Abstract
Main conclusion
We found a new in vivo route to produce maternal doubled haploid of Brassica napus . The pollen donor, an allooctaploid rapeseed, acts as a DH inducer.
Inbred line has a powerful advantage in cultivar breeding and genetic analysis. Compared to the traditional breeding methods, doubled haploid production can save years off the breeding process. Though genotype-dependent tissue culture methods are widely used in the Brassica crops, seed-based in vivo doubled haploid developing systems are rare in nature and in the laboratory. As interspecific cross and interploid hybridization play an important role in genome evolution and plant speciation, we created a new Brassica artificial hybrid, a Brassica allooctaploid (AAAACCCC, 2n = 8× = 76), by interspecific crossing and genome doubling. A homozygous line was observed at the third self-generation of a synthesized Brassica allohexaploid (AAAACC, 2n = 6× = 58). Crosses between B. napus as female and Brassica allooctaploid as pollen donor were conducted, which yielded maternal doubled haploid B. napus that were identified based on phenotype, ploidy, and molecular analysis. The Brassica octaploid acted as a maternal doubled haploid inducer and had a relatively high induction rate. Our research provides a new insight for generation of homozygous lines in vivo using a single-step approach, as well as promotes the understanding in breeding programs and genetic studies involving the Brassicas.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Polyploidy is a widespread phenomenon in angiosperms and is considered to play a major role in genome evolution (Ramsey and Schemske 1998; Wendel 2000). Most flowering plant lineages reflect one or more rounds of ancient polyploidization events (Jiao et al. 2011), often followed by massive silencing and elimination of duplicated genes (Adams and Wendel 2005). Duplicated genes can be lost, retained, or maintained as duplicates, which often undergo subfunctionalization and neofunctionalization (Comai 2005). In addition to divergence and functional diversification following genome duplication, polyploidy can lead to immediate and extensive changes in gene expression through gene dosage effects, altered regulatory interactions, and genetic and epigenetic changes (Adams and Wendel 2005; Comai 2005; Jiao et al. 2011).
Brassica species, which have a propensity for genome duplication and genome mergers (Boulos et al. 2014), have been used as a model for studying the evolution of polyploidy genomes, mechanisms of duplicated gene loss, neo- and sub-functionalization, and its associated impact on morphological diversity and species differentiation (Boulos et al. 2014; Liu et al. 2014). Interspecific crosses among the Brassicas have significantly contributed to plant evolution by generating new ecotypes or new species and by allowing gene exchanges (Leflon et al. 2006).
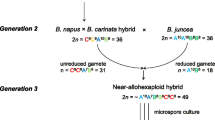
B. napus (AACC, 2n = 4× = 38) is formed by the natural hybridization between B. rapa (AA, 2n = 20) and B. oleracea (CC, 2n = 18), followed by chromosome doubling. Oilseed rape is tetraploid, thereby rendering it difficult to generate homozygous lines, which usually need at least 6–8 years. On the other hand, haploid plants can be generated and subsequently doubled to produce true-breeding lines that are entirely homozygous within two generations. Since 1982, isolated microspore cultures have been successfully applied to the generation of B. napus haploids (Lichter 1982), rendering it is a useful and common breeding tool. However, this technique is tedious, expensive, and restricted to certain genotypes (Seymour et al. 2012). In some other species, wide crosses or intraspecific crosses using lines with specific genetic determinants yield haploid embryos because the chromosomes of the haploid inducer (HI) are lost during postzygotic mitotic divisions, whereas those of the non-inducer parent are retained (Zhao et al. 2013). HIs have been reported in maize (Coe 1959), wheat (Dorota et al. 2005), barley (Sanei et al. 2011), potato (Liu and Douches 1993), Arabidopsis thaliana (Ravi et al. 2014), and many other plants. However, no HI has been detected in B. napus.
Here, we create two new Brassica artificial hybrids, a Brassica allohexaploid (AAAACC, 2n = 6× = 58) that was produced by genome doubling of a Brassica triploid hybrid and a Brassica allooctaploid (AAAACCCC, 2n = 8× = 76) that was generated by genome duplication of B. napus. At the third generation of selfing from the Brassica allohexaploid, a homozygous B. napus line was observed. Maternal B. napus doubled haploid (DH) were produced by interploid hybridization between B. napus and Brassica octaploid, which, to our knowledge, is a phenomenon that has not been reported in the literature. We hereby describe for the first time a doubled haploid inducer of B. napus and a Brassica octaploid as pollen donor, which yields homozygous line after one generation without colchicine treatment, and thus may significantly accelerate rapeseed breeding and genetic research.
