Abstract
Acyl–acyl carrier protein (ACP) thioesterases are enzymes that control the termination of intraplastidial fatty acid synthesis by hydrolyzing the acyl–ACP complexes. Among the different thioesterase gene families found in plants, the FatA-type fulfills a fundamental role in the export of the C18 fatty acid moieties that will be used to synthesize most plant glycerolipids. A reverse genomic approach has been used to study the FatA thioesterase in seed oil accumulation by screening different mutant collections of Arabidopsis thaliana for FatA knockouts. Two mutants were identified with T-DNA insertions in the promoter region of each of the two copies of FatA present in the Arabidopsis genome, from which a double FatA Arabidopsis mutant was made. The expression of both forms of FatA thioesterases was reduced in this double mutant (fata1 fata2), as was FatA activity. This decrease did not cause any evident morphological changes in the mutant plants, although the partial reduction of this activity affected the oil content and fatty acid composition of the Arabidopsis seeds. Thus, dry mutant seeds had less triacylglycerol content, while other neutral lipids like diacylglycerols were not affected. Furthermore, the metabolic flow of the different glycerolipid species into seed oil in the developing seeds was reduced at different stages of seed formation in the fata1 fata2 line. This diminished metabolic flow induced increases in the proportion of linolenic and erucic fatty acids in the seed oil, in a similar way as previously reported for the wri1 Arabidopsis mutant that accumulates oil poorly. The similarities between these two mutants and the origin of their phenotype are discussed in function of the results.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The de novo fatty acid synthesis in plants is catalyzed by a fully dissociated type-2 fatty acid synthase (FAS) complex (Ohlrogge and Jaworski 1997). This process takes place in the stroma of plastids or chloroplasts and it involves the sequential elongation of acyl intermediates that are esterified to an acidic protein, the acyl carrier protein (ACP), by the action of the aforementioned FAS complex. The newly synthesized acyl chains feed the intraplastidial prokaryotic lipid synthesis pathway (Roughan and Slack 1982). However, in most plant seeds glycerolipids are synthesized in the endoplasmic reticulum through a eukaryotic pathway. This means it is necessary to export the newly synthesized acyl moieties to the cytoplasm, a process that involves the hydrolysis of the acyl–ACP complexes by acyl–ACP thioesterases, which release free reduced ACP and free fatty acids (Browse and Somerville 1991). Recent evidence indicates that free fatty acids are quickly transported to the cytosol in the form of acyl-CoAs due to the action of an acyl-CoA synthetase located in the outer plastidial membrane (Koo et al. 2004). This export model involving free fatty acid intermediates created certain controversy when it was first hypothesized, and alternative models suggest that acyl-CoA or acyl-carnitine derivatives are exported instead (Joyard and Stumpf 1980; Thomas et al. 1983; McLaren et al. 1985). However, 18O labeling experiments demonstrated that unesterified acyl moieties were indeed released during the fatty acid biosynthesis in plants (Pollard and Ohlrogge 1999).
Acyl–ACP thioesterases are encoded by nuclear genes and they are imported into the plastid following maturation by hydrolysis of a N-terminal transit peptide. These enzymes are classified into two gene families, FatA and FatB (Jones et al. 1995), of which the FatA thioesterases (or oleoyl-thioesterases) are found ubiquitously in plants, displaying high specificity for oleoyl-ACP (Hellyer et al. 1992; Dörmann et al. 1994; Salas and Ohlrogge 2002; Sánchez-García et al. 2010). These enzymes are highly conserved between different plant species and there is a consensus regarding their role as the general housekeeping thioesterases responsible for the export of most C18 fatty acids from plastids. The FatB thioesterases are much more variable and they can be classified into two subfamilies: FatB1 and FatB2. FatB1 thioesterases are highly specific towards palmitoyl-ACP and oleoyl-ACP, and they are widely spread across the plant kingdom (Jones et al. 1995; Dörmann et al. 2000). In Arabidopsis, only one copy of the FatB gene exists and when it is knocked out, reduced amounts of saturated fatty acids accumulate in all plant tissues. Based on the composition of the different tissues, it was concluded that half of the saturated fatty acids in this plant are exported via FatB (Bonaventure et al. 2003). Examining the lipid composition of all the tissues in this mutant plant, it is difficult to define what causes the dwarf phenotype of the plant, although this growth defect has since been attributed to a shortage in palmitoyl-CoA for sphingolipid synthesis (Chen et al. 2006). The FatB2 enzymes are much less common and they are found in species that accumulate high levels of medium chained fatty acids in their seed oils. This subfamily of enzymes displays high activity towards saturated C8–C14 acyl–ACPs (Dehesh et al. 1996; Voelker et al. 1997) and enzymes with such unusual specificity are especially important in plant lipid biotechnology. Thus, when Cuphea californica FatB2 was expressed at high levels in rapeseed, it was able to interrupt the fatty acid pathway and induce the accumulation of medium chain fatty acids in this crop (Voelker et al. 1992).
