ambrose, T., Nagamatsu, L. S., Voss, M. W., Khan, K. M., & Handy, T. C. (2012). Resistance training and functional plasticity of the aging brain: A 12-month randomized controlled trial. Neurobiology of Aging, 33(8), 1690–1698. https://doi.org/10.1016/j.neurobiolaging.2011.05.010.
Lustig, C., Shah, P., Seidler, R., & Reuter-Lorenz, P. A. (2009). Aging, training, and the brain: A review. Neuropsychology Review, 19, 504–522.
Mavros, Y., Gates, N., Wilson, G. C., Jain, N., Meiklejohn, J., Brodaty, H., Wen, W., Singh, N., Baune, B. T., Suo, C., Baker, M. K., Foroughi, N., Wang, Y., Sachdev, P. S., Valenzuela, M., & Fiatarone Singh, M. A. (2017). Mediation of cognitive function improvements by strength gains after resistance training in older adults with mild cognitive impairment: Outcomes of the study of mental and resistance training. Journal of the American Geriatrics Society, 65, 550–559. https://doi.org/10.1111/jgs.14542.
Middleton, L., Manini, T., Simonsick, E., Harris, T., Barnes, D., Tylasvsky, F., Brach, J., Everhart, J., & Yaffe, K. (2011). Activity energy expenditure and incident cognitive impairment in older adults. Archives of Internal Medicine, 171, 1251–1257. https://doi.org/10.1001/archinternmed.2011.277.
Moreau, D., Morrison, A. B., & Conway, A. R. (2015). An ecological approach to cognitive enhancement: Complex motor training. Acta Psychologica, 157, 44–55. https://doi.org/10.1016/j.actpsy.2015.02.007.
Nagamatsu, L. S., Handy, T. C., Hsu, C. L., Voss, M., & Liu-Ambrose, T. (2012). Resistance training promotes cognitive and functional brain plasticity in seniors with probable mild cognitive impairment. American Medical Association, 172, 2013–2015.
Nagamatsu, L. S., Chan, A., Davis, J. C., Beattie, B. L., Graf, P., Voss, M. W., et al. (2013). Physical activity improves verbal and spatial memory in older adults with probable mild cognitive impairment: a 6-month randomized controlled trial. Journal of Aging Research. https://doi.org/10.1155/2013/861893.
Nelson, M. E., Rejeski, W. J., Blair, S. N., Duncan, P. W., & Judge, J. O. (2007). Physical activity and public health in older adults: Recommendation from the American College of Sports Medicine and the American Heart Association. Circulation, 116, 1094–1105. https://doi.org/10.1161/circulationaha.107.185650.
Ortega, F. B., Ruiz, J. R., Castillo, M. J., & Sjöström, M. (2008). Physical fitness in childhood and adolescence: A powerful marker of health. International Journal of Obesity, 32, 1–11. https://doi.org/10.1038/sj.ijo.0803774.
Ouellette, M. M., LeBrasseur, N. K., Bean, J. F., Phillips, E., Stein, J., Frontera, W. R., & Fielding, R. A. (2004). High-intensity resistance training improves muscle strength, self-reported function, and disability in long-term stroke survivors. Stroke, 35, 1404–1409. https://doi.org/10.1161/01.STR.0000127785.73065.34.
Paillard, T. (2015). Preventive effects of regular physical exercise against cognitive decline and the risk of dementia with age advancement. Sports Medicine Open, 1, 1–6. https://doi.org/10.1186/s40798-015-0016-x.
Penedo, F. J., & Dahn, J. R. (2005). Exercise and well-being: A review of mental and physical health benefits associated with physical activity. Current Opinion in Psychiatry, 18, 189–193. https://doi.org/10.1097/00001504-200503000-00013.
Perrig-chiello, P., Perrig, W. J., Ehrsam, R., & Staehelin, H. B. (1998). The effects of resistance training onwell-being and memory in elderly volunteers. Age and Ageing, 27, 469–475.
Peterson, M. D., Rhea, M. R., & Alvar, B. A. (2005). Applications of the dose–response for muscular strength development. The Journal of Strength and Conditioning Research, 19, 950–958. https://doi.org/10.1519/00124278-200511000-00038.