Materials and methods
Plant materials
F009 (B. napus, AACC, 2n = 38, allotetraploid) is a steady double-low (low erucic acid and low glucosinolate content) line that is bred by the Crop Research Institution of the Chengdu Academy of Agricultural and Forest Sciences. Ya’an Huang (YH, B. rapa, AA, 2n = 20) is a native variety originating from Ya’an City (N28°51′10″–30°56′40″, E101°56′26″–103°23′28″), Sichuan Province, has a short life period, and is characterized by yellow seed skin and high oil content (~45%). The Brassica triploid hybrids (AAC, 2n = 3× = 29) were produced from a cross between F009 as female and YH as male. Brassica hexaploid hybrids were produced by colchicine treatment of Brassica triploid hybrid. Self-pollination of the hexaploid was performed, which yielded a homozygous tetraploid B. napus line that we designated as P3-2 that showed steady growth and development in third generation, and was subsequently identified in the fourth generation. Zhongshuang 11 (ZS11) is a homozygous B. napus variety that was bred by the Oilcrops Research Institute, Chinese Academy of Agricultural Sciences and certified by the National Crop Variety Approval Committee of China in 2008.
Y3560 is a Brassica octaploid plant, 4247, 1365 and 1325 are homozygous B. napus lines, and 3321, 3323, WLA and 0068A are homozygous Polima cytoplasmic male sterile B. napus lines. The whole plant of WLA is waxless and bright green in color. 3209, 3304, and 3118 are steady genic male sterile lines of B. napus, which are controlled by a pair of recessive genes.
Chromosomes doubling
Brassica hexaploid and Brassica octaploid were generated by colchicine treatment. Seeds were sowed on an MS medium with 30 mg/L colchicine until the hypocotyl of the seedlings grew to a height of 1–2 cm. The hypocotyl of the seedlings was cut and cultured in the MS medium supplemented with 30 mg/L of colchicine to generate DH plants.
Analysis of chromosome number during mitotic metaphase
The number of chromosomes in Brassica triploid, hexaploid, and Brassica octaploid was determined using root tip somatic cells and chromosome spread technique. Young root tips of approximately 0.5 cm were soaked in 8-hydroxyquinoline at 4 °C for 24 h, followed by immersion in Carnoy’s fixative (alcohol:acetic acid = 3:1 v/v) for 24 h, and then in 70% alcohol for long-term storage. Before observation under a microscope, the root tips were washed with distilled until cleared then immersed in 1 mol/L HCl for 8–10 min in a 60 °C water bath. The numbers of chromosome were determined under a microscope after staining with carbol fuchsin.
Cytogenetic studies during meiosis
Pollen mother cells (PMCs) were used to study the behavior of chromosomes of the hexaploid and octaploid plants. Floral tissues were fixed in Carnoy’s solution. The anthers were dissected from fixed flower buds using a dissecting needle in a drop of staining solution (carbol fuchsin). After removing other floral tissues, the anthers were fully submerged in 10–20 µL of staining solution that was placed onto the slide. A thin coverslip was then placed on the slide and gently pressed with the plunger end of the needle until the microspores were finely extruded out from the anther sacs.
Pollen viability observation
Pollen was stained with 1% acetocarmine (Jugulam et al. 2015; Kuligowska et al. 2015; Wang et al. 2015) and observed in an inverted microscope. When the pollen grain is stained with acetocarmine, viable pollen appears stained and spherical in shape; conversely, non-viable pollen is colorless.
Flow cytometry analysis
To determine the genome size flow, cytometry analyses were performed using Vieira et al. (2014) method for the induced generations using young leaf tissues. Approximately 100 mg of fresh leaf tissues from young plants was selected and used in flow cytometry analysis. The leaves were washed with distilled water, blotted with filter paper, and then placed in a pre-cooling Petri dish. Then, 2 mL of pre-cooling cell lysis buffer (MOPS, 20 mmol/L; sodium citrate, 30 mmol/L; MgCl2, 45 mmol/L; Triton X-100, 0.2% v/v; pH 7) was added into the Petri dish, and immediately followed by cutting up the leaf with a sharp shaver. The mixture was then filtered twice with a 30-mesh strainer. Approximately 1 mL of the filtrate was then centrifuged at 152.94g for 5 min at 4 °C. The supernatant was discarded and 20 µL of RNase (500 µg/mL) and 400 µL of propidium iodide solution (50 µg/mL) were added to the Eppendorf tubes, and then left to stand for 30 min in the dark. Flow cytometry analysis was performed on an Accuri C6 (BD Company, Franklin Lake, NJ, USA). About 20,000 cells per sample were collected. The Accuri C software was used for the analysis of the results.
A linear regression between the chromosome number of the cells of the root tips of B. napus and flow cytometric data was performed according to Frédérique et al. (1997) and Warwick et al. (2003). Leflon et al. (2006) previously assessed Brassica triploid hybrid chromosome inheritance using flow cytometry and estimated a 0.99 correlation between DNA content and number of mitotic chromosomes. The flow cytometry results of B. rapa, the Brassica triploid, B. napus, the Brassica hexaploid, and the Brassica octaploid were showed in Fig. 1.