Acyl–ACP thioesterases have not yet been crystallized, although their structure has been modeled on the basis of their similarity towards Escherichia coli 4-hydroxybenzoyl-CoA thioesterase. Accordingly, it was hypothesized that the active site of these enzymes displays a hot-dog fold pattern, with a catalytic triad of amino acids similar to that in papain (Mayer and Shanklin 2005; Serrano-Vega et al. 2005).
Of all the thioesterases, FatA is probably that which is essential for plant viability. Moreover, their role in diverting acyl chains to extraplastidial glycerolipid synthesis makes them quite important when determining the metabolic flux into triacylglycerols in oil seeds. Arabidopsis thaliana contains two copies of FatA in its genome (At3g25110 or FatA1 and At4g13050 or FatA2). These thioesterase isoforms are expressed similarly and they seem to be ubiquitously expressed in all tissues (Beisson et al. 2003). Here we have studied the effect of a reduction in FatA activity in Arabidopsis. Thus, two lines with T-DNA insertions in the promoter regions of the two copies of FatA were identified and crossed to obtain a double mutant. This double mutant displayed changes in the amount and composition of the seed oil similar to that reported for the Arabidopsis wri1 mutant (Focks and Benning 1998). The Arabidopsis wri1 mutant exhibits a deficient glucose metabolism in seeds that reduced oil accumulation and hence, the reasons for these similarities are discussed in function of these data.
Materials and methods
Biological material and growth condition
The A. thaliana homozygous fata1 (WiscDsLox357F07) and fata2 (SALK_050939) mutant lines from the European Arabidopsis Stock Center were crossed to produce the fata1 fata2 double mutant. These plants were genotyped and a homozygous A. thaliana fata1 fata2 line was selected for use in this study along with the Columbia-0 (Col-0) ecotype. Arabidopsis seeds were surface sterilized, cold-treated at 4°C and imbibed in the dark for 4 days on 1% phytoagar plates containing 1% sucrose and 0.5 MS media. The seeds were then germinated and grown in soil at 20°C under a 16 h day/8 h night photoperiod.
Genotyping
PCR reactions were performed on genomic DNA to detect the homozygosity of the T-DNA insertions in the mutant fata1 and fata2 lines. T-DNA-specific primers (p745 for At3g25110 or LBb1.3 for At4g13050) and At3g25110 or At4g13050 gene-specific RP primers (N852633RP or N550939RP, respectively) were used to detect the existence of the T-DNA (Table 1). The T-DNA signal was only present in the mutant lines. The LP (N852633LP for At3g25110 and N550939LP for At4g13050) and RP primers specific for the gene sequences near the left and right borders of T-DNA insertion were used to amplify the intact gene (Table 1). The mutant lines lacking signal were considered homozygous.
RNA preparation and cDNA synthesis
Approximately 0.25 g of Arabidopsis siliques containing embryos at walking-stick stage were ground in liquid nitrogen using a precooled sterile mortar and pestle, and total RNA was isolated using the RNeasy Mini Kit (Qiagen). RNA samples were treated with DNAseI (Promega) and this DNA-free RNA (1 μg) was reverse transcribed with SuperScript II RT (Invitrogen) using an oligo (dT) primer.
Quantitative real time PCR
First strand cDNA samples were amplified in quantitative real time PCR (QRT-PCR) reactions using the following gene-specific primers: AtFatA1-F and AtFatA1-R2 for FatA1, AtFatA2 and FAtFatA2-R2 for FatA2, AtActin-F and AtActin-R for the Arabidopsis actin gene (Table 1). The Livak and Schmittgen (2001) method was applied to calculate comparative expression levels between samples and the Actin gene was used as the internal reference to normalize the relative amount of cDNAs for all samples.
