Abstract
Background
Recent research has revealed a beneficial impact of chronic resistance exercise (RE) on brain function. However, it is unclear as to whether RE is also effective in an acute setting.
Objective
To investigate the immediate effects of a single RE session on cognitive performance in healthy adults.
Methods
A multilevel meta-analysis with random effects meta-regression model was used to pool the standardized mean differences (SMD) between RE and no-exercise (NEX) as well as between RE and aerobic exercise (AE). In addition to global cognitive function, effects on reported sub-domains (inhibitory control, cognitive flexibility, working memory, attention) were examined.
Results
Twelve trials with fair methodological quality (PEDro scale) were identified. Compared to NEX, RE had a positive effect on global cognition (SMD: 0.56, 95% CI 0.22–0.90, p = 0.004), but was not superior to AE (SMD: − 0.10, 95% CI 0.01 to − 0.20, p = 0.06). Regarding cognitive sub-domains, RE, compared to NEX, improved inhibitory control (SMD: 0.73, 95% CI 0.21–1.26, p = 0.01) and cognitive flexibility (SMD: 0.36, 95% CI 0.17–0.55, p = 0.004). In contrast, working memory (SMD: 0.35, 95% CI − 0.05 to 0.75, p = 0.07) and attention (SMD: 0.79, 95% CI − 0.42 to 2.00, p = 0.16) remained unaffected. No significant differences in sub-domains were found between RE and AE (p > 0.05).
Conclusion
RE appears to be an appropriate method to immediately enhance cognitive function in healthy adults. Further studies clearly elucidating the impact of effect modifiers such as age, training intensity, or training duration are warranted.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Previous literature shows that several weeks of resistance training induces moderate improvements in cognitive function. |
A single bout of resistance exercise leads to moderate improvements in cognitive function when compared to a no-exercise control. |
The acute effects of resistance exercise are not superior to those occurring after aerobic exercise. |
The impact of effect modifiers such as age, training duration, or training intensity needs to be further elucidated. |
1 Introduction
Engagement in physical activity represents a well-established method to elicit health-beneficial effects in a variety of peripheral organs such as the skeletal muscles, the heart, or the lungs [1]. Over recent decades, the potential impact of regular movement on brain morphology and function has evolved as another focus of research. Accumulating evidence suggests the occurrence of training-induced cerebral adaptations that may help to prevent or delay cognitive decline and neurodegenerative diseases; according to data from animal experiments, chronic exercise promotes synaptic plasticity, angiogenesis, and neurogenesis [2,3,4]. Human studies have, furthermore, demonstrated the expression of brain-derived neurotrophic factor (BDNF), as well as increases in hippocampal brain volume, in response to several weeks of training [5]. It has been hypothesized that the described exercise-induced changes in the brain, functionally, result in enhanced cognitive performance. This seems plausible as, for instance, angiogenesis allows enhanced perfusion and the hippocampus is related to learning and long-term memory [6].
To date, most studies examining the effects of physical activity on brain function have used aerobic-type exercise. Several meta-analyses, which included both acute and chronic interventions, detected small to moderate training-induced improvements in domains such as processing speed, attention, executive function, and memory [7,8,9,10,11,12,13,14,15,16,17]. In view of the positive effects observed, recent research has also been dedicated to the potential impact of resistance exercise. Compared to endurance training, which typically consists of the repetitive execution of one specific movement pattern (e.g., running or cycling), resistance training often involves a series of different exercises in both the upper and lower limbs. It may, therefore, be speculated that strength training, with its higher variability, may stimulate the brain at least to a similar degree as aerobic exercise does.
Existing systematic reviews found mixed, but mainly small, positive effects of resistance exercise on different measures of cognition [18,19,20,21]. Their findings are in line with data reported by two meta-analyses of trials recruiting healthy older adults for chronic training interventions. Kelly et al. [22] did not detect an effect of resistance exercise on working memory or attention, but did observe a large improvement in reasoning. However, their sample was relatively small as they identified only a maximum of three studies per cognitive dimension. Northey et al. [15] were able to pool the results of 13 trials; the authors reported moderate effects of resistance exercise on executive function (standardised mean difference [SMD]: 0.49), memory (SMD: 0.54), and working memory (SMD: 0.49).
In contrast to the data available for chronic regimes, evidence is scarce with regard to the immediate effects of one training session. The only existing systematic analysis of the literature examining this issue [23] is rather old, did not provide a quantitative data synthesis, and was focussed on children. The present meta-analysis, therefore, aimed to investigate the acute effects of a single resistance exercise bout on cognitive function in healthy adults.
2 Methods
A systematic review with multilevel meta-analysis and a random effect meta-regression model was performed according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines [24]. It followed the recommendations for ethical publishing of systematic reviews proposed by Wager and Wiffen [25]. The study was registered in the PROSPERO database (CRD42018089914).
2.1 Search Strategy
Two independent investigators (JW, FG) performed a systematic literature search between February and April 2018. Relevant articles were identified using the online databases MEDLINE (PubMed), ScienceDirect, Web of Science, Cochrane Central, and Google Scholar. For Google Scholar, an approach described in previous systematic reviews of our work group was used [26, 27]. In addition to the database search, the reference lists of all included studies were checked in order to identify other eligible papers [28].
2.2 Inclusion Criteria
Randomized controlled trials (crossover or parallel-group design) with accessible full text were considered for inclusion. Further criteria were (1) enrolment of healthy adults, (2) performance of resistance exercise, (3) testing of acute effects on cognitive function (measurement starting within 5 min post-exercise), and (4) publication in the English language and in a peer-reviewed journal. All studies investigating chronic effects, other training methods, or persons with diseases were excluded.
2.3 Data Extraction
Using a standardized assessment sheet, two investigators (KK, FG) independently performed the data extraction. They retrieved the following information: study design, sample size, participant characteristics, interventions, measured outcomes (tests and related cognitive sub-dimension; Table 1) and results (pre-post changes plus standard deviations of each intervention arm). The primary outcome of the meta-analysis was global cognition, which included pooling of all clinically validated measures of cognitive function. If a study performed more than one cognitive test, or had more sub-domains in one test, all effect sizes (ES) were extracted.
2.4 Data Synthesis and Statistics
For each intervention arm of the parallel-group studies, the mean pre to post changes plus standard deviations (SDs) were retrieved. If reporting was incomplete (i.e., missing SDs of the changes from baseline), the corresponding authors of the trials were contacted to request the missing information. If no values could be obtained, missing data were determined from figures or imputed according to the recommendations in the Cochrane handbook, using the formula \({\text{SD}}_{\text{change}} = \surd \left( {{\text{SD}}_{\text{baseline}}^{2} + {\text{SD}}_{\text{postintervention}}^{2} } \right) - \left( { 2\times {\text{Corr}} \times {\text{SD}}_{\text{baseline}} \times {\text{SD}}_{\text{postintervention}} } \right)\), where Corr = 0.7. The value chosen for Corr has previously been recommended as a conservative estimate of the correlation between the baseline and post-treatment SDs [32]. For crossover trials, the SD of the difference between the two relevant conditions’ pre-post changes, the correlation of the respective pre-post changes, and the standard error were calculated. If the correlation coefficient could not be extracted from publications or raw data, a conservative value of 0.5 was assumed, which also fits with the known correlations of the other included studies. When combining the results from parallel-group and crossover studies, we used appropriate formulae for standardized mean ES and standard errors [33].
The following potential moderators of the treatment effect were coded as numerical data: cognitive domain (inhibitory control, cognitive flexibility, working memory, attention), study design (parallel-group, crossover trial), training duration (short: ≤ 30 min, long: > 30 min), training intensity (light: < 50% of the 1-repetition maximum (1 RM), moderate: 50–75% 1 RM, high: 75–100% 1 RM) and age (young: ≤ 40 years, old > 40 years).
A multilevel meta-analysis with a robust random effects meta-regression model was used to pool the SMD and 95% confidence intervals (CIs) between resistance exercise (RE) and aerobic exercise (AE), as well as between RE and no-exercise control (NEX [34]). Dependency of ES was taken into account by nesting the term “study” as a random factor in the model. Potential moderators were identified with separate models: (1) estimating the significance of each level by means of the 95% CI and (2) testing for differences between the respective levels [15]. The between-study variance component was determined by means of Tau2, using the method-of-moments estimate; for within-study variance (more than one dependent effect size), omega2 (ω2) was calculated [34]. p values < 0.05 were considered significant. The software employed was R (R Foundation for Statistical Computing, Vienna, Austria), packages meta (G Schwarzer), and robumeta (version 2.0, [35]).
2.5 Sensitivity Analyses and Risk of Bias
To test the robustness of imputed values, sensitivity analyses were performed for each outcome; this was achieved by means of comparing the result of the primary meta-analyses (studies with fully available data plus studies with imputed SDs) against a secondary analysis including only trials with fully available data. If the conclusions drawn, based on the ES and confidence intervals, were identical, then the findings of the primary meta-analysis were deemed robust [36]. Publication bias was examined by means of visual inspection of funnel plots (ES against standard errors) and optional sensitivity analyses excluding potential outliers.
The methodological quality of the included trials was rated by means of the PEDro scale [29], which has been demonstrated to represent a reliable and valid assessment tool [29,30,31]. The sum score of the instrument is based on the rating of 10 items assessing potential sources of bias. Two independent examiners (JW, FG) performed the quality scoring. In case of disagreement, a third investigator (KK) provided the decisive vote.
3 Results
3.1 Search Results
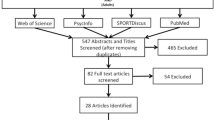
A flow diagram of the literature search is displayed in Fig. 1. The algorithms used returned a total of 1611 records. Twelve studies [34,35,36,37,38,39,40,41,42,43,44,45] met the eligibility criteria and were included in the review.
3.2 Characteristics of the Studies
All included papers collectively evaluated 447 participants (239 men and 208 women) with mean ages ranging from 20.4 to 72.3 years (Table 2). Eleven studies [37,38,39,40,41,42,43,44, 46,47,48] compared the acute effects of RE and CON, whereas five studies [39, 40, 45,46,47] compared the impact of RE and AE on brain function. Complete data were available or obtained from figures for seven studies [40, 42,43,44, 46,47,48], whilst imputation was needed for five trials [37,38,39,40,41, 45].
3.3 Methodological Quality
The two reviewers agreed on 116 (96.3%) of the 120 criteria scored by means of the PEDro scale. All disagreements were resolved by discussion without consulting the third investigator. The methodological quality of the 12 trials included ranged from 2 to 5 out of 10 and the mean score (3.7 ± 1) was classified as fair. Most studies reported randomization, had comparable baseline values between conditions or groups, analyzed group differences, and included point and variability measures (Table 3). Since some studies used a crossover design, a substantial share of the sample was not able to satisfy the criterion of concealed allocation because it was a priori evident that all participants would receive each intervention.
3.4 Resistance Exercise versus No Exercise
A medium ES favouring RE over NEX was found (n = 86 ES, SMD: 0.56, 95% CI 0.22–0.90, p = 0.004, Tau2: 0.38, ω2: 0). According to the moderator analysis (Table 4), RE was more effective in some of the sub-domains, improving inhibitory control (SMD: 0.73, 95% CI 0.21–1.26, p = 0.01, Fig. 2) and cognitive flexibility (SMD: 0.36, 95% CI 0.17–0.55, p = 0.004, Fig. 3). A tendency approaching but failing statistical significance was found in working memory (SMD: 0.35, 95% CI − 0.05 to 0.75, p = 0.07, Fig. 4). For attention, no difference between RE and NEX was found (SMD: 0.79, 95% CI − 0.42 to 2.00, p = 0.16). With regard to the other moderators, factors statistically associated with a superiority of RE were parallel-group study design, a higher treatment duration, younger age, and a low and high (but not moderate) exercise intensity (p < 0.05). However, the ES and p values of the other levels of these variables were mostly similar, which implies a rather marginal impact (Table 4). This was confirmed by the fact that no between-level differences were found in any of the potential moderators.
Effects of resistance exercise vs. no-exercise control in the domain of inhibitory control. Forest plots with pooled standardized mean differences (SMD), standard errors (SEs), and 95% confidence intervals (CIs) are displayed. Inc incongruent condition, int interference score, LI low intensity, MI moderate intensity, HI high intensity, RT reaction time, acc accuracy, RE random effects
Effects of resistance exercise vs. no-exercise control in the domain of working memory. Forest plots with pooled standardized mean differences (SMDs), standard errors (SEs), and 95% confidence intervals (CIs) are displayed. PASAT Paced Auditory Serial Addition Test, LI low intensity, MI moderate intensity, HI high intensity, acc accuracy, RT reaction time, RE random effects
3.5 Resistance Exercise versus Aerobic Exercise
Although the position of the confidence interval almost suggested a trend for the slight superiority of AE, there was no significant difference between RE and AE (SMD: − 0.10, 95% CI 0.01 to − 0.20, p = 0.06, Tau2: 0.03, ω2: 0, n = 26 ES). Similarly, no condition was more effective in the investigated sub-domains. Further moderator analyses were possible for the variables study design and participants’ age (Table 4). While there were no differences between levels, the factors crossover-design and old age of participants were significant (p < 0.05).
3.6 Risk of Bias
Sensitivity analyses for imputed data did not yield any indication of bias. Visual inspection of the funnel plot (Fig. 5) suggested a potential publication bias due to the large effects and standard errors in one study [42]. A sensitivity analysis without this study, however, showed that the results were robust; only the effect of RE on attention became significant (SMD 0.39, 95% CI 0.16–0.62, p = 0.008). The findings including this trial (which was kept in the model) are therefore a conservative estimate of the treatment effects.
Funnel plot of the overall effect of resistance exercise on measures of cognitive function (effect sizes against standard error). Note the outliers on the right. Sensitivity analyses without these data from Chang et al. [42] showed that the result was robust
4 Discussion
This systematic review is the first to summarize the available evidence for the acute effects of RE on cognitive function. Our results demonstrate that a single training session can induce moderate improvements in performance. Previous meta-analyses investigating the long-term effects following chronic RE interventions mostly reported smaller ES [11, 18,19,20,21]. The hypothesized mechanisms by which exercise may affect cognitive function could explain this difference. Acute AE has been shown to enhance the blood flow in the brain [49, 50]. A similar effect may occur following RE. However, while the main modulators of cerebral perfusion after AE are neuronal demand, cardiac output, and partial pressure of arterial carbon dioxide, RE can be speculated to rather cause blood flow variations through oscillations and/or peaks in blood pressure [50]. Another potential factor relates to alterations in serum cortisol level. In their study, Tsai et al. [43] measured higher concentrations of the stress hormone after RE. Interestingly, these increases were associated with higher arousal, a psycho-physiological state of being awake and attentive, which, in turn, could influence brain function.
While circulatory processes (blood flow, hormones) appear to moderate acute exercise-induced changes in cognitive performance, the long-term response may rather be explained by structural adaptations. It has been speculated that chronic RE triggers adult neurogenesis, which is supported by recent data. For example, when Yarrow et al. [51] examined the association between RE and expression of BDNF they detected elevated serum concentrations of the substance immediately post-training. Furthermore, following a 5-week intervention period, the exercise-induced increases in BDNF were even more pronounced, suggesting the importance of repeated stimuli [51]. In another study by Tsai et al. [52], higher serum levels of insulin growth factor 1 (IGF-1), which is related to neurogenesis and synaptogenesis [53], were measured after a 12-month RE intervention. Finally, Best et al. [54] demonstrated that 52 weeks of RE reduced age-related white matter atrophy in older women [54].
Our review detected no difference between RE and AE in acute effects on brain function. This result is in line with the meta-analysis of Northey et al. [15], who investigated the effects of chronic exercise on cognition in adults aged 50 years and older. Pooling data from 39 studies, these authors calculated comparable SMDs for RE (0.29) and AE (0.25). Notwithstanding, our result of the similar effectiveness of RE and AE in an acute setting should be interpreted with caution. The lower limit of the comparison’s confidence interval (− 0.01 to 0.20, SMD of 0.10 in favour of AE) was very close to zero and, hence, one additional study favouring AE may have been sufficient to alter the meta-analytic outcome. Additional research is necessary in order to answer the question of whether AE could be slightly more effective.
Acute enhancements of cognitive function may be of value in different contexts. The use of resistance exercises during warm-up could play a role in the prevention of sports injuries because, in most situations potentially causing trauma, athletes are required to rapidly integrate and process a multitude of sensory information on a supraspinal level, developing and adjusting motor plans under high time constraints. The tested cognitive domains (particularly inhibitory control and cognitive flexibility) represent key factors within this framing [55]. Moreover, although data from prospective trials are still sparse, a study by Wilkerson [56] found that neurocognitive reaction time could be used to predict lower extremity injuries (relative risk: 2.2). Besides helping to prevent musculoskeletal injury, cognitive improvements induced by RE could also aid game-related decision-making by athletes and, with this, increase sports performance. In a cross-sectional study, Huijgen et al. [57] demonstrated that elite youth football players display superior cognitive flexibility and inhibitory control when compared to sub-elite players. Outside the sports setting, acute RE sessions can be of use in occupational or academic settings, as work- and study-related abilities, such as cognitive flexibility and working memory, may be increased by active breaks, i.e., during lunchtime.
Despite the promising fields of application, several aspects call for further research. The studies included mainly focused on the effects of RE on different aspects of executive function. However, it would be intriguing to elucidate its impact on other domains such as episodic memory. Furthermore, while our systematic review provides compelling evidence for the occurrence of acute RE-induced increases in cognitive performance, little is known about the sustainability of the effects. Only two of the 12 included studies performed a follow-up measurement. In the first of these, Pontifex et al. [39] did not find enhancements of cognitive function at 30 min post-RE. The second trial by Johnson et al. [45] found persisting, but non-significant, increases at 30 and 60 min in some outcomes, which were ascribed to high data variability. The most important aspect warranting additional investigation relates to the impact of effect modifiers. We made a strong effort to include a series of potentially relevant variables in the moderator analysis. However, the value of the conclusions to be drawn is limited. With regard to training parameters and participant characteristics, there was little variability, often only allowing a binary classification. For instance, in the majority of the included trials, the participants exercised for around 30 min. Only one study used a very short duration (10 min [45]), and none of the trials performed sessions longer than 45 min. The same (small between-study variation) applied to the factor age. It is, hence, not surprising that the ES and p values of the different levels were similar even if one of them was significant. Another issue that needs to be noted became evident for training intensity. Whilst some trials used percentages of 1 RM, others used 10RM. Due to this and because of the large CI in the effects of RE on cognitive function at moderate intensity, the suggested U-shaped relationship (superiority of low- and high-intensity exercise) should be considered with great care.
In sum, contrary to the general effects of RE on cognitive function and related sub-domains, the optimal training parameters (intensity, duration, repetitions) and conditions (age, sex) are still unclear and are yet to be elucidated.
5 Conclusions
Based on the available evidence, resistance exercise appears to represent an adequate method to acutely improve cognitive function. This finding may be of value for athletes aiming to prevent injury and to improve performance in team sports, or employees seeking to advance job performance. Future research should examine dose-response relationships and the sustainability of the effects of RE on cognitive function.
References
Löllgen H, Böckenhoff A, Knapp G. Physical activity and all-cause mortality: an updated meta-analysis with different intensity categories. Int J Sports Med. 2009;30:213–24.
Vaynman S, Gomez-Pinilla F. License to run: exercise impacts functional plasticity in the intact and injured central nervous system by using neurotrophins. Neurorehabil Neural Repair. 2016;19:283–95.
Thomas AG, Dennis A, Bandettini PA, et al. The effects of aerobic activity on brain structure. Front Psychol. 2012;3.
Liu PZ, Nusslock R. Exercise-mediated neurogenesis in the hippocampus via BDNF. Front Neurosci. 2018;12:450.
Kandola A, Hendrikse J, Lucassen PJ, et al. Aerobic exercise as a tool to improve hippocampal plasticity and function in humans: practical implications for mental health treatment. Front Hum Neurosci. 2016;10:373.
Howland JG, Wang YT. Chapter 8 Synaptic plasticity in learning and memory: stress effects in the hippocampus. Essence of memory. Amsterdam: Elsevier; 2008. p. 145–58.
Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults. Psychol Sci. 2016;14:125–30.
Smith PJ, Blumenthal JA, Hoffman BM, et al. Aerobic exercise and neurocognitive performance: a meta-analytic review of randomized controlled trials. Psychosom Med. 2010;72:239–52.
Hindin SB, Zelinski EM. Extended practice and aerobic exercise interventions benefit untrained cognitive outcomes in older adults: a meta-analysis. J Am Geriatr Soc. 2012;60:136–41.
McMorris T, Sproule J, Turner A, et al. Acute, intermediate intensity exercise, and speed and accuracy in working memory tasks: a meta-analytical comparison of effects. Physiol Behav. 2011;102:421–8.
Chang Y, Labban J, Gapin J, et al. The effects of acute exercise on cognitive performance: a meta-analysis. Brain Res. 2012;1453:87–101.
Ludyga S, Gerber M, Brand S, et al. Acute effects of moderate aerobic exercise on specific aspects of executive function in different age and fitness groups: a meta-analysis. Psychophysiology. 2016;53:1611–26.
Groot C, Hooghiemstra A, Raijmakers P, et al. The effect of physical activity on cognitive function in patients with dementia: a meta-analysis of randomized control trials. Ageing Res Rev. 2016;25:13–23.
Firth J, Stubbs B, Rosenbaum S, et al. Aerobic exercise improves cognitive functioning in people with schizophrenia: a systematic review and meta-analysis. Schizophr Bull. 2016;43:546–56.
Northey JM, Cherbuin N, Pumpa KL, et al. Exercise interventions for cognitive function in adults older than 50: a systematic review with meta-analysis. Br J Sports Med. 2018;52:154–60.
de Greeff JW, Bosker RJ, Oosterlaan J, et al. Effects of physical activity on executive functions, attention and academic performance in preadolescent children: a meta-analysis. J Sci Med Sport. 2018;21:501–7.
Song D, Yu DS, Li PW, et al. The effectiveness of physical exercise on cognitive and psychological outcomes in individuals with mild cognitive impairment: a systematic review and meta-analysis. Int J Nurs Stud. 2018;79:155–64.
van Uffelen JGZ, Chin Paw MJM, Hopman-Rock M, et al. The effects of exercise on cognition in older adults with and without cognitive decline: a systematic review. Clin J Sport Med. 2008;18:486–500.
Snowden M, Steinman L, Mochan K, et al. Effect of exercise on cognitive performance in community-dwelling older adults: review of intervention trials and recommendations for public health practice and research. J Am Geriatr Soc. 2011;59:704–16.
Loprinzi PD, Frith E, Edwards MK. Resistance exercise and episodic memory function: a systematic review. Clin Physiol Funct Imaging. 2018;8:e76301.
Sáez de Asteasu ML, Martínez-Velilla N, Zambom-Ferraresi F, et al. Role of physical exercise on cognitive function in healthy older adults: a systematic review of randomized clinical trials. Ageing Res Rev. 2017;37:117–34.
Kelly ME, Loughrey D, Lawlor BA, et al. The impact of exercise on the cognitive functioning of healthy older adults: a systematic review and meta-analysis. Ageing Res Rev. 2014;16:12–31.
Sibley BA, Etnier JL. The relationship between physical activity and cognition in children: a meta-analysis. Pediatr Exerc Sci. 2003;15:243–56.
Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535.
Wager E, Wiffen PJ. Ethical issues in preparing and publishing systematic reviews. J Evid Based Med. 2011;4:130–4.
Wilke J, Krause F, Vogt L, et al. What is evidence-based about myofascial chains: a systematic review. Arch Phys Med Rehabil. 2016;97:454–61.
Krause F, Wilke J, Vogt L, et al. Intermuscular force transmission along myofascial chains: a systematic review. J Anat. 2016;228:910–8.
Horsley T, Dingwall O, Sampson M. Checking reference lists to find additional studies for systematic reviews. Cochrane Database Syst Rev. 2011;73:505.
Maher CG, Sherrington C, Herbert RD, et al. Reliability of the PEDro scale for rating quality of randomized controlled trials. Phys Ther. 2003;83:713–21.
Foley CF, Bhogal SK, Teasell RW, et al. Estimates of quality and reliability with the physiotherapy evidence-based database scale to assess the methodology of randomized controlled trials of pharmacological and nonpharmacological interventions. Phys Ther. 2006;86(6):817–24.
de Morton NA. The PEDro scale is a valid measure of the methodological quality of clinical trials: a demographic study. Aust J Physiother. 2009;55:129–33.
Rosenthal R. Meta-analytic procedures for social research. Newbury Park: Sage Publications; 1993.
Curtin F, Altman DG, Elbourne D. Meta-analysis combining parallel and cross-over clinical trials I: continuous outcomes. Stat Med. 2002;21:2131–44.
Hedges LV, Tipton E, Johnson MC. Robust variance estimation in meta-regression with dependent effect size estimates. Res Synth Methods. 2010;1:39–65.
Fisher Z, Tipton E. Robumeta. an r package for robust variance estimation in meta-analysis. https://arxiv.org/abs/1503.02220.
Radford JA, Burns J, Buchbinder R, et al. Does stretching increase ankle dorsiflexion range of motion? A systematic review. Br J Sports Med. 2006;40:870–5.
Chang Y, Etnier JL. Effects of an acute bout of localized resistance exercise on cognitive performance in middle-aged adults: a randomized controlled trial study. Psychol Sport Exerc. 2009;10:19–24.
Chang Y, Etnier JL. Exploring the dose-response relationship between resistance exercise intensity and cognitive function. J Sport Exerc Psychol. 2009;31:640–56.
Pontifex MB, Hillman CH, Fernhall BO, et al. The effect of acute aerobic and resistance exercise on working memory. Med Sci Sports Exerc. 2009;41:927–34.
Alves CRR, Gualano B, Takao PP, et al. Effects of acute physical exercise on executive functions: a comparison between aerobic and strength exercise. J Sport Exerc Psychol. 2012;34(4):539–49.
Chang Y, Ku P, Tomporowski PD, et al. Effects of acute resistance exercise on late-middle-age adults’ goal planning. Med Sci Sports Exerc. 2012;44:1773–9.
Chang Y, Tsai C, Huang C, et al. Effects of acute resistance exercise on cognition in late middle-aged adults: general or specific cognitive improvement? J Sci Med Sport. 2014;17:51–5.
Tsai C, Wang C, Pan C, et al. Executive function and endocrinological responses to acute resistance exercise. Front Behav Neurosci. 2014;8:283.
Hsieh S, Chang Y, Hung T, et al. The effects of acute resistance exercise on young and older males’ working memory. Psychol Sport Exerc. 2016;22:286–93.
Johnson L, Addamo PK, Selva Raj I, et al. An acute bout of exercise improves the cognitive performance of older adults. J Aging Phys Act. 2016;24:591–8.
Chang H, Kim K, Jung Y, et al. Effects of acute high-intensity resistance exercise on cognitive function and oxygenation in prefrontal cortex. J Exerc Nutr Biochem. 2017;21(2):1–8.
Dunsky A, Abu-Rukun M, Tsuk S, et al. The effects of a resistance vs an aerobic single session on attention and executive functioning in adults. PLoS One. 2017;12(4):0176092.
Tsukamoto H, Suga T, Takenaka S, et al. An acute bout of localized resistance exercise can rapidly improve inhibitory control. PLoS One. 2017;12(9):e0184075.
Querido JS, Sheel AW. Regulation of cerebral blood flow during exercise. Sports Med. 2007;37(9):765–82.
Ogoh S, Ainslie PN. Cerebral blood flow during exercise: mechanisms of regulation. J Appl Physiol. 2009;107(5):1370–80.
Yarrow JF, White LJ, McCoy SC, et al. Training augments resistance exercise induced elevation of circulating brain derived neurotrophic factor (BDNF). Neurosci Lett. 2010;479:161–5.
Tsai C, Wang C, Pan C, et al. The effects of long-term resistance exercise on the relationship between neurocognitive performance and GH, IGF-1, and homocysteine levels in the elderly. Front Behav Neurosci. 2015;9:471.
Nieto-Estévez V, Defterali Ç, Vicario-Abejón C. IGF-I: a key growth factor that regulates neurogenesis and synaptogenesis from embryonic to adult stages of the brain. Front Neurosci. 2016;10:2896.
Best JR, Chiu BK, Liang Hsu C, et al. Long-term effects of resistance exercise training on cognition and brain volume in older women: results from a randomized controlled trial. J Int Neuropsychol Soc. 2015;21:745–56.
Grooms D, Appelbaum G, Onate J. Neuroplasticity following anterior cruciate ligament injury: a framework for visual-motor training approaches in rehabilitation. J Orthop Sports Phys Ther. 2015;45:381–93.
Wilkerson GB. Neurocognitive reaction time predicts lower extremity sprains and strains. Int J Athl Ther Train. 2012;17:4–9.
Huijgen BCH, Leemhuis S, Kok NM, et al. Cognitive functions in elite and sub-elite youth soccer players aged 13–17 years. PLoS One. 2015;10:e0144580.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No sources of funding were used to assist in the preparation of this article.
Conflict of interest
Jan Wilke, Florian Giesche, Kristina Klier, Lutz Vogt, Eva Herrmann, and Winfried Banzer declare that they have no conflicts of interest relevant to the content of this review.
Rights and permissions
About this article
Cite this article
Wilke, J., Giesche, F., Klier, K. et al. Acute Effects of Resistance Exercise on Cognitive Function in Healthy Adults: A Systematic Review with Multilevel Meta-Analysis. Sports Med 49, 905–916 (2019). https://doi.org/10.1007/s40279-019-01085-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40279-019-01085-x