Flow cytometric values of YH (AA, 2n = 2× = 20), Brassica triploid (AAC, 2n = 3× = 29), P3-2 (AACC, 2n = 4× = 38), Brassica hexaploid (AAAACC, 2n = 6× = 58), Brassica octaploid Y3560 (AAAACCCC, 2n = 8× = 76) and Brassica octaploid Y3380 (AAAACCCC, 2n = 8× = 76). The channel value of each flow chart was about 150,000 (a), 360,000 (b), 480,000 (c), 720,000 (d), 960,000 (e) and 960,000 (f), respectively
Molecular genetic analysis
Simple sequence repeat (SSR) molecular markers have been extensively used to test the hybridity and paternity due to co-dominance and high level of polymorphism (Nelson et al. 2009; Pradhan et al. 2010). Genomic DNA was extracted from young leaf tissues using the cetyl-trimethyl-ammonium bromide method as described elsewhere (Kidwell and Osborn 1992). SSR primer pairs were used to assess the homozygosity of the P3-2 line (Suppl. Table S1), as well as to determine differences in microsatellites between octaploid males and B. napus females (Suppl. Table S2 and Table S3). The SSR primer pairs with different amplified bands between parents were used to analyze the first induced generations (F1). SSRs were amplified by PCR using the following cycling conditions: 95 °C for 5 min; 40 cycles of 95 °C for 30 s, 50 °C for 45 s, 72 °C for 1.5 min; and 72 °C for 8 min. Approximately 3 µL of the amplified products was separated by electrophoresis in 2.5% (w/v) agarose gels (Uzunova and Ecke 1999).
Statistical analyses
ANOVA was performed using SPSS 21.0 for Windows at the 5% level of significance. To calculate the induced rate of Y3560 to common B. napus female, the plants of the F1 generation with the same phenotype, same ploidy, and same SSR amplified bands as that of their mother were counted, and the ratio between the selected plants and the total F1 plants was determined to be the induction rate.
Results
Characterization of the Brassica hexaploid (AAAACC)
The initial meiosis of PMCs of triploid hybrids derived from the cross between B. rapa (♀) and B. napus (♂) was unbalanced. The pollens of Brassica triploid were small and irregularly shaped (Fig. 2a), and the viability of most pollen of triploid hybrids was very low that ultimately lead to low seed setting percentage. Root tip cells and PMCs of the chromosome-doubled hybrids (Suppl. Fig. S1) indicated that all triploid hybrids were not successfully reduplicated to hexaploids and showed somatic cells harboring 40–58 chromosomes. Compared to the Brassica triploid, the plants with reduplicated chromosomes have significantly greener, more crinkled, and thicker leaves, wider stems, lower plant height, larger flower organs with higher fertility (Fig. 2b), and increased resistance phenotypically (Suppl. Fig. S1). Haploids, tetraploids, hexaploids, and mixoploids were found in the F2 selfed generation of Brassica hexaploid (Fig. 3). A conspicuously tetraploid phenotype was selected for selfing, and its offspring (F3) showed uniform growth, leaf color, leaf style, height, flower size, reproduction and hereby designated as P3-2. We deduced that P3-2 belongs to a homozygous line of B. napus.
Pollen viability of Brassica triploid hybrid, Brassica hexaploid, P3-2, ZS11, Brassica octaploid Y3380 and Brassica octaploid Y3560. Triploids (a) had small and irregular pollens. The majority of the pollens of the hexaploids (b) was large and regularly shaped. Most of the pollens of P3-2(c), ZS11 (d), Y3380 (e) and Y3560 (f) were large and oval. The pollen diameter of octaploids was about 1.2 times of that of the tetraploids. Scale bar 200 μm
The performance of F2 generation plants derived from self-pollination of Brassica hexaploid in the presence of parathion. a Seedlings of the F2 population. b Flower buds and crinkled leaves of mixoploidy plant. c Flowering phase of haploid plant. d Inflorescence of normal tetraploid plant (B. napus)
Identification of homozygosity in P3-2 strain
To verify that P3-2 was truly of B. napus and homozygous, its phenotype, cytology, and molecular characterizations were observed. P3-2 showed uniform growth, and the phenotypes of seedling to mature stages were extremely similar to that of B. napus. Similar to B. napus, the somatic cells of P3-2 comprised 38 chromosomes (Suppl. Fig. S2a). The meiosis in PMCs of P3-2 found no abnormal chromosome behaviors (Suppl. Fig. S2b). Five SSR pairs of primers with specificity between parents F009 and YH were used to verify the homozygosity of P3-2. The SSR assays of 26 plants from the P3-2 line (Suppl. Fig. S2c) indicated identical amplification of bands also testified that P3-2 was a homozygous strain.
P3-2 is a semi-winter rapeseed with 214-d growth period when planted in the Chengdu Plain of Sichuan Province. The plants showed rapid and uniform growth, and developed large flowers, numerous pollen, and high oil content (~55%). P3-2 also had harder stalks with greater resistance to Plasmodiophora brassicae Woron and Sclerotinia sclerotiorum than F009 and YH. The number of seeds per silique, the 1000-seed weight, and yield per plant of P3-2 were higher than those of the parents, F009 and YH (Table 1).