Preparation of acyl–ACP substrates
Labeled acyl–ACP substrates were prepared using a recombinant acyl–ACP synthetase from E. coli. Acylation reactions with a final volume of 0.5 ml contained 50 μg of recombinant ACP from E. coli (Sigma-Aldrich), 180 kBq (approx. 0.1 μmol) of [1-14C] fatty acid ammonium salt ([3H] fatty acid in the case of 16:1Δ9), 5 mM ATP, 2 mM DTT, 400 mM LiCl2, 10 mM MgCl2, 100 mM Tris–HCl [pH 8.0] and 10 μg acyl–ACP synthetase. Reactions were carried out at room temperature for 3 h and the acyl–ACPs were purified and concentrated by ion exchange chromatography on DEAE-Sepharose as described by Rock and Garwin (1979).
Acyl–ACP thioesterase assays
Siliques with walking-stick embryos were harvested and 1 g of tissue was ground in 10 ml extraction buffer containing: 50 mM Tris–HCl [pH 8.0] and 5 mM DTT (Martínez-Force et al. 2000). The protein concentration in the crude extracts was measured using the Bio-Rad Protein Assay (Hercules, CA, USA) according to the manufacturer’s instructions and using BSA as the standard. Thioesterase activity was assayed in 0.1 ml reactions containing 50 mM Tris–HCl [pH 8.0], 5 mM DTT, 2–6 μg of the protein preparation and 0.07 nmol (150 Bq approx.) of acyl–ACP substrate. Reactions were carried out at room temperature for 5 min and stopped by the addition of 0.25 ml of 1 M acetic acid in 2-propanol. Unesterified fatty acids were then extracted twice with 0.3 ml hexane, and the radioactivity in the pooled organic phase was determined in a calibrated liquid scintillation counter (Rackbeta II; LKB).
Lipid analysis
Developing seeds or whole young plants leaves were harvested and ground for lipid analysis. The samples’ fatty acid composition was determined via acid-catalyzed transmethylation and gas-chromatography with flame ionization detection (GC8000 Top, Thermoquest Separation Products, Manchester, UK), fitted with a 30 m long 0.25 mm ID SGE BPX70 column (SGE, Milton Keynes, UK). Helio was used as a carrier gas at 1 ml min−1 with a 30:1 split ratio. The oven was run isothermally at 110°C for 1 min, then ramped to 180°C at 20°C min−1 then to 221°C at 2.5°C min−1 (Larson and Graham 2001). The pool of acyl-CoAs was determined by HPLC using a modified version of the method of Larson and Graham ( 2001; Larson et al. 2002), and neutral lipids were analyzed by LC/MS/MS according to Burgal et al. (2008).
Results
Generation of the fata1 fata2 double mutant line
All the available sequenced knockout libraries of Arabidopsis were screened for insertions in the At3g25110 (FatA1) and At4g13050 (FatA2) loci, and mutants with T-DNA insertions in the 5′ untranslated regions were acquired from the European Arabidopsis Stock Center. The T-DNA insertions were located 100 bp upstream of the start of codon (Fig. 1) and homozygous lines for the insertions were obtained. Both homozygous lines were crossed and the resulting offspring was screened to identify double mutants by PCR. A double mutant line (fata1 fata2) was fixed in the third generation and when these fata1 fata2 plants were grown in parallel with wild type (Col-0) plants. Like in the single mutant lines, fata1 and fata2, no morphological differences were evident in the double mutant. Accordingly, the fata1 fata2 plants had a normal rosette diameter and number of leaves throughout the vegetative period (data not shown).
Expression of FatA genes and acyl–ACP thioesterase activity
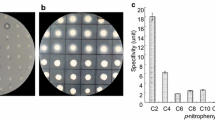
The FatA1 and FatA2 genes are most strongly expressed in Arabidopsis in late torpedo to early walking-stick embryos (Schmid et al. 2005). Siliques containing embryos of the Col-0, fata1, fata2 and fata1 fata2 lines at those stages were harvested and FatA gene expression was analyzed by RT-quantitative PCR (RT-Q-PCR). In the double mutant, the T-DNA insertions in the gene promoter regions diminished the expression of both genes by about 50% with respect to the Col-0 line (Fig. 2). This reduction in the number of FatA gene transcripts induced an important decrease in the acyl–ACP thioesterase activity in crude extracts from developing siliques (Fig. 3). Thus, the 18:1-ACP substrate was hydrolyzed in fata1 fata2 mutant line at a rate that was 40% that found in Col-0 plants. No significant decrease in activity was observed for the other acyl-ACP species assayed.