Physical Activity [WWW Document], (2016). Retrieved from 11 October 2016, from https://www.healthypeople.gov/2020/topics-objectives/topic/Physical-Activity/objectives#5071.
Pluijm, S. M. F., Smit, J. H., Tromp, E. A. M., Stel, V. S., Deeg, D. J. H., Bouter, L. M., & Lips, P. (2006). A risk profile for identifying community-dwelling elderly with a high risk of recurrent falling: Results of a 3-year prospective study. Osteoporosis International, 17, 417–425. https://doi.org/10.1007/s00198-005-0002-0.
Radak, Z., Chung, H. Y., & Goto, S. (2008). Systemic adaptation to oxidative challenge induced by regular exercise. Free Radical Biology and Medicine, 44, 153–159. https://doi.org/10.1016/j.freeradbiomed.2007.01.029.
Rhea, M.R., Ball, S.D., Phillips, W.T., & Burkett, L.N. (2003). A comparison of linear and daily undulating periodized programs with equated volume and intensity for strength. The Journal of Strength and Conditioning Research 17, 82–87. https://doi.org/10.1519/1533-4287(2003)017%3C0082:ACOLAD%3E2.0.CO;2
Rovio, S., Kåreholt, I., Helkala, E. L., Viitanen, M., Winblad, B., Tuomilehto, J., Soininen, H., Nissinen, A., & Kivipelto, M. (2005). Leisure-time physical activity at midlife and the risk of dementia and Alzheimer’s disease. Neurology, 4, 705–711. https://doi.org/10.1016/S1474-4422(05)70198-8.
Sala, G., & Gobet, F. (2017). Does far transfer exist? Negative evidence from chess, music, and working memory training. Current Directions in Psychological Science, 26, 515–520. https://doi.org/10.1177/0963721417712760.
Sale, D. G. (1988). Neural adaptation to resistance training. Medicine and Science in Sports and Exercise, 20, 135–145.
Sibley, B. A., & Etnier, J. L. (2003). The relationship between physical activity and cognition in children: A meta-analysis. Pediatric Exercise Science, 15, 243–256.
Singh, N., Clements, K. M., & Fiatarone Singh, M.A. (1997). A randomized controlled trial of progressive resistance training in depressed elders. The Journals of Gerontology Series A Biological Sciences and Medical Sciences, 52, M27–M35. https://doi.org/10.1093/gerona/52A.1.M27.
Skriver, K., Roig, M., Lundbye-Jensen, J., Pingel, J., Helge, J. W., Kiens, B., & Nielsen, J. B. (2014). Acute exercise improves motor memory: Exploring potential biomarkers. Neurobiology of Learning and Memory. https://doi.org/10.1016/j.nlm.2014.08.004.
Smith, P. J., Blumenthal, J. A., Hoffman, B. M., Strauman, T. A., Welsh-bohmer, K., Jeffrey, N., & Sherwood, A. (2010). Aerobic exercise and neurocognitive performance: A meta-analytic review of randomized controlled trials. Psychosomatic Medicine, 72, 239–252. https://doi.org/10.1097/PSY.0b013e3181d14633.Aerobic.
Smolarek, A. C., Boiko Ferreira, L. H., Gomes Mascarenhas, L. P., McAnulty, S. R., Varela, K. D., Dangui, M. C., et al. (2016). The effects of strength training on cognitive performance in elderly women. Clinical Interventions in Aging, 11, 749–754. https://doi.org/10.2147/CIA.S102126.
Stroth, S., Hille, K., Spitzer, M., & Reinhardt, R. (2009). Aerobic endurance exercise benefits memory and affect in young adults. Neuropsychological Rehabilitation, 19, 223–243. https://doi.org/10.1080/09602010802091183.