Morphological and cytological observation of Brassica octaploid
Two ploidy-stable octaploid rapeseed lines, namely Y3560 and Y3380 showed relatively high levels of thioglycoside (>40 mg/g) and erucic oil (15–20%). The detailed breeding schematic diagrams were shown in Suppl. Fig. S3. Compared to tetraploid B. napus, the Brassica octaploid plants had dwarfed, thicker and severely crinkled leaves (Fig. 4a). The size of the flower buds rapeseeds was relatively bigger, with a rougher bud surface, and the flower stalks were shorter; furthermore, sizes of the petals, stamens, and pistils of octaploid rapeseeds were also larger (Fig. 4b–d). The siliques of the octaploid rapeseed were shorter and wider than that of tetraploid B. napus (Fig. 4d). Octaploid Y3560 and Y3380 exhibited significantly higher amount of pollen and pollen viability (Fig. 2e, f) than the Brassica triploid, as well as larger volume than B. napus (Fig. 2c, d).
Mitotic analysis of root tip cells and meiotic analysis of PMCs of Y3560 was conducted (Fig. 5). Y3560 showed relatively normal mitosis (Fig. 5a–c), whereas some abnormal behaviors were observed during the meiosis. Trivalents and tetravalents were observed at the diakinesis stage (Fig. 5f). In addition, chromosome bridges were detected at anaphase I in some of the cells (Fig. 5g). A few PMCs showed tetrads that had divided into two cells prior to the formation of mature pollen grain (Fig. 5i), whereas the majority of cells comprised normal tetrads. The seed abortion rate was relatively high in crosses between B. napus and Brassica octaploid (Suppl. Fig. S4).
Hereditary characteristics of Brassica octaploid Y3560 and Y3380
Hybridizations between Brassica octaploid as pollen donor and conventional B. napus as female were conducted to assess the hereditary characteristics of Brassica octaploid. Upon morphological inspection, plants of the F1 generation were extremely similar to female parent ZS11 and showed uniform growth. The F2 population traits were highly similar to that of ZS11; individuals within this population showed uniform growth and no heterologous plants were observed. Because ZS11 is a homozygous inbred line, any hybridizations occurring between Y3560 and ZS11 would yield heterozygous offspring, thereby each allele segregate in F2 generation. Thus, we considered that genome of Brassica octaploid was not integrated into egg of ZS11, but the genome of ZS11 gametes had doubled through an unknown mechanism to produce a homozygous DH. So octaploid Y3560 have the capacity to induce apomixis in tetraploid B. napus.
To further confirm that octaploid Brassica possessed the ability to generate doubled haploids, WLA was crossed with Y3560. WLA is a homozygous male sterile line, its whole plant surface is bright green and waxless, and these traits are controlled by two recessive genes (Tatlioglu 1989; Mo et al. 1995). In the cross between WLA and Y3560, approximately 435 of the 456 plants (Table 2) of F1 generation displayed bright green coloration, and no wax powder as similar to that in WLA (Fig. 6a). Flow cytometry analysis (Suppl. Fig. S5c, d) showed that all 435 plants were tetraploid. Of the 435 plants, 29 plants had heterozygous microsatellite amplified bands of Y3560 and WLA, whereas the rest of the 406 plants showed bands that were similar to that of the female WLA (Fig. 7a). Therefore, WLA was successfully induced by Y3560 to produce DHs at an induced efficiency of 89.04%. Another pure B. napus line 0068A was crossed with Y3560. The F1 seeds yielded 103 seedlings, of which 99 were male sterile and phenotypically similar to 0068A (Fig. 6b), whereas the other four plants were fertile (Table 2). The 99 sterile plants were confirmed by flow cytometry as tetraploids (Suppl. Fig. S5e, f). SSR analysis revealed that only 1 out of the 99 plants was a hybrid of 0068A and Y3560, as indicated by the presence of heterozygous bands (Fig. 7b). Hence, the octaploid Y3560 could induce cytoplasmic male sterility to produce homozygous offsprings. The induction rate of Y3560 to 0068A was 95.15%. Same crosses were conducted between other B. napus line and Y3560, including 3209, 3304, 3118, 3321, and 3323 (Table 2; Figs. 6, 7; Suppl. Fig. S5). Similarly, the induced frequency of Y3560 to 3209, 3304, 3118, 3321, and 3323 was 39.90, 34.10, 44.07, 90.00, and 98.66%, respectively.
SSR analysis of the Brassica octaploid male, B. napus females, and part of their F1 generations. a Amplified bands of Y3560, WLA and their F1, the arrow showed hybrid offspring, the rest showed bands that were identical to their female parent, namely tetraploid homozygous B. napus. b Amplified bands of Y3560, 0068A and the generations. c Amplified bands of Y3560, 3204 and their F1. d Amplified bands of Y3560, 3304 and the F1. e Amplified bands of Y3380, 0068A and the F1. f Amplified bands of Y3380, WLA and the F1. The primers used were BrGMS70, SSRRa2-F11, BrGMS70, and BoGMS0057, SSRRa2-F11 and BrGMS70, respectively
In cross of WLA × Y3380, 410 out of the 419 plants were sterile and waxless; among the sterile progenies, 401 plants were tetraploids, six plants were haploids, and three plants were octaploids, and three progenies were hybrids of WLA × Y3380 (Table 2; Fig. 7f; Suppl. Fig. S5r–t). The induction rate of Y3380 to WLA and 0068A was 94.99 and 93.33%, respectively. There was no significant difference in induction ratio between Y3560 and Y3380.
Discussion
Artificially synthesized Brassica allopolyploid hybrids have some novel features
Interspecific hybridization and polyploidy crosses play an important role in plant speciation. Allotetraploidy is the predominant ploidy in Brassica, however, no Brassica allohexaploid specie occurs naturally (Chen et al. 2011). Tsai and Chen (1964) obtained two allohexaploids (AACCCC, 2n = 56; AAAACC, 2n = 58) by colchicine treatment and used B. napus × B. oleracea and B. napus × B. chinesis as parents, respectively. Mcnaughton (1973) also artificially developed Brassica hexaploid named B. napocampestris (2n = 58, AAAACC). Brassica hexaploids were morphologically characterized by its gigantic appearance than corresponding triploids. Fairly stable meiosis was observed in PMCs, with fewer univalents and more trivalents and quadrivalents, leading to that the fertility of Brassica hexaploid was recovered but not complete.
In the present study, compared to their triploid progenitor, Brassica hexaploid was genetically stable, fertile, and vigorous that was in agreement with the previous studies (Tsai and Chen 1964; Mcnaughton 1973). F2 individuals of B. napocampestris include haploids, tetraploids, and mixoploids that were attributed to homologous and homoeologous recombinations between genomes of A–A, A–C and C–C during meiosis and unbalanced gametes segregation. Crossovers and genome duplication in Brassica hexaploid may significantly impact in creation of novel allelic combinations. Moreover, this occasional phenomenon was very rare but was very fortunate and may associated with unreduced gametes that causes relatively higher frequencies of hexaploid than in parent Brassica species (Mason et al. 2014).
It has been proposed that interspecific hybridization and polyploidy in plants are the evolutionary mechanisms underlying the formation of new species with greater heterosis than their progenitor species (Otto and Whitton 2000), which in turn might be due to the contribution of extra sets of alleles from multiple genomes. Artificial synthesis and study of Brassica allooctaploid (AAAACCCC, 2n = 76) was first conducted by Ge and Zhang (1979a). Because of multiple sets of genome A and C, Brassica allooctaploid exhibited abnormal meiosis including synaptic variation, univalent, multivalent, chromosomal bridge, chromosomal lagging, chromosomal fragments, chromosomal unequal distribution, asynchronous cell division, and abnormal tetrad formation in the PMCs of the Brassica octaploid hybrid. Meiotic abnormalities generally lead to low vigor gametes and seed setting rate, but this is genotype-dependent (Li et al. 2015). The Brassica octaploid Y3560 and Y3380 showed very high pollen viability but low setting rate, indicating that most ovules underwent abortion after fertilization.
Preservation of ploidy-stable Brassica allooctaploid hybrids
Chromosome stability is the major factor for the establishment and persistence of polyploids in plants (Geng et al. 2013). Since meiotic behavior in the Brassica is controlled by various QTLs (Liu et al. 2006), hence selection for octaploid chromosome number may be the most effective way in improving meiotic stability of new Brassica species. Moreover, instability in chromosome number during selfing occurs in Brassica octaploid but its progenies showed normal chromosome pairing and segregation over several selfing generations and further could be selected to preserve. Ge and Zhang (1979b) selected ploidy-stable Brassica octaploid from the selfing progenies, and some Brassica allohexaploids have been maintained over ten generations of selfing (Takeda and Takahata 1996). Many researches showed that the stability of polyploids was significantly improved after several generations of selfing (Larter and Gustafson 1980; Comai 2000; Liu et al. 2001; Zhang et al. 2004; Tian et al. 2010). So, long-term preservation and breeding of the Brassica octaploid Y3560 and Y3380 is practical and feasible.
DH induction and possible mechanism of Brassica octaploid hybrids
In the cross of Brassica octaploid as pollen donor and B. napus as female, the progenies contained haploids, tetraploids, octaploids, and mixoploids, showing the complexity of the results of interploidy hybridization. A few number of tetraploid hybrids with biparental SSR polymorphic bands indicated that double fertilization occurred, and genes of two gametes underwent exchanges. SSR marker results showed that each polymorphic locus carried by some tetraploid hybrids with 38 chromosomes originated from the same female. The production of presumed maternal B. napus DH, which only showed genetic composition of the female, was indicative of the elimination of the paternal genome. As in DH induction, there were no significant differences between the two Brassica octaploid Y3560 and Y3380, but the stability of DH induction of Y3560 is higher than that of Y3380.
The induction rate of Brassica octaploid to the cytoplasmic male sterile B. napus lines was around 90%, whereas that of Y3560 to the genic male sterile B. napus lines was nearly 40%, indicating that the induction rate depends on the genotype of female B. napus. As earlier studies proposed that spontaneous chromosome doubling during adventitious somatic regeneration is a relatively frequent phenomenon; however, it is genotype-dependent (Jakše et al. 2010). In specific barley cultivars, the rate of spontaneous chromosome doubling during microspore culture reached up to 87%, thereby skipping the artificial reduplication step and in turn directly creating DHs (Hoekstra et al. 1993), whereas the duplication rate in B. napus microspore cultures ranged from 10 to 40% (Henry 1999). In maize and Arabidopsis, the haploid induction rate ranges from 1.2 to 16% (Rotarenco et al. 2010), 25–45% (Ravi and Chan 2010), respectively. In our study, the DH induction frequencies of Y3560 and Y3380 were comparatively higher, which may have huge practical and economic value.
Taken together, we inferred that in the interploid crosses between Brassica octaploid and B. napus, the paternal genome was eliminated after fertilization, and subsequently, the maternal genome spontaneously doubled, thereby forming homozygous B. napus DH. Complete elimination of chromosomes from one parent during the mitotic divisions of zygotes has been reported in barley (Kasha and Kao 1970), maize (Coe 1959), and Arabidopsis (Ravi and Chan 2010). In Arabidopsis, chromosomes with altered CENH3 proteins, including chimeric CENH3, CENH3 from diverged species, and point mutation in CENH3 are eliminated when crossed with the wild-type, which in turn results in the production of maternal or paternal haploid progeny (Ravi and Chan 2010; Ravi et al. 2014; Kuppu et al. 2015). In Hordeum vulgare × Hordeum bulbosum, centromere inactivity of H. bulbosum chromosomes triggers the mitosis-dependent process of uniparental chromosome elimination in unstable hybrids (Sanei et al. 2011). In maize, haploid induction is triggered by a frame-shift mutation in MATRILINEAL (MTL), a pollen-specific phospholipase (Kelliher et al. 2017). The underlying mechanism for the production of maternal B. napus DH from interploidy crosses need to be further studied.
A similar phenomenon has been reported in rice and cucumber. In the F2 generation, a genetically stable rice line was selected from the crosses between autotriploid rice as female and diploid rice as pollen donor (Li et al. 2004). Similarly, a genetically stable diploid cucumber population was chosen from the crosses between autotriploid and DH cucumber (Diao 2009). These findings indicate that homozygous diploid progenies may be a common consequence of interploidy hybridization.
Conclusions
In conventional breeding, around 5–7 years of selfing are required to generate a homozygous rapeseed line from two hybrid parents, whereas our method requires only 1–2 years. This research describes a new one-step pathway for the creation of homozygous Brassica lines that excludes the process of genome doubling, but may still significantly improve rapeseed breeding and advance polyploid genetic studies. This methodology may also accelerate rapeseed breeding as well as improve breeding efficiency.
Author contribution statement
LY, SF, JY, TF and MW conceived and designed the experiments. LY, SF, MX, YL and AA performed the experiments. LY, MX, JS, BY, and JW analyzed the results. QZ, JW, LT, ZK and RT contributed oilseed rape cultivation and reagents. LY, MX, MW, and AA contributed to the writing of the manuscript. All authors read and approved the manuscript.
Abbreviations
- DH:
-
Doubled haploid
- HI:
-
Haploid inducer
- PMCs:
-
Pollen mother cells
- ZS11:
-
Zhongshuang 11
References
Adams KL, Wendel JF (2005) Polyploidy and genome evolution in plants. Curr Opin Plant Biol 8:135–141
Boulos C, France D, Liu S et al (2014) Early allopolyploid evolution in the post-Neolithic Brassica napus oilseed genome. Science 345:950–953
Chen S, Nelson MN, Chèvre A-M et al (2011) Trigenomic bridges for Brassica improvement. Crit Rev Plant Sci 30:524–547
Coe EH (1959) A line of maize with high haploid frequency. Am Nat 93:381–382
Comai L (2000) Genetic and epigenetic interactions in allopolyploid plants. Plant Mol Biol 43:387–399
Comai L (2005) The advantages and disadvantages of being polyploid. Nat Rev Genet 6:836–846
Diao W (2009) Creation and genetic analysis of cucumber (Cucumis sativus L.) materials with different ploidies. Dissertation, Nanjing Agricultural University
Dorota G, Twan R, Alok V et al (2005) Uniparental chromosome elimination at mitosis and interphase in wheat and pearl millet crosses involves micronucleus formation, progressive heterochromatinization, and DNA fragmentation. Plant Cell 17:2431–2438
Frédérique E, Letanneur J, Anne-Marie C (1997) Chromosome number of oilseed rape (Brassica napus)–wild radish (Raphanus raphanistrum) spontaneous hybrids and of their progeny estimated by flow cytometry. Cruciferae Newslett 19:17–18
Ge K, Zhang A (1979a) Synthesis of a new species and cytogenetic studies in oleiferous Brassica. V. The cytogenetic studies in auto-allooctoploidy of Brassica napus L. var. Oleifera. Acta Agron Sin 5:13–20
Ge K, Zhang A (1979b) Synthesis of new species and cytogenetic studies in Brassica. V. Artificial synthesis of an autoallooctoploid in Brassica napus var Oleifera. Acta Genet Sin 6:62
Geng X, Chen S, Astarini I et al (2013) Doubled haploids of novel trigenomic Brassica derived from various interspecific crosses. Plant Cell Tissue Organ Culture 113:501–511. doi:10.1007/s11240-013-0292-4
Henry Y (1999) Origin of microspore-derived dihaploid and polyhaploid in vitro plants. Plant Tissue Culture Biotechnol 4:127–135
Hoekstra S, van Zijderveld MH, Heidekamp F et al (1993) Microspore culture of Hordeum vulgare L.: the influence of density and osmolality. Plant Cell Rep 12:661–665
Jakše M, Hirschegger P, Bohanec B et al (2010) Evaluation of gynogenic responsiveness and pollen viability of selfed doubled haploid onion lines and chromosome doubling via somatic regeneration. J Am Soc Hortic Sci 135:67–73
Jiao Y, Wickett NJ, Saravanaraj A et al (2011) Ancestral polyploidy in seed plants and angiosperms. Nature 473:97–100
Jugulam M, Ziauddin A, So KKY et al (2015) Transfer of dicamba tolerance from Sinapis arvensis to Brassica napus via embryo rescue and recurrent backcross breeding. PLoS ONE 10:e0141418
Kasha KJ, Kao KN (1970) High frequency haploid production in barley (Hordeum vulgare L.). Nature 225:874–876
Kelliher T, Starr D, Richbourg L et al (2017) MATRILINEAL, a sperm-specific phospholipase, triggers maize haploid induction. Nature 542:105
Kidwell KK, Osborn TC (1992) Simple plant DNA isolation procedures. In: Beckman J, Osborn TC (eds) Plant genomes: methods for genetic and physical mapping. Kluwer Academic Publishers/Springer, Dordrecht, pp 1–13. doi:10.1007/978-94-011-2442-3
Kuligowska K, Lütken H, Christensen B et al (2015) Evaluation of reproductive barriers contributes to the development of novel interspecific hybrids in the Kalanchoë genus. BMC Plant Biol 15:1–15
Kuppu S, Tan EH, Nguyen H et al (2015) Point mutations in centromeric histone induce post-zygotic incompatibility and uniparental inheritance. PLoS Genet 11:e1005494
Larter EN, Gustafson JP (1980) Triticale. Hybridization of crop plants. American Society of Agronomy/Crop Science Society of America, Madison
Leflon M, Eber F, Letanneur JC et al (2006) Pairing and recombination at meiosis of Brassica rapa (AA) × Brassica napus (AACC) hybrids. Theor Appl Genet 113:1467–1480
Li Y, Zhang HY, Wang XZ et al (2004) Identification and analysis of F2 stable population derived from the cross of triploid × diploid in rice. Acta Genet Sin 31:604–608
Li H, Guo X, Wang C, Ji W (2015) Spontaneous and divergent hexaploid triticales derived from common wheat × rye by complete elimination of D-genome chromosomes. PLoS ONE 10:e0120421
Lichter R (1982) Induction of haploid plants from isolated pollen of Brassica napus. Z Pflanzenphysiol 105:427–434
Liu C, Douches DS (1993) Production of haploids of potato (Solanum tuberosum subsp. tuberosum) and their identification with electrophoretic analysis. Euphytica 70:113–126
Liu B, Brubaker CL, Mergeai G et al (2001) Polyploid formation in cotton is not accompanied by rapid genomic changes. Genome 44:321–330
Liu Z, Adamczyk K, Manzanaresdauleux M et al (2006) Mapping PrBn and other quantitative trait loci responsible for the control of homeologous chromosome pairing in oilseed rape (Brassica napus L.) haploids. Genetics 174:1583–1596
Liu S, Liu Y, Yang X et al (2014) The Brassica oleracea genome reveals the asymmetrical evolution of polyploid genomes. Nat Commun 5:3930
Mason AS, Nelson MN, Takahira J et al (2014) The fate of chromosomes and alleles in an allohexaploid Brassica population. Genetics 197:273–283
Mcnaughton IH (1973) Brassica napocampestris L. (2n = 58). 1. Synthesis, cytology, fertility and general considerations. Euphytica 22:301–309
Mo J, Li W, Yu Q et al (1995) Inheritance of the waxless character of Brassica napus Nilla glossy. Can J Plant Sci 75:893–894
Nelson MN, Mason AS, Castello MC et al (2009) Microspore culture preferentially selects unreduced (2n) gametes from an interspecific hybrid of Brassica napus L. × Brassica carinata Braun. Theor Appl Genet 119:497–505
Otto SP, Whitton J (2000) Polyploid incidence and evolution. Annu Rev Genet 34:401–437
Pradhan A, Plummer JA, Nelson MN et al (2010) Trigenomic hybrids from interspecific crosses between Brassica napus and B. nigra. Crop Pasture Sci 61:464–474
Ramsey J, Schemske DW (1998) Pathways, mechanisms, and rates of polyploid formation in flowering plants. Annu Rev Ecol Syst 29:467–501
Ravi M, Chan SW (2010) Haploid plants produced by centromere-mediated genome elimination. Nature 464:615–618
Ravi M, Marimuthu MPA, Tan EH et al (2014) A haploid genetics toolbox for Arabidopsis thaliana. Nat Commun 5:5334
Rotarenco V, Dicu G, State D et al (2010) New inducers of maternal haploids in maize. Maize Genet Cooperation Newslett 84:1–7
Sanei M, Pickering R, Kumke K et al (2011) Loss of centromeric histone H3 (CENH3) from centromeres precedes uniparental chromosome elimination in interspecific barley hybrids. Proc Natl Acad Sci USA 108:498–505
Seymour DK, Filiault DL, Henry IM et al (2012) Rapid creation of Arabidopsis doubled haploid lines for quantitative trait locus mapping. Proc Natl Acad Sci USA 109:4227–4232
Takeda T, Takahata Y (1996) Production of alloplasmic Chinese cabbage using synthesized trigenomic hexaploid (AABBCC) in Brassica. Acta Hortic 407:151–154
Tatlioglu T (1989) Inheritance of waxlessness in kohlrabi (Brassica oleracea L. var. Gongyloides) and its utilization in hybrid seed production. Plant Breed 102:215–221
Tian E, Jiang Y, Chen L et al (2010) Synthesis of a Brassica trigenomic allohexaploid (B. carinata × B. rapa) de novo and its stability in subsequent generations. Theor Appl Genet 121:1431–1440
Tsai I, Chen P (1964) Synthesis of new species and cytogenetic studies in oleiferous Brassica—II. Synthesis of two auto-allohexaploids from Brassica napus L. and its two primary species. J Fudan Univ 9:413–428
Uzunova MI, Ecke W (1999) Abundance, polymorphism and genetic mapping of microsatellites in oilseed rape (Brassica napus L.). Plant Breed 118:323–326
Vieira P, Clercq AD, Stals H et al (2014) The cyclin-dependent kinase inhibitor KRP6 induces mitosis and impairs cytokinesis in giant cells induced by plant-parasitic nematodes in Arabidopsis. Plant Cell 26:2633
Wang S, Zhang G, Zhang Y et al (2015) Comparative studies of mitochondrial proteomics reveal an intimate protein network of male sterility in wheat (Triticum aestivum L.). J Exp Bot 66:6191
Warwick SI, Simard MJ, Legere A et al (2003) Hybridization between transgenic Brassica napus L. and its wild relatives: Brassica rapa L., Raphanus raphanistrum L., Sinapis arvensis L., and Erucastrum gallicum (Willd.) OE Schulz. Theor Appl Genet 107:528–539
Wendel JF (2000) Genome evolution in polyploids. Plant Mol Biol 42:225
Zhang L, Liu D, Yan Z et al (2004) Rapid changes of microsatellite flanking sequence in the allopolyploidization of new synthesized hexaploid wheat. Sci China Ser C 47:553–561
Zhao X, Xu X, Xie H et al (2013) Fertilization and uniparental chromosome elimination during crosses with maize haploid inducers. Plant Physiol 163:721–731
Acknowledgements
This work was financially supported by the “13th Five-year” National Key Research Projects (China; Grant no. JFYS2016 ZY03002156), the “13th Five-year” National Key Research Projects “Utilization of heterosis technology in oilseed rape and creation of superior hybridization” (China; Grant no. 2016YFD0101300), Sichuan Province Breeding Project “Breakthrough innovation of oil crops materials and methods” (China; Grant no. 2016NYZ0031), and the project of Chengdu Academy of Agricultural and Forestry Sciences “Integrated application demonstration and scientific research innovation” (China; Grant no. 2017Y2500W06).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Fu, S., Yin, L., Xu, M. et al. Maternal doubled haploid production in interploidy hybridization between Brassica napus and Brassica allooctaploids. Planta 247, 113–125 (2018). https://doi.org/10.1007/s00425-017-2772-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-017-2772-y