Seed lipid content and composition
The storage lipid content of seeds was carefully analyzed in Col-0 and fata1 fata2 plants in order to assess the impact of the decrease in FatA activity on the metabolic flux into oil. The total fatty acid, triacylglycerol (TAG) and diacylglycerol (DAG) content were determined in seeds at different stages of development and in seedlings (Fig. 4). The lipid content was studied at different embryonic stages (walking stick, green cotyledon, mature seed and dry seed) and in seedlings at 12 h and 5 days after imbibition. Remarkably, the average quantities of the neutral lipid species and total fatty acids studied were always lower in the double mutant, although these differences were only relevant in some cases (Fig. 4). This difference was most evident at the green cotyledon stage, which accumulated significantly less of the three lipids (in the range of 40%) in the fata1 fata2 line. In addition, there were less TAGs in fata1 fata2 dry seeds, although the differences were lower than in green cotyledons (around 10%).
Furthermore, the fatty acid composition in the fata1 fata2 seeds was distinct to that in the controls (Fig. 5) and these alterations were even more important that those found in the total amount of lipids. The most notable difference during the development of the seeds was the stronger accumulation of linolenic acid at all developmental stages, except in the green cotyledon. This increase took place at the expense of C18 fatty acids with a lower degree of unsaturation, oleic and linoleic acids, both in terms of the absolute amounts of fatty acids (data not shown) or the relative compositions of the developing seeds (Fig. 5). Moreover, the content of very long chain fatty acids (C20–C24) was also altered in the fata1 fata2 line and specifically, erucic acid accumulated in much higher proportions (30–40% higher) in the mutant plants.
Fatty acid composition of Arabidopsis Col-0 and fata1 fata2 seeds and seedlings at different stages of development. The data corresponded to the average and standard deviation of five determinations. a Walking stick. b Green cotyledon. c Mature seed. d Dry seed. e 12 h after imbibition. f 5 days after imbibition. 16:0, palmitic acid; 16:1n9, palmitoleic acid; 16:1n7, 9-hexadecenoic acid; 18:0, stearic acid; 18:1n9c, oleic acid; 18:1n7c, asclepic acid; 18:2n6c, linoleic acid; 18:3n6, γ-linolenic acid; 18:3n3, α-linolenic acid; 20:0, araquic acid; 20:1n9, gondoic acid; 20:1n7, 13-eicosenoic acid; 20:2n6, 11,14-eicosadienoic acid; 20:3n3, 11,14,17-eicosatrienoic acid; 22:0, behenic acid; 22:1n9, erucic acid; 22:2n6, 13,16-docosadienoic acid; 24:0, lignoceric acid; and 24:1n9, nervonic acid
Composition of the acyl-CoA pool
The changes observed in the fatty acid composition were also reflected in the acyl-CoA pool of the seeds (Fig. 6). In the case of developing green cotyledons (Fig. 6a) the differences between the control and mutant plants concerned to the very long chain fatty acids. So, there was a higher conversion of 20:0-CoA to 22:0-CoA in the seeds of the double mutant. No statistically significant differences were found in the case of CoA esters from polyunsaturated fatty acids in that stage. In the case of dry cotyledons (Fig. 6b), there was an increase of the 18:3-CoA at the expense of the less unsaturated precursors, 18:2-CoA and 18:1-CoA, although this increment was small than that found in seed TAGs. The C22 acyl-CoA derivatives were also more abundant in the double mutant seeds, in conjunction with lower C20 CoA content. This increase was especially important in the case of erucic acid that augmented twofold in the fata1 fata2 line.
TAG and DAG molecular species
As expected, the TAG species in the dry seeds of the fata1 fata2 line were distinct to those from the Col-0 line (Fig. 7). The differences in TAGs species between the two lines mainly affected those containing linolenic acid, all 13 species of TAGs that were more abundant in the seeds of the double mutant contained one, two or three 18:3 residues. By contrast, TAGs species containing 18:1 and 18:2 acyl moieties were less abundant in the fata1 fata2 seeds. The differences between the two lines studied were not so clear in the case of very long chain fatty acids.
The same tendency was found with the DAGs in dry seeds (Fig. 8) and again, the species carrying esterified linolenic acid were more abundant in the fata1 fata2 seeds, whereas DAGs containing 18:2 and 18:1 were less strongly represented when compared with those mentioned above. In general, the very long chain fatty acid content of DAGs was much lower than that in TAG species.
Discussion
Unlike FatB, there have been no studies to date on the depletion of FatA thioesterases, the main contributors to the acyl metabolic flux out of plastids, in function of their level of activity, specificity and expression (Jones et al. 1995). Arabidopsis plants contain two copies of the FatA gene in their genome and thus, we screened all the available sequenced KO mutant databases to identify insertional FatA mutants. As a result of this screening, two mutants were identified with insertions in their promoter region that maintained the coding regions intact (Fig. 1). The absence of mutants with insertions in either exons or introns may indicate that they provoked lethality, although we have no direct evidence to support this hypothesis.
There were no differences in the number of leaves, growth rate, or number and viability of the seeds between control Col-0, single mutants, and double mutant fata1 fata2 lines. Hence, the insertions did not appear to interfere with the synthesis of lipids necessary for the vegetative development of the plant. Accordingly, most of the present study focused on oil accumulation in the developing seeds since this was the plant tissue that most actively synthesized lipids and any change in their metabolic flux could influence the amount and composition of the TAGs accumulated in this organ. Therefore, the expression of both FatA1 and FatA2 genes was studied by Q-RT-PCR to determine the impact of the insertions on the regulation of these genes (Fig. 2). A reduction in the expression of both genes was evident in developing siliques, which in the case of the double mutant represented a reduction to half the number of transcripts found in the control line. This reduction of FatA expression was far from that found for other insertional thioesterase mutants. However, the expression in the FatB knockout mutant described previously was 150-fold lower than that of control plants (Bonaventure et al. 2003), and consequently, the mutant described here should be considered a mutant deficient in FatA expression but not a knockout FatA mutant.
The reduction in the expression of both FatA genes significantly affected the levels of acyl–ACP thioesterase activity in crude extracts from developing siliques. Thus, a slowing of the rate of oleoyl–ACP hydrolysis was found in the mutant lines that agreed well with this reduction in the expression of both FatA genes. Such a reduction in activity was not significant for the other substrates assayed, which could be affected by hydrolyzing activities like FatB or other unspecific enzymes. Although there was no impact on plant growth, the rates of lipid accumulation in seeds were affected in the double mutants. The profiles of TAG, DAG and total fatty acid accumulation were investigated throughout seed development in both control and mutant plants. In all cases the lipid content analyzed was lower in the double FatA deficient mutant. While these differences were small and often not significant in the green cotyledons, the lipids accumulated in the mutant were generally around 50% of those found in the control plants. At later stages of development, the TAG, DAG and total fatty acid accumulation reached similar levels, although significant differences were found in the TAGs in dry seeds. The levels of lipids in seedlings were also examined and there were larger amounts of lipids in the control Col-0 line. This profile of lipid accumulation is compatible with a reduced flux of lipid synthesis in the fata1 fata2 line. Thus, there were important differences between the lines at the beginning of this process, the Col-0 line having a higher flux and accumulating more lipids than the double FatA mutant. At later stages the amount of lipids accumulated becomes more even. The decrease in the flux of lipid synthesis associated with the reduction in FatA activity in developing seeds was more apparent in the green cotyledon stage and it was later compensated for by lipid deposition.
The reduction in FatA expression causes changes in the fatty acid composition. Taking into consideration the profiles of Arabidopsis FatA and FatB specificity, the expected changes in fatty acid composition in a FatA deficient mutant would affect the ratio between C16 and longer fatty acids. Thus, a decrease of FatA while the activity of FatB remained constant would decrease the relative amount of palmitic acid incorporated to glycerolipids. However, there was no important decrease in C16 fatty acids at any stage of development of Arabidopsis seeds, and the proportion of palmitic acid was only slightly higher and did not exhibit significant differences in the samples analyzed. This contrasted with the Arabidopsis FatB mutant where there was important decrease in palmitic acid in all tissues, which altered the relative C16 fatty acid content in the accumulated oil (Bonaventure et al. 2003). This difference between the two mutants was probably caused by the specific expression of the repressed thioesterase genes described above. The differences in fatty acid composition between the FatA deficient mutant and the control line mainly concerned the levels of unsaturation of C18 fatty acids. These differences were especially high in mature and dry seeds where there were significant differences in the linolenic acid content, which was more abundant in the fata1 fata2 line at the expense of oleic and linoleic acids. Furthermore, a similar tendency was observed with C22 fatty acids, which were more prominent in the fata1 fata2 than in the Col-0 plants. Interestingly, the changes in the fatty acid composition were not related to the de novo synthesis but rather to the later steps of fatty acid modification. This probably means that the changes were not a direct consequence of the reduction in FatA activity but rather to a metabolic response of the plant to the alterations caused by the insertions.
The changes in seed fatty acid content also affected the acyl-CoA pool. In this case, the increase in the C22 fatty acids was more important than the changes in the total fatty acid content, probably due to the elongation of C20 and C22 fatty acids in the acyl-CoA substrates. This was observed in both developing green cotyledons and dry seeds (Fig. 6). Thus, almost all the 20:0-CoA was converted to C22 derivatives in the fata1 fata2 line, which displayed twice the amount of 22:1-CoA than control dry seeds. The increased accumulation of linolenic acid in the fata1 fata2 was more important in dry seeds and affected their TAG composition, so the species containing multiple linolenic acid residues were more prevalent in the double mutant at the expense of those containing oleic or linoleic acid. In terms of the DAGs, the last precursor of TAGs, all species containing 18:3 were more abundant in the mutant. It was also notable that DAGs displayed a smaller proportion of 22:1, and in general, of all the very long chain fatty acids known to be present in Arabidopsis, which means that these fatty acids are mostly incorporated into TAGs in the last step of synthesis, via DAG-acyl-CoA acyltransferase (DAGAT).
All these modifications in fatty acid composition were unexpected and were not caused directly by the decrease of FatA activity but rather, by the metabolic response of the plant to a diminished fatty acid flux caused by the reduction in such activity. The response of plant metabolism to the depletion of FatB thioesterase has been studied previously (Bonaventure et al. 2004), indicating that plants try to compensate for the lack of saturated fatty acids by increasing the rates of synthesis and degradation of fatty acids. However, the FatB mutant displayed alterations that were mainly related to saturated fatty acids exported by FatB, with a substrate specificity that differed from that of FatA.
Thus, it seems that the regulatory pathways activated by FatA depletion were essentially different to those activated in the FatB knockout mutant. Indeed, the alterations found in the double FatA mutant were more similar to those of Arabidopsis mutants deficient in oil accumulation, such as wri1 (reduced transcription factor expression; Focks and Benning 1998), pkp1 (reduced plastidial pyruvate kinase activity; Andre et al. 2007; Baud et al. 2007), and BCCP2 (reduced acetyl-CoA carboxylase activity; Thelen and Ohlrogge 2002) mutant plants. In particular, wri1 mutant displayed a reduced oil content caused by an embryonic deficiency in the conversion of sucrose to precursors for TAG biosynthesis. Indeed, the mutation affected the AP2/EREB domain of a transcription factor involved in the biosynthesis of a storage compound (Cernac and Benning 2004). Interestingly, the fatty acid composition of wri1 plants was similar to that reported here, with increased the linolenic and erucic acid content. Indeed, there are AW-boxes presumptively recognized by WRI1 in both FatA promoter regions (Maeo et al. 2009; and Martínez-Force, personal communication). This suggests that in the wri1 mutant, that shows a reduction of total lipid content due to the lower expression of glycolytic and fatty acid biosynthetic genes, among the affected genes the FatA genes are included.
Fatty acid synthesis in Arabidopsis involves the export of saturated and monounsaturated fatty acids via intraplastidial thioesterases, and the subsequent desaturation by reticular oleate and linoleate desaturases that act on fatty acids esterified to the sn-2 position of phosphatidylcholine. Acyl-CoA derivatives can also be elongated by fatty acid elongases to C20 or C22 fatty acids typically found in the seeds of this plant. Each enzymatic step might be associated with given fatty acids and thus, it should be possible to establish the relative flux of each enzyme and compare the values obtained in the control and mutant lines (as shown in Table 2). Indeed, the relative activities of the β-ketoacyl-ACP synthase II (KASII) and stearoyl-ACP desaturase (SAD) appear to be similar in both lines, whereas linoleate desaturase (LDS) and fatty acid elongase (FAE) activity is higher in the double mutant. These data suggest that the decrease in metabolic flux caused by FatA gene depletion involves a general down-regulation of some genes involved in fatty acid synthesis, such as KASII and SAD, whereas other genes retained normal levels of activity (ODS, LDS and FAE). As a result there is an increase in the proportion of linolenic and erucic acid at the expense of their direct precursors in the fata1 fata2 mutant. This also seemed to occur in wri1 mutants when gene expression was studied in microarrays (Ruuska et al. 2002). During seed development in the wri1 Arabidopsis mutant, different genes are expressed distinctly, indicating that they regulated independently. When the expression pattern of these genes in the WT plant was compared with those in wri1 mutants, the expression of less than 1% was affected. However, amongst the genes that suffered alterations in their expression were some encoding for central fatty acid synthesis enzymes, like KASII and SAD. Hence, these genes appear to be regulated according the metabolic flux, and a reduction in the rate of glycolysis induced a down-regulation in the fatty acid synthesis pathway. Nevertheless, the expression of some genes involved in lipid synthesis was not altered in the mutant. These were typically abscisic-acid regulated genes like FAE1 and LDS, which displayed similar levels of expression in both wild type and mutant lines. Accordingly, the products of these enzymes (linolenic acid and C22 fatty acids) were more prominent in the oil of mutant plants as an indirect consequence of the changes in metabolic flux. This phenotype was similar to that found in the double FatA mutant reported here, indicating that the reduced expression of the two FatA forms induced a reduction of the metabolic flux into TAGs. This effect was associated with the down-regulation of most of the enzymes in this pathway, while FAE or LDS were not affected by this modulation of metabolic flux.
The resulting increase in linolenic and erucic fatty acids was like that seen in the wri1 mutant and therefore, in Arabidopsis fatty acid synthesis appears to be regulated in function of the metabolic flux. The reduction of FatA activity could produce a temporal acyl–ACPs accumulation in the plastid provoking a feedback inhibition of fatty acid biosynthesis. Actually, in E. coli, the main mechanism for the regulation of fatty acid synthesis is through feedback inhibition by long-chain acyl–ACPs (Jiang and Cronan 1994). These long-chain acyl–ACPs affect the pathway at three discrete steps by inhibiting acetyl-CoA carboxylase, β-ketoacyl-ACP synthase III (FabH) and enoyl-ACP reductase (FabI) (Lu et al. 2004). Therefore, the reduction of FatA activity stimulated these mechanisms, provoking a slightly lower rate of lipid accumulation and changes in the fatty acid composition of the seeds. These alterations were associated with an increase in the products of the enzymes encoded by genes whose expression was not altered. Since the resulting phenotype was similar to that reported for the wri1 mutant, this mechanism is also likely to be related to the phenotype of that plant. How this mechanism is activated and controlled has not yet been defined but it would be of great interest to explain how the metabolic flux in seeds is associated with the production of carbohydrates or oil in plants. This process could be an important tool when contemplating altering the oil content of seeds to obtain improved crops.
Abbreviations
- TE:
-
Acyl–ACP thioesterase
- KASII:
-
β-Ketoacyl-ACP synthase II
- SAD:
-
Stearoyl-ACP desaturase
- ODS:
-
Oleate desaturase
- LDS:
-
Linoleate desaturase
- FAE:
-
Fatty acid elongase
References
Andre C, Froehlich JE, Moll MR, Benning C (2007) A heteromeric plastidic pyruvate kinase complex involved in seed oil biosynthesis. Plant Cell 19:2006–2022
Baud S, Wuillème S, Dubreucq B, de Almeida A, Vuagnat C, Lepiniec L, Miquel M, Rochat C (2007) Function of plastidial pyruvate kinase in seeds of Arabidopsis thaliana. Plant J 52:405–419
Beisson F, Koo AJK, Ruuska S, Schwender J, Pollard M, Thelen JJ, Paddock T, Salas JJ, Savage L, Milcamps A, Mhaske VB, Cho Y, Ohlrogge JB (2003) Arabidopsis genes involved in acyl lipid metabolism. A 2003 census of the candidates, a study of the distribution of expressed sequence tags in organs, and a Web-based database. Plant Physiol 132:681–697
Bonaventure G, Salas JJ, Pollard MR, Ohlrogge JB (2003) Disruption of the FATB gene in Arabidopsis demonstrates an essential role of saturated fatty acids in plant growth. Plant Cell 15:1020–1033
Bonaventure G, Bao X, Ohlrogge J, Pollard M (2004) Metabolic responses to the reduction in palmitate caused by disruption of the FATB gene in Arabidopsis. Plant Physiol 135:1269–1279
Browse J, Somerville CR (1991) Glycerolipid synthesis: biochemistry and regulation. Annu Rev Plant Physiol Plant Mol Biol 42:467–506
Burgal J, Shockey J, Lu C, Dyer J, Larson T, Graham I, Browse J (2008) Metabolic engineering of hydroxy fatty acid production in plants: RcDGAT2 drives dramatic increases in ricinoleate levels in seed oil. Plant Biotechnol J 8:819–831
Cernac A, Benning C (2004) WRINKLED1 encodes an AP2/EREB domain protein involved in the control of storage compound biosynthesis in Arabidopsis. Plant J 40:575–585
Chen M, Han G, Dietrich CR, Dunn TM, Cahoon EB (2006) The essential nature of sphingolipids in plants as revealed by the functional identification and characterization of the Arabidopsis LCB1 subunit of serine palmitoyltransferase. Plant Cell 18:3576–3593
Dehesh K, Jones A, Knutzon DS, Voelker TA (1996) Production of high levels of 8:0 and 10:0 fatty acids in transgenic canola by overexpression of Ch FatB2, a thioesterase cDNA from Cuphea hookeriana. Plant J 9:167–172
Dörmann P, Kridl JC, Ohlrogge JB (1994) Cloning and expression in Escherichia coli of a cDNA coding for the oleoyl-acyl carrier protein thioesterase from coriander (Coriandrum sativum L.). Biochim Biophys Acta Lipids Lipid Metab 1212:134–136
Dörmann P, Voelker TA, Ohlrogge JB (2000) Accumulation of palmitate in Arabidopsis mediated by the acyl-acyl carrier protein thioesterase FATB1. Plant Physiol 123:637–644
Focks N, Benning C (1998) Wrinkled1: a novel, low-seed-oil mutant of Arabidopsis with a deficiency in the seed-specific regulation of carbohydrate metabolism. Plant Physiol 118:91–101
Hellyer A, Leadlay PF, Slabas AR (1992) Induction, purification and characterisation of acyl-ACP thioesterase from developing seeds of oil seed rape (Brassica napus). Plant Mol Biol 20:1573–5028
Jiang P, Cronan JE (1994) Inhibition of fatty-acid synthesis in Escherichia coli in the absence of phospholipid-synthesis and release of inhibition by thioesterase action. J Bacteriol 176:2814–2821
Jones A, Davies HM, Voelker TA (1995) Palmitoyl-acyl carrier protein (ACP) thioesterase and the evolutidnary-origin of plant acyl-ACP thioesterases. Plant Cell 7:359–371
Joyard J, Stumpf PK (1980) Characterization of an acyl-coenzyme A thioesterase associated with the envelope of spinach chloroplasts. Plant Physiol 65:1039–1043
Koo AJK, Ohlrogge JB, Pollard M (2004) On the export of fatty acids from the chloroplast. J Biol Chem 279:16101–16110
Larson TR, Graham IA (2001) A novel technique for the sensitive quantification of acyl CoA esters from plant tissues. Plant J 25:115–125
Larson TR, Edgell T, Byrne J, Dehesh K, Graham IA (2002) Acyl CoA profiles of transgenic plants that accumulate medium-chain fatty acids indicate inefficient storage lipid synthesis in developing oilseeds. Plant J 32:519–527
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408
Lu YJ, Zhang YM, Rock CO (2004) Product diversity and regulation of type II fatty acid synthases. Biochem Cell Biol 82:145–155
Maeo K, Tokuda T, Ayame A, Mitsui N, Kawai T, Tsukagoshi H, Ishiguro S, Nakamura K (2009) An AP2-type transcription factor, WRINKLED1, of Arabidopsis thaliana binds to the AW-box sequence conserved among proximal upstream regions of genes involved in fatty acid synthesis. Plant J 60:476–487
Martínez-Force E, Cantisán S, Serrano-Vega MJ, Garcés R (2000) Acyl–acyl carrier protein thioesterase activity from sunflower (Helianthus annuus L.) seeds. Planta 211:673–678
Mayer KM, Shanklin J (2005) A structural model of the plant acyl-acyl carrier protein thioesterase FatB comprises two helix/4-stranded sheet domains, the N-terminal domain containing residues that affect specificity and the C-terminal domain containing catalytic residues. J Biol Chem 280:3621–3627
McLaren I, Wood C, Jalil MNH, Yong BCS, Thomas DR (1985) Carnitine acyltransferases in chloroplasts of Pisum sativum L. Planta 163:197–200
Ohlrogge JB, Jaworski JG (1997) Regulation of fatty acid synthesis. Annu Rev of Plant Physiol Plant Mol Biol 48:109–136
Pollard M, Ohlrogge J (1999) Testing models of fatty acid transfer and lipid synthesis in spinach leaf using in vivo oxygen-18 labeling. