Abstract
Main conclusion
The kinetics of acyl-ACP thioesterases from sunflower importantly changed when endogenous ACPs were used. Sunflower FatB was much more specific towards saturated acyl-ACPs when assayed with them.
Acyl carrier proteins (ACPs) are small (~9 kDa), soluble, acidic proteins involved in fatty acid synthesis in plants and bacteria. ACPs bind to fatty acids through a thioester bond, generating the acyl-ACP lipoproteins that are substrates for fatty acid synthase (FAS) complexes, and that are required for fatty acid chain elongation, acting as important intermediates in de novo fatty acid synthesis in plants. Plants, usually express several ACP isoforms with distinct functionalities. We report here the cloning of three ACPs from developing sunflower seeds: HaACP1, HaACP2, and HaACP3. These proteins were plastidial ACPs expressed strongly in seeds, and as such they are probably involved in the synthesis of sunflower oil. The recombinant sunflower ACPs were expressed in bacteria but they were lethal to the prokaryote host. Thus, they were finally produced using the GST gene fusion system, which allowed the apo-enzyme to be produced and later activated to the holo form. Radiolabelled acyl-ACPs from the newly cloned holo-ACP forms were also synthesized and used to characterize the activity of recombinant sunflower FatA and FatB thioesterases, important enzymes in plant fatty acids synthesis. The activity of these enzymes changed significantly when the endogenous ACPs were used. Thus, FatA importantly increased its activity levels, whereas FatB displayed a different specificity profile, with much high activity levels towards saturated acyl-CoA derivatives. All these data pointed to an important influence of the ACP moieties on the activity of enzymes involved in lipid synthesis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fatty acid synthesis is catalyzed by fatty acid synthase (FAS, EC 2.3.1.85) complexes (Wakil 1989). Plants and bacteria use type II FAS complexes, which are made up of discrete peptides that catalyze the individual steps in the elongation reaction (White et al. 2005). In these discrete type II systems, the acyl chains under elongation bind to a small (~9.5 kDa), soluble, acidic proteins, the acyl carrier proteins (ACP), and thus, all the metabolic intermediates of FAS from malonate to long chain fatty acids exist as acylated-ACPs in plant cells. Moreover, acyl-ACPs are the substrates for other important enzymes within plant lipid metabolism, such as stearoyl-ACP desaturases (EC 1.14.99.6), plastidial acyltransferases (EC 2.3.1.15) and acyl-ACP thioesterases (EC 3.1.2.14; Ohlrogge and Jaworski 1997). ACP proteins are highly conserved across different eukaryote and plant species, and their structure has been resolved by the solution of NMR and X-ray crystallography, consisting of four α-helices connected by three loops. Furthermore, functional ACPs (holo-ACPs) contain a 4′-phosphopanthotenic acid (4-PP) molecule as the prosthetic group that binds to a serine residue located at the junction of the α1α2 loop near the N-terminal region of helix II (Holak et al. 1988; Li et al. 2003). This is an essential moiety as fatty acids bind to the thiol group present in this molecule. Moreover, the presence of this prosthetic group stabilizes the structure of the protein, allowing greater flexibility during the interaction with the FAS complex (Kim et al. 2006). Inactive apo-ACP is transferred to the 4-PP group from a coenzyme-A molecule in a reaction that is catalyzed by the holo-ACP synthase (EC 2.7.8.7: Lambalot and Walsh 1995).
ACPs are strongly expressed in bacteria and unicellular algae, where they are often encoded by a single gene. In plants, several ACP isoforms are found and they are expressed at distinct levels in different tissues (Battey and Ohlrogge 1990; Rawlings and Cronan 1992). Thus, there are eight genes encoding different ACPs in Arabidopsis thaliana, five expressed in plastids and three expressed in the mitochondria (Beisson et al. 2003). Mitochondrial ACPs are involved in the synthesis of lipoic acid and importantly, they have diverged from the plastidial ACPs involved in fatty acid synthesis (Hiltunen et al. 2009). All ACPs are encoded by nuclear genes, and thus, ACPs must be transported to their corresponding organelle as they mature after translation. To date, the influence of ACPs on the different aspects of intraplastidial fatty acid synthesis has not been investigated in depth. In this regard, ACPs from different plants expressing unusual acyl-ACP desaturases (∆4 and ∆6 palmitoyl-ACP desaturases from Coriadrum sativum and Thumbergia alata, respectively) have been purified and studied in terms of their activity and specificity (Suh et al. 1999). In assays of substrate competition, the unusual fatty acids were produced faster from the endogenous palmitoyl-ACP derivative. Furthermore, ∆4 desaturase from C. sativum displayed fourfold and tenfold more activity with coriander palmitoyl-ACP than with the spinach and E. coli equivalents, respectively. These differences were not observed when coriander ∆9-stearate desaturase was assayed with the different ACPs, which indicated a role of ACP in the production of unusual monoenes in these species. Despite these interesting results, there are few studies into the influence of specific ACP isoforms on the different plastidial enzymes and usually, commercial or recombinant E. coli ACP is used in biochemical studies.
Here, the genes encoding for three different ACPs were isolated from developing sunflower seeds (HaACP1, HaACP2, and HaACP3) and they were overexpressed in bacteria. These ACPs were used to synthesise different acyl-ACPs to study their effect on the specificity of FatA and FatB acyl-ACP thioesterases from sunflower (HaFatA and HaFatB). Acyl-ACP thioesterases are important enzymes in plant fatty acid synthesis, as they terminate the plastidial reactions on acyl-ACPs by hydrolyzing the acyl-ACP derivatives (Ohlrogge and Jaworski 1997). At this point the free ACP protein is reused and the released free fatty acids are exported out of the plastids to be incorporated into glycerolipids in the endoplasmic reticulum. Thus, these enzymes to some extent determine the fatty acid composition of the TAGs that accumulate in the seeds, and they are therefore of interest in plant biotechnology (Voelker 1996; Thelen and Ohlrogge 2002). Two thioesterase gene families have been described in plants: FatA, which displays high specificity towards oleoyl-ACP; and FatB, with a broader substrate specificity profile and strong activity towards oleoyl-ACP and palmitoyl-ACP (Jones et al. 1995). While FatA is broadly accepted as an essential house-keeping enzyme (Moreno-Pérez et al. 2012), FatB is necessary to supply the lipids in the cell with the necessary amount of saturated fatty acids (Bonaventure et al. 2003). However, in all these studies of specificity, the ACP forms used for enzymatic characterization came from bacteria (E. coli) or other plant sources (spinach), and no studies on the impact of the endogenous ACP forms on acyl-ACP thioesterases have been reported to date.
As indicated above, here three forms of ACP expressed in developing sunflower embryos were cloned and characterized: HaACP1, HaACP2 and HaACP3. These cofactors were expressed in E. coli, purified and used to synthesize the corresponding acyl-ACPs to assay recombinant HaFatA and HaFatB activities. The resulting specificity and kinetic profiles of these enzymes with endogenous ACPs was compared with those obtained using recombinant ACP from E. coli (EcACP). The results obtained demonstrate that the protein part of the acyl-ACP substrates were as determinant as the acyl moiety to define enzyme specificity. The consequences of this finding for earlier and future studies on ACP-dependent enzymes are discussed.
Materials and methods
Plant material
Sunflower seeds from the CAS-6 common sunflower line (Sunflower Collection of Instituto de la Grasa, CSIC, Seville, Spain) were germinated in wet perlite at 25 °C and then moved to a germination chamber for 2 weeks. Subsequently, the seedlings were transferred to growth chambers, and grown in bags endowed with fertilizer supply at 25°/15 °C (day/night). They were then grown on a 16 h photoperiod, and with a photon flux density of 250 μmol m−2 s−1 until the vegetative tissues or developing seeds were harvested.
Cloning sunflower ACP cDNAs
Developing sunflower embryos (~0.3 g) were harvested at 15–18 days after flowering (DAF), and they were ground in liquid N2 in a precooled sterile mortar. The mRNA was isolated from the developing embryos as described by González-Thuillier et al. (2015), and cDNAs were obtained using a Ready-To-Go TPrimed First Strand Kit (Amersham Bioscience, Roosendaal, The Netherlands). Arabidopsis thaliana ACP protein encoded by the At3g05020 gene (strongly expressed in seeds) was used to search sunflower expressed sequence tags (ESTs) in public databases to identify putative mRNAs encoding sunflower ACP homologues using the TBLASTN algorithm (Altschul et al. 1997). Two ESTs encoding whole ACPs were detected, as well as a partial sequence of a third gene. Specific primers were designed to amplify the former two ESTs (ACP1-F/ACP1-R and ACP2-F/ACP2-R: Table 1), which were named HaACP1 and HaACP2. A partial sequence of the 5 ′ end of the third gene (HaACP3) was obtained using the Smart™-RACE cDNA amplification kit (Clontech, Mountain View, CA, USA) and the specific internal reverse oligonucleotides, ACP3-rac1rev and ACP3-rac2rev (Table 1). The 3′ end of the HaACP3 cDNA was obtained by PCR using the externally added FA2Z primer, complementary to the sequences incorporated during the initial cDNA synthesis, and the specific internal primers ACP3-rac1for and ACP3-rac2for (Table 1). Once the complete sequence of HaACP3 was determined the whole gene was amplified using the ACP3-F/ACP3-R primer pair (Table 1). The PCR fragments obtained using different combinations of these forward and reverse primers were cloned into the pMBL-T vector (Genaxxon BioScience GmbH, Biberach, Germany) and several clones were sequenced on both strands (SECUGEN, Madrid, Spain).
The HaACP1, HaACP2 and HaACP3 genes were amplified without the peptide signal using the pair of primers pGEXACP1-F/pGEXACP1-R, pGEXACP2-F/pGEXACP2-R and pGEXACP3-F/pGEXACP3-R (Table 1), respectively. For cloning into the pGEX-4T (GST) expression vector (GE Healthcare), the PCR products contained the restriction sites for the enzymes BamHI and XhoI at their 5′ and 3′ ends, respectively. The resulting vectors pGEX-4::HaACP1, pGEX-4::HaACP2 and pGEX-4::HaACP3 were transformed into competent E. coli BL21 strain to produce the apo-ACPs fused to a glutathione-S-transferase protein (GST), as described by Smith and Johnson (1988).
Cloning E. coli holo-ACP synthase
The E. coli holo-ACP synthase (EcacpS) was cloned as described by Lambalot and Walsh (1995), after amplifying it from E. coli using the acpS-F and acpS-R primers (Table 1) that included the SphI and HindIII sites. The resulting DNA fragment was cloned into the pQE80L plasmid where a poly histidine tag was added to facilitate its purification by Ni–NTA affinity chromatography.
RT-qPCR studies of gene expression
The cDNAs from vegetative tissues (leaf, stem and roots), developing seeds (12, 15, 20, 25, and 30 DAF) and cotyledons (2, 5, and 7 days after imbibition) were obtained as described above. The expression of the three sunflower ACP genes in these tissues was quantified by RT-qPCR using gene-specific primer pairs: QHaACP1-F/IQHaACP1R for HaACP1; QHaACP2-F/QACP2-R for HaACP2; and QHaACP3-F/QHaACP3R for HaACP3 (Table 1). The fluorescence resulting from the SYBR Green I system (QuantiTect™ SYBR® Green PCR Kit; Qiagen, Crawley, UK) was monitored in a MiniOpticon apparatus (Bio-Rad, Hercules, CA). The reaction mixture was heated to 50 °C for 2 min and then to 95 °C for 15 min before subjecting it to 40 PCR cycles: 94 °C for 15 s; 61 °C for 30 s; and 72 °C for 15 s. The calibration curves were drawn up using sequential dilutions of the cDNAs and the Livak method was employed to calculate the comparative expression of the samples (Livak and Schmittgen 2001). Amplification of the sunflower HaACT1 actin gene with QHaActin-F4/QHaActin-R4 primers (GenBank Accession number FJ487620) served as a reference (Table 1).
Protein expression in E. coli and affinity purification
Different recombinant proteins were produced by overexpressing the corresponding gene in the pGEX-4T-1 (GE Healthcare) or pQE80L (Quiagen) vectors using E. coli K27 or BL21 strains, respectively. As a general rule, bacteria were grown at 37 °C until the optical density at 600 nm reached a value of 0.5. The cultures were then induced with 0.5 mM of isopropyl-beta-d-thiogalactopyranoside (IPTG) and allowed to grow for an additional 5–6 h. Then, bacteria were recovered by centrifugation, resuspended in Phosphate-buffered saline [PBS: 20 mM K2HPO4 (pH 7.3), 140 mM NaCl, 2.7 mM KCl] and treated with 1 mg/mL lysozyme for 1 h at 37 °C. The bacteria were then sonicated on ice using a probe sonicator (Branson B12), applying 10 pulses of 10″ (20 kHz, 75 W) with pauses of 45″. Then 1 mg/mL of DNAse was added, it was incubated for 30 min at 37 °C and the lysate was centrifuged for 20 min at 12,000×g using the resulting crude extracts for subsequent protein purification.
The polyhistidine tagged holo-ACP synthases were purified from E. coli (EcacpS) by affinity chromatography, loading 5 mL of the crude extract onto a 1 mL column of Ni–NTA agarose [equilibrated in 50 mM sodium phosphate (pH 7.5), 300 mM NaCl and 30 mM imidazole] having previously filtered it through a 0.45 µm membrane. The column flow through was collected and passed twice more through the column to maximize protein recovery, and the column was then eluted with 10 mL of elution buffer to remove all the unbound protein. Subsequently, the purified proteins were recovered by eluting the column with 50 mM sodium phosphate (pH 7.5), 300 mM NaCl and 250 mM imidazole, collecting four fractions of 0.5 mL that were assayed for their protein content. The columns were then regenerated by passing 10 mL of distilled water, and they were stored at 4 °C in 20 % ethanol/water. The imidazole associated with the purified proteins was removed using Millipore ultrafiltration units of 30 or 3 K depending on their size, and the histidine tagged holo-ACP synthase from E. coli was stored in 50 µL aliquots at −80 °C.
To purify the recombinant HaACPs, the cleared lysate was loaded onto a GSA-agarose affinity column (GE Healthcare) containing 1 mL of the hydrated GSA-agarose equilibrated in PBS. A volume of 1 mL of the crude extract was loaded onto the column and incubated for 1 h at room temperature on a rocking plate. The column flow through was discarded and it was washed with an additional 5 mL of PBS. Subsequently, 1 mL of PBS containing 10 units of Thrombin (GE Healthcare) was added to the columns, and they were again incubated for 1 h at room temperature on the rocking plate. The column flow through containing the sunflower apo-ACPs was then collected and the columns were washed with additional 1 mL of PBS. The columns were then regenerated with 5 mL of 10 mM glutathione dissolved in 50 mM Tris–HCl (pH 8). The thrombin present in the fraction was removed using 30 K ultrafiltration (Millipore) units, and the apo-ACPs were then concentrated using 3 k ultrafiltration units (Millipore) prior to activation.
Activation of the mature sunflower ACPs
The activation reaction was carried out on 400 µg of apo-ACP in 50 mM Tris (pH 8.8), 1 mM CoA, 5 mM DTT, 10 mM MgCl2 and 200 nmol of EcacpS in a final volume of 0.5 mL, and it was run for 4 h at 37 °C. In these conditions virtually all the apo-ACP was activated to the holo-ACP form, it was confirmed on native PAGE gels and with a radioactive assay based on the acyl-ACP synthetase reaction (Kuo and Ohlrogge 1984).
ACP Production in E. coli
The EcACP clone used in this work was kindly supplied by Dr Penny von Wettstein-Knowles (University of Copenhagen). This gene was cloned into the pQE80L vector and overexpressed in E. coli. The polyhistidine EcACP was purified by Ni–NTA agarose affinity chromatography and fully activated by a reaction with EcacpS, as described above.
Preparation of radiolabelled acyl-ACP substrates
Labelled acyl-ACP substrates were prepared using a recombinant acyl-ACP synthetase from E. coli kindly supplied by Dr J. Shanklin (Brookhaven National Laboratory). Acylation reactions were carried out on 50 μg of holo-ACP, with 180 kBq (~0.1 μmol) of (1-14C) fatty acid ammonium salt [(3H) fatty acid in the case of 16:1Δ9], 5 mM ATP, 2 mM DTT, 400 mM LiCl, 10 mM MgCl2, 100 mM Tris–HCl (pH 8.0) and 10 μg acyl-ACP synthetase. Reactions were carried out in a final volume of 500 µL at room temperature for 3–6 h, and the acyl-ACPs were then purified and concentrated by ion exchange chromatography on DEAE-Sepharose (as described by Rock and Garwin 1979).
Production of recombinant HaFatA and HaFatB thioesterases
The sunflower thioesterase genes have been cloned and studied previously by us (Serrano-Vega et al. 2003, 2005). The genes encoding the mature HaFatA and HaFatB polypeptides were cloned into the pQE80L vector for overexpression in E. coli. In the case of HaFatB, the domain involved in membrane anchoring was removed to recover the enzyme in the soluble fraction. Recombinant HaFatA and HaFatB thioesterases tagged with a 3′ polyhistidine tail were recovered in the soluble phase and purified on a Ni–NTA affinity column, as indicated above. Purified thioesterases (up to 85 % purity when assessed by SDS-PAGE) were used for activity assays.
Acyl-ACP thioesterase assays
Thioesterase activity was assayed in a final volume of 0.1 ml containing 50 mM Tris–HCl (pH 8.0), 5 mM DTT plus 0.2–12 μg of the protein preparation, and an amount of acyl-ACP substrate ranging from 0.02 to 0.08 nmol (30–170 Bq approx.). The reactions were run at room temperature for 5 min and quenched by adding 0.25 ml of 1 M acetic acid in 2-propanol. The free fatty acids were then extracted twice with 0.3 ml hexane, and the radioactivity in the pooled organic phase was determined in a calibrated liquid scintillation counter (Rackbeta II; LKB). The data from thioesterase assays were fitted to the Hill equation by non-linear least-squares regression analysis using OriginPro 8 software and they were correlated at P < 0.005 as determined by Student’s t test. Both the V max and K m were derived from these curves. Protein concentrations were measured using the colorimetric Bradford (1976) method.
Bioinformatics
ACP protein sequences from public databases were aligned to identify regions of homology using the ClustalX v2.1 program (Larkin et al. 2007) and a phylogenetic tree was constructed using MEGA 5.0 software (Tamura et al. 2011). The prediction of the transit peptides of the three HaACPs was carried out using the ChloroP (http://www.cbs.dtu.dk/services/ChloroP/) and Signal IP (http://www.cbs.dtu.dk/services/SignalP/) programmes. The tertiary structure of sunflower ACPs were modelled using the SWISSMODEL server (Guex and Peitsch 1997) based on the structure of Spinacia oleracea (spinach) ACP (UniProtKB/Swiss-Prot: P07854.2; Zornetzer et al. 2006).
Results
ACPs from sunflower
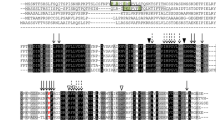
Three new sunflower ACPs were cloned from developing sunflower seeds that were named HaACP1, HaACP2 and HaACP3. These genes were around 400 bp long and they encoded proteins of 139, 132, and 139 amino acids with molecular weights of 14.71, 14.15, and 14.76 kDa, and with isoelectric points of 4.69, 4.48, and 5.01, respectively. They were deposited in the gene bank with accession numbers HM013970 (HaACP1), HM13971 (HaACP2) and HM013972 (HaACP3). These sequences were launched into the public ChloroP and Signal IP prediction servers, which confirmed that the three proteins were targeted to chloroplasts, and that each of them had predicted transit peptides of approximately 55 amino acids (Fig. 1). The mature HaACP1 and HaACP2 proteins displayed 80 % identity, whereas they maintained 60 % identity with HaACP3, also displaying high levels of identity when aligned with ACP from Arabidopsis (At3g05020) and E. coli (Fig. 1).
Alignment of the sunflower ACPs. Alignment of the deduced amino acid sequences of the H. annuus acyl carrier proteins, HaACP1, HaACP2, and HaACP3, along with the closely related sequences from A. thaliana, AtACP4 (gi|15234875|), and E. coli, EcACP (gi|955473707|). Identical residues are highlighted in black and highly conserved residues in light grey. The serine residue involved in 4′-phosphopanthotenic acid binding is indicated with a black triangle and those residues participating in the acyl binding site are indicated with a rectangular box. The putative N-terminal plastid transit peptides of HaACP1, HaACP2 and HaACP3 are underlined (56, 49 and 58 amino acids, respectively)
Structural analysis of HaACPs
The three-dimensional structures of HaACPs were modelled using the SWISS-MODEL server (Guex and Peitsch 1997) and the data in the RSC PDB protein database (Berman et al. 2000). The structure of the HaACPs corresponded to that of a globular protein with four α-helices that generate a hydrophobic region at the surface of the protein (Fig. 2). The serine residue that binds to the 4-PP moiety remains in the external region of the protein.
Structure of the sunflower ACP. Proposed structural model for sunflower acyl carrier protein HaACP1 modelled from the Spinacia oleracea ACP (2fvf: Zornetzer et al. 2006). Models a and c represent ACP ribbon diagrams with stearate. Models b and d show views of the molecular surfaces. The serine residue bound to 4′-phosphopanthotenic acid is shown in green in the molecular surface models, and positive and negative residues are shown in blue and red, respectively
Phylogenetic analysis of HaACPs
A phylogenetic tree was drawn up to study the relationship between the three new ACPs and those described in other plant species using the whole sequences of the ACP proteins (Fig. 3). All three forms were situated within the group of chloroplastic/plastidic ACP isoforms, clearly differentiated from the mitochondrial forms. HaACP1 and HaACP2 clustered together in the tree, very close to HaACP3. Moreover, all three resided in a group near to the ACPs from tomato (Solanum lycopersicum) and castor bean (Ricinus communis).
Phylogenetic comparison of chloroplast and mitochondrial ACPs using ACPs from Nostoc and E. coli as an outgroup to root the tree. The tree was constructed using the neighbour-joining method. The plant species included in the phylogenetic tree are Ha, H. annuus; Os, Oryza sativa; Rc, Ricinus communis; and Sl, Solanum lycopersicum
Gene expression
The expression of the HaACPs in different vegetative tissues, in germinating cotyledons and in developing seeds was studied by RT-QPCR at different developmental stages (Fig. 4). The three HaACPs were expressed in all the organs studied. In vegetative tissues, HaACP1 and HaACP2 predominated in the roots and stems, whereas HaACP1 and HaACP3 were the genes with most transcripts in green leaves. There was a little change in ACP expression in germinating cotyledons at 1, 4, and 7 days after imbibition (DAI), with a clear predominance of the HaACP1 and HaACP2 forms over that of HaACP3. The strongest expression of these three proteins was found in the developing embryos in seeds, especially during the period of oil accumulation 16–25 days after anthesis (DAA), when all the three ACP forms were expressed at similar levels, indicating their involvement in the process of oil synthesis.
Influence of HaACPs on the substrate specificity of HaFatA and HaFatB thioesterases
The influence of the protein group of the acyl-ACP lipoprotein substrate on HaFatA and HaFatB thioesterase substrate specificity was examined by assaying these enzymes at a constant substrate concentration and comparing the data with those obtained with acyl-ACPs containing EcACP. Recombinant HaFatA displayed the highest thioesterase activity towards 18:1-ACP derivatives in all cases (Fig. 5a), although the final level of activity reached with that substrate depended strongly on the ACP form used. Thus, the activity achieved with 18:1-EcACP was half that measured with the endogenous 18:1-HaACP1 and 18:1-HaACP2, and fourfold lower than that found with the 18:1-HaACP3 derivative. Two different patterns were found for the other substrates assayed. Thus, the activity of HaACP1 and HaACP2 increased from the 16:0 to the 18:0 derivative, with the 16:1 derivative lying in between these (16:0 < 16:1 < 18:0). Furthermore, acyl-ACP derivatives synthesized with EcACP and HaACP3 displayed higher activity towards 16:1-ACP, which was hydrolyzed at a higher rate than the saturated 16:0 and 18:0 acyl-ACP derivatives.
In the case of HaFatB, the specificity profile changed significantly in function of the ACP form assayed (Fig. 5b). Thus, the activity of EcACP was much lower than that measured with the endogenous forms of ACP, also displaying a very different specificity profile. The HaFatB enzyme displayed strong hydrolytic activity towards saturated derivatives when acyl-HaACPs were used as the substrates. Thus, the strongest activity was found for 16:0-HaACP2, followed by 16:0-HaACP1 and 16:0-HaACP3. Activities displayed by the enzyme towards 18:0-HaACP derivatives were also high, this time the 18:0-HaACP3 being the substrate hydrolysed at the highest rate. The activities detected were much lower when monounsaturated 16:1 and 18:1 acyl-ACPs were assayed. In contrast, the profile obtained with EcACP was very different. Accordingly, the levels of activity were much lower than those found with the endogenous substrates, as much as 10-fold lower in the case of 16:0-ACP, and the enzyme displayed similar activity towards 16:0, 18:0 and 18:1-ACPs, and a weaker activity on the 16:1-ACP derivative.
Kinetics parameters of HaFatA and HaFatB thioesterases
Certain kinetic parameters of the HaFatA and HaFatB thioesterases towards the acyl-ACP substrates synthesized with the different ACP forms were determined. In the case of HaFatA, the maximum K cat values corresponded to the 18:1-ACP substrates, as expected, with similar values for all the three forms of the 18:1-HaACPs between 17.3 and 25.5 s−1 (Table 2). These values were four to sixfold higher than for the saturated 16:0-ACP and 18:0-ACP acyl intermediates. The K m values were similar for all substrates assayed and ranged from 2.1 to 5.2 µM, so the specificity factors (K cat/K m) followed a similar pattern to the K cat values. When the kinetic parameters of HaFatB with the different HaACP derivatives were assessed (Table 3), the maximum K cat values corresponded to the saturated 16:0-ACP and 18:0-ACP acyl-ACP derivatives, with lower values towards 18:1-ACP derivatives. Similarly, the maximum K cat values were recorded for 18:0 derivatives, in a range between 20 and 26 s−1 for the three HaACP forms. The values for 16:0 derivatives were lower but still remained high, around 9 s−1. The K m values were in a micromolar order, being slightly higher for 18:0-HaACPs and lower for 18:1-HaACPs. The kinetic characterization of HaFatB was also carried out with EcACP derivatives to better assess the impact of using endogenous forms of that protein. The kinetic profile of the HaFatB using the bacterial ACP form was quite different (Table 3) with K cat values remaining in a much lower range than those found with HaACPs. Thus, the K cat values for 16:0-EcACP were around 20-fold lower than those found for 16:0-HaACP. Such differences were even higher in the case of 18:0 derivatives (80-fold higher) and even greater for the 18:1 derivatives. The maximum K cat values of this enzyme were obtained with 18:1-EcACP and 16:0-EcACP. Again, the K m values all stayed within a micromolar range.
Discussion
In this regards, it is unclear whether the different plastidial isoforms of ACPs have different characteristics and functions in plants. Here, three plastidial ACP isoforms were cloned from developing sunflower seeds and thus, they are very probably involved in the synthesis of fatty acids associated to TAG accumulation in that species. The modelling of the three sunflower ACPs reveals that they display an appropriate functional structure, without displaying any unexpected or aberrant domain.
The three sunflower ACPs are ubiquitously expressed proteins whose transcripts are found in all the tissues assayed, although all they were expressed more strongly in the developing seeds. This is consistent with the role of these proteins in the synthesis of fatty acids, which takes place in all plant tissues but especially in the oil accumulating embryos (Fig. 4). Hence, HaACP3 may be more strongly implicated in the synthesis of fatty acids associated to TAG accumulation in seeds due to display of higher expression levels in developing seeds and lower ones in vegetative tissues. Differentiation between them was also supported by their position in the phylogenetic tree (Fig. 3). In this regard, data of Arabidopsis plastidial ACP expression available on http://aralip.plantbiology.msu.edu/ shows that there are some forms of ACP that are specifically expressed in developing seeds (At3g05020 and At5g27200), vegetative tissues (At4g25050) and with ubiquitous expression (At1g54630 and At1g54580), pointing to a relative specialization of certain forms of these proteins that would be in agreement with results in sunflower.
The heterologous expression of these genes in E. coli was initially carried out in the protein expression plasmid pQE80L. However, the induction of protein expression provoked the death of the bacteria, probably because the HaACPs interact with the endogenous fatty acid metabolism and they hinder the native lipid synthesis. Moreover, the concentration of these recombinant proteins in the soluble fraction was very low when expressed in yeast (data not shown). Since it was necessary to produce milligram amounts of these proteins, we produced fusion proteins in the pGEX-4T-1 plasmid that generated recombinant ACPs fused to the large soluble GST protein. This system is particularly suited to express proteins targeted to inclusion bodies or those that cause lethality. Thus, the peptide resulting of the fusion of ACP and Glutathione S-transferase did not interacted with E. coli metabolism and was expressed strongly in the soluble phase of the bacteria and they could be purified by affinity chromatography. In that form, the 4-pp group was not esterified to the ACPs, so after the HaACP moieties were released by digestion of the fusion protein with thrombin, the resulting apo-HaACP had to be activated in vitro to the holo forms. That was achieved by incubation with the recombinant EcacpS in the presence of CoA, resulting in the quantitative conversion to holo-HaACPs. These recombinant holo-HaACPs were used to produce different acyl-ACP substrates to assay the sunflower acyl-ACP thioesterases.
All plants possess two types of thioesterases, FatAs and FatBs. In this regard, thioesterases from many very different sources have been characterized in terms of their substrate specify and their activity towards different acyl derivatives bound to the same ACP (Pollard et al. 1991; Hawkins and Kridl 1998; Salas and Ohlrogge 2002; Wu et al. 2009; Sánchez-García et al. 2010). The effect of the ACP on the activity of these enzymes has not been studied in detail, despite the great variety of ACP forms that can be found in plants. In the present study, we hypothesized that ACP could have an important role on the acyl-ACP thioesterase activity, as it has the acyl moiety. So we investigated the specificity and kinetic parameters of these enzymes using endogenous acyl-ACP derivatives and acyl-EcACPs as the control.
From the first studies into plant acyl-ACP thioesterases, the specificity of FatAs indicated that they participate in the export of the 18:1 derivatives to synthesize glycerolipids from the plastids to the cytosol and endoplasmic reticulum. This makes it a very important housekeeping enzyme as most of the C18 fatty acids present in plant tissues are derived from these de novo synthesized fatty acids. From the point of view of oil TAG synthesis in seeds, this enzyme was also of importance since most oil seeds accumulate oleic acid or fatty acids derived from oleic acid by desaturation (linoleic and linolenic) or elongation/desaturation (gondoic and erucic). This means that FatA enzymes have to support very high metabolic fluxes, allowing the synthesis and accumulation of large amounts of storage TAGs. When sunflower FatA was assayed with the E. coli and endogenous acyl-ACPs the18:1-ACP derivatives were those hydrolyzed at the highest rate. However, the specificities and catalytic efficiencies were higher with sunflower ACPs (Fig. 5a; Table 2), which indicated that there was an influence of the protein moiety of the substrate on this enzyme, and that endogenous ACP should be used to measure the kinetic parameters of these enzymes and at estimating their performance and contribution to metabolic flux.
FatB thioesterases were first described in common plants like Arabidopsis by Dörmann et al. (1995, 2000), this enzyme was hypothesized to play a role in the export of saturated fatty acids present in these plants. However, the specificity of Arabidopsis FatB showed strong preference for 18:1-ACP and a weaker preference for 18:0-ACP, which challenged this hypothesis and pointed that this enzyme did not complement the specificity of FatA within plant lipid metabolism. However, a decisive approach to FatB functionality was made when a FatB knockout mutant was isolated and characterized in Arabidopsis. The analysis of the lipid composition of that mutant showed that the suppression of this enzyme drastically decreased the proportion of saturated 16:0 and 18:0 fatty acids in all plant tissues (Bonaventure et al. 2003). This deficiency in saturated fatty acids caused dwarf phenotype in mutant plants, pointing that saturated fatty acids are essential for normal plant development. Later studies pointed that a deficiency in saturated fatty acids could alter long chain bases synthesis pathway, producing a lack of sphingolipids that could hamper plant growth (Chen et al. 2006). Thus, while this enzyme appears to be involved in the synthesis of these fatty acids, its specificity profile did not support this function. Studies on FatB enzymes from other sources did not clarified these contradictory results. Thus, FatBs from coriander (Salas and Ohlrogge), castor (Sánchez-garcía et al. 2010), Jatropha curcas (Wu et al. 2009), Madhuca longifolia (Ghosh et al. 2007) and Macadamia tetraphylla (Moreno-Pérez et al. 2011) were cloned and characterized yielding profiles similar to those reported by Dörmann et al. (2000) for the Arabidopsis form. The data presented in this work showed that FatB from sunflower was much more active with the endogenous saturated acyl-ACP substrates, displaying K cat values 20-fold larger for 16:0 derivatives and almost 100-fold larger for 18:0 ones (Fig. 5b; Table 3). On the contrary, it displayed lower activities towards 18:1 derivatives. This result indicate that plant FatB thioesterases are probably very specific towards saturated acyl-ACP substrates, and that the specificity profiles reported elsewhere for this enzyme are probably due to the use of non-endogenous ACPs to assay this enzyme activity. This aspect of acyl-ACP thioesterases should be taken into consideration in future when ACP-dependent enzymes are characterized. Another interesting issue is that HaFatB was more efficient at exporting 18:0 than 16:0 in sunflower. In this regard, FatB should be responsible of most of the saturated fatty acid flow that are stored in TAGs by developing seeds, so it is an important enzyme in plant lipid biotechnology. The production of sunflower oils enriched in stearic acid has been a priority in our group due it could be a source of healthy solid fats. Thus, the partial inhibition of sunflower stearate desaturase produced an increase of the accumulation of stearic acid in sunflower (Pérez-Vich et al. 1999), which means that this plant should have the necessary enzymatic machinery to export this fatty acid from plastids at high rates. Moreover, the stearoyl-ACP thioesterase activity of sunflower seeds plastids was measured in vivo by labelling experiments (Pleite et al. 2006), resulting in a considerable capacity of that enzyme to hydrolize the mentioned substrate, which does not fitted well with the specificity profile reported for FatB from sunflower and other sources, which very often show low levels of activity towards stearoyl-ACP. Data in the present work explain that results and supported that most of the export of stearic acid from sunflower seed plastids is undertaken by HaFatB thioesterase, which was highly specific for endogenous stearoyl-ACPs. Furthermore, the gene coding for HaFatB could be related with the improved high-stearic traits of some sunflower mutants (Pérez-Vich et al. 1999). On the contrary, the low stearic acid content of common sunflower can be easily explained by the competition of this enzyme with the very active sunflower stearoyl-ACP desaturase.
Conclusions
The main conclusion of this work was that the nature of the ACP protein can alter the specificity and catalytic efficiency of non-divergent enzymes of fatty acid metabolism, as was the case of HaFatA and HaFatB thioesterases. The endogenous ACPs enhanced HaFatA activity with respect to substrates prepared from EcACP, and the resulting specificity and kinetic parameters were similar for the three sunflower ACPs. In the case of HaFatB, the use of endogenous ACP caused important changes on enzyme specificity, which displayed much stronger activity and catalytic efficiency with these ACPs and was highly specific towards saturated acyl-ACPs. This was in very good agreement with the reverse genetic results obtained in Arabidopsis (Bonaventure et al. 2003) and suggests that plant FatB1 like enzymes are highly specialized for the export of de novo synthesized saturated fatty acids from plastids. Moreover, these results demonstrate that the nature of ACPs used is very important when studying intraplastidial biochemistry. Indeed, the results obtained with alternative ACPs could change substantially when using physiological substrates, a fact that must be taken into consideration in future experiments in the field.
Author contribution statement
Jose A. Aznar-Moreno did the work of cloning, gene and protein expression and biochemical determinations. Mónica Venegas-Calerón supervised and organized the work of cloning and gene expression. Enrique Martínez-Force did the phylogenetic tree and the protein modelling work. Rafael Garcés managed to get funds for the project and also general coordination of tasks. Joaquín J. Salas did the manuscript writing and edition and also supervision and organization of the protein expression and biochemical work.
Abbreviations
- ACP:
-
Acyl carrier protein
- DAF:
-
Days after flowering
- DAG:
-
Diacylglycerol
- FAS:
-
Fatty acid synthases
- PBS:
-
Phosphate-buffered saline
- TAG:
-
Triacylglycerol
References
Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucl Acids Res 25:3389–3402
Battey JF, Ohlrogge JB (1990) Evolutionary and tissue-specific control of expression of multiple acyl-carrier protein isoforms in plants and bacteria. Planta 180:352–360
Beisson F, Koo AJK, Ruuska S, Schwender J, Pollard MR, Thelen JJ, Paddock T, Salas JJ, Savage L, Milcamps A, Mhaske VB, Cho Y, Ohlrogge JB (2003) Arabidopsis genes involved in acyl lipid metabolism. A 2003 census of the candidates, a study of the distribution of expressed sequence tags in organs, and a web-based database. Plant Physiol 132:681–697
Berman HM, Westbrook J, Feng Z, Gilliland G, Bhat TN, Weissig H, Shindyalov IN, Bourne PE (2000) The protein data bank. Nucl Acids Res 28:235–242
Bonaventure G, Salas JJ, Pollard MR, Ohlrogge JB (2003) Disruption of the FATB gene in arabidopsis demonstrates an essential role of saturated fatty acids in plant growth. Plant Cell 15:1020–1033
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Chen M, Han G, Dietrich CR, Dunn TM, Cahoon EB (2006) The essential nature of sphingolipids in plants as revealed by the functional identification and characterization of the Arabidopsis LCB1 subunit of serine palmitoyltransferase. Plant Cell 18:3576–3593
Dörmann P, Voelker VA, Ohlrogge JB (1995) Cloning and expression in Escherichia coli of a novel thioesterase from arabidopsis thaliana specific for long-chain acyl-acyl carrier proteins. Arch Biochem Bioph 316:612–618
Dörmann P, Voelker TA, Ohlrogge JB (2000) Accumulation of palmitate in arabidopsis mediated by the acyl-acyl carrier protein thioesterase FATB1. Plant Physiol 123:637–644
Ghosh SK, Bhattacharjee A, Jha JK, Mondal AK, Maiti MK, Basu A, Ghosh D, Ghosh S, Sen SK (2007) Characterization and cloning of a stearoyl/oleoyl specific fatty acyl–acyl carrier protein thioesterase from the seeds of Madhuca longifolia (latifolia). Plant Physiol Biochem 45:887–897
González-Thuillier I, Venegas-Calerón M, Garcés R, Wettstein-Knowles P, Martínez-Force E (2015) Sunflower (Helianthus annuus) fatty acid synthase complex: enoyl-(acyl carrier protein)-reductase genes. Planta 241:43–56
Guex N, Peitsch MC (1997) SWISS-MODEL and the Swiss-Pdb Viewer: an environment for comparative protein modelling. Electrophoresis 18:2714–2723
Hawkins DJ, Kridl JC (1998) Characterization of acyl-ACP thioesterases of mangosteen (Garcinia mangostana) seed and high levels of stearate production in transgenic canola. Plant J 13:743–752
Hiltunen JK, Schonauer MS, Autio KJ, Mittelmeier TM, Kastaniotis AJ, Dieckmann CL (2009) Mitochondrial fatty acid synthesis type II: more than just fatty acids. J Biol Chem 284:9011–9015
Holak TA, Kearsley SK, Kim Y, Prestegard JH (1988) Three-dimensional structure of acyl carrier protein determined by NMR pseudoenergy and distance geometry calculations. Biochemistry 27:6135–6142
Jones A, Davies HM, Voelker TA (1995) Palmitoyl-acyl carrier protein (ACP) thioesterase and the evolutionary origin of plant acyl-ACP thioesterases. Plant Cell 7:359–371
Kim Y, Kovrigin EL, Eletr Z (2006) NMR studies of Escherichia coli acyl carrier protein: dynamic and structural differences of the apo- and holo-forms. Biochem Bioph Res Co 341:776–783
Kuo TM, Ohlrogge JB (1984) Acylation of plant acyl carrier proteins by acyl-acyl carrier protein synthetase from Escherichia coli. Arch Biochem Bioph 230:110–116
Lambalot RH, Walsh CT (1995) Cloning, overproduction, and characterization of the Escherichia coli holo-acyl carrier protein synthase. J Biol Chem 270:24658–24661
Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG (2007) Clustal W and clustal X version 2.0. Bioinformatics 23:2947–2948
Li Q, Khosla C, Puglisi JD, Liu CW (2003) Solution structure and backbone dynamics of the holo form of the frenolicin acyl carrier protein. Biochemistry 42:4648–4657
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2 − ΔΔCT method. Methods 25:402–408
Moreno-Pérez AJ, Sánchez-García A, Salas JJ, Garcés R, Martínez-Force E (2011) Acyl-ACP thioesterases from macadamia (Macadamia tetraphylla) nuts: cloning, characterization and their impact on oil composition. Plant Physiol Biochem 49:82–87
Moreno-Pérez AJ, Venegas-Calerón M, Vaistij FE, Salas JJ, Larson TR, Garcés R, Graham IA, Martínez-Force E (2012) Reduced expression of FatA thioesterases in Arabidopsis affects the oil content and fatty acid composition of the seeds. Planta 235:629–639
Ohlrogge JB, Jaworski JG (1997) Regulation of fatty acid synthesis. Annu Rev Plant Biol 48:109–136
Pérez-Vich B, Garcés R, Fernández-Martínez JM (1999) Genetic control of high stearic acid content in the seed oil of the sunflower mutant CAS-3. Theor Appl Genet 99:663–669
Pleite R, Martínez-Force E, Garcés R (2006) Increase of the stearic acid content in high-oleic sunflower (Helianthus annuus) Seeds. J Agric Food Chem 54:9383–9388
Pollard MR, Anderson L, Fan C, Hawkins DJ, Davies MH (1991) A specific acyl-ACP thioesterase implicated in medium-chain fatty acid production in immature cotyledons of Umbellularia californica. Arch Biochem Bioph 284:306–312
Rawlings M, Cronan JE (1992) The gene encoding Escherichia coli acyl carrier protein lies within a cluster of fatty acid biosynthetic genes. J Biol Chem 267:5751–5754
Rock CO, Garwin JL (1979) Preparative enzymatic synthesis and hydrophobic chromatography of acyl-acyl carrier protein. J Biol Chem 254:7123–7128
Salas JJ, Ohlrogge JB (2002) Characterization of substrate specificity of plant FatA and FatB acyl-ACP thioesterases. Arch Biochem Biophys 403:25–34
Sánchez-García A, Moreno-Pérez AJ, Muro-Pastor AM, Salas JJ, Garcés R, Martínez-Force E (2010) Acyl-ACP thioesterases from castor (Ricinus communis L.): an enzymatic system appropriate for high rates of oil synthesis and accumulation. Phytochemistry 71:860–869
Serrano-Vega MJ, Venegas-Calerón M, Garcés R, Martínez-Force E (2003) Cloning and expression of fatty acids biosynthesis key enzymes from sunflower (Helianthus annuus L.) in Escherichia coli. J Chromatog B 786:221–228
Serrano-Vega MJ, Garcés R, Martínez-Force E (2005) Cloning, characterization and structural model of a FatA-type thioesterase from sunflower seeds (Helianthus annuus L.). Planta 221:868–880
Smith DB, Johnson KS (1988) Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene 67:31–40
Suh MC, Schultz DJ, Ohlrogge JB (1999) Isoforms of acyl carrier protein involved in seed-specific fatty acid synthesis. Plant J 17:679–688
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731–2739
Thelen JJ, Ohlrogge JB (2002) Metabolic engineering of fatty acid biosynthesis in plants. Metab Eng 4:12–21
Voelker T (1996) Plant Acyl-ACP Thioesterases: chain-length determining enzymes in plant fatty acid biosynthesis. Genetic Eng 18:111–133
Wakil SJ (1989) Fatty acid synthase, a proficient multifunctional enzyme. Biochemistry 28:4523–4530
White SW, Zheng J, Zhang Y-M, Rock CO (2005) The structural biology of type II fatty acid biosynthesis. Annu Rev Biochem 74:791–831
Wu P-Z, Li J, Wei Q, Zeng L, Chen Y-P, Li MR, Jiang HW, Wu G-J (2009) Cloning and functional characterization of an acyl-acyl carrier protein thioesterase (JcFATB1) from Jatropha curcas. Tree Physiol 29:1299–1305
Zornetzer GA, Fox BG, Markley JL (2006) Solution structures of spinach acyl carrier protein with decanoate and stearate. Biochemistry 45:5217–5227
Acknowledgments
This work was funded by the “Ministerio de Economia y Competitividad” and FEDER (Project AGL2014-53537-R and JAE-CSIC to J.A. A-M).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Aznar-Moreno, J.A., Venegas-Calerón, M., Martínez-Force, E. et al. Acyl carrier proteins from sunflower (Helianthus annuus L.) seeds and their influence on FatA and FatB acyl-ACP thioesterase activities. Planta 244, 479–490 (2016). https://doi.org/10.1007/s00425-016-2521-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-016-2521-7