Suo, C., Singh, M. F., Gates, N., Wen, W., Sachdev, P., Brodaty, H., Saigal, N., Wilson, G. C., Meiklejohn, J., Singh, N., Baune, B. T., Baker, M., Foroughi, N., Wang, Y., Mavros, Y., Lampit, A., Leung, I., & Valenzuela, M. J. (2016). Therapeutically relevant structural and functional mechanisms triggered by physical and cognitive exercise. Molecular Psychiatry, 21, 1633–1642. https://doi.org/10.1038/mp.2016.19.
Simons, D.J., Boot, W.R., Charness, N., Gathercole, S.E., Chabris, C.F., Hambrick, D.Z., Stine-Morrow, E.A.L., (2016). Do “Brain-Training” programs work? Psychology Science Public Interest. 17, 103–186. https://doi.org/10.1177/1529100616661983.
ten Brinke, L. F., Bolandzadeh, N., Nagamatsu, L. S., Hsu, C. L., Davis, J. C., Miran-Khan, K., & Liu-Ambrose, T. (2015). Aerobic exercise increases hippocampal volume in older women with probable mild cognitive impairment: A 6-month randomised controlled trial. British Journal of Sports Medicine, 49, 248–254. https://doi.org/10.1136/bjsports-2013-093184.
Timinkul, A., Kato, M., Omori, T., Deocaris, C. C., Ito, A., Kizuka, T., Sakairi, Y., Nishijima, T., Asada, T., & Soya, H. (2008). Enhancing effect of cerebral blood volume by mild exercise in healthy young men: A near-infrared spectroscopy study. Neuroscience Research, 61, 242–248. https://doi.org/10.1016/j.neures.2008.03.012.
Vasques, P. E., Moraes, H., Silveira, H., Deslandes, A. C., & Laks, J. (2011). Acute exercise improves cognition in the depressed elderly: The effect of dual-tasks. Clinics, 66, 1553–1557. https://doi.org/10.1590/S1807-59322011000900008.
Venturelli, M., Lanza, M., Muti, E., & Schena, F. (2010). Positive effects of physical training in activity of daily living-dependent older adults. Experimental Aging Research, 36(2), 190–205. https://doi.org/10.1080/03610731003613771.
Verburgh, L., Königs, M., Scherder, E. J. A., & Oosterlaan, J. (2014). Physical exercise and executive functions in preadolescent children, adolescents and young adults: A meta-analysis. British Journal of Sports Medicine, 48, 973–979. https://doi.org/10.1136/bjsports-2012-091441.
Viechtbauer, W. (2010). Conducting meta-analysis in R with the metafor package. Journal of Statistical Software, 36, 1–48.
Voss, M. W., Nagamatsu, L. S., Liu-ambrose, T., & Kramer, A. F. (2011). Exercise, brain, and cognition across the life span. Journal of Applied Physiology, 111, 1505–1513. https://doi.org/10.1152/japplphysiol.00210.2011.
Warburton, D. E. R., Nicol, C. W., & Bredin, S. S. D. (2006). Health benefits of physical activity: The evidence. CMAJ, 174, 801–809. https://doi.org/10.1503/cmaj.051351.
Yerokhin, V., Anderson-Hanley, C., Hogan, M. J., Dunnam, M., Huber, D., Osborne, S., & Shulan, M. (2012). Neuropsychological and neurophysiological effects of strengthening exercise for early dementia: A pilot study. Aging, Neuropsychology, and Cognition, 19, 380–401. https://doi.org/10.1080/13825585.2011.628378.
Yoon, D. H., Kang, D., Kim, H., Kim, J.-S., Song, H. S., & Song, W. (2016). Effect of elastic band-based high-speed power training on cognitive function, physical performance and muscle strength in older women with mild cognitive impairment. Geriatrics and Gerontology International. https://doi.org/10.1111/ggi.12784.
Yoon, D. H., & Song, W. (2018). Effects of resistance exercise training on cognitive function and physical performance in cognitive frailty. A Randomized Controlled Trial, 22, 944–951.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Landrigan, JF., Bell, T., Crowe, M. et al. Lifting cognition: a meta-analysis of effects of resistance exercise on cognition. Psychological Research 84, 1167–1183 (2020). https://doi.org/10.1007/s00426-019-01145-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00426-019-01145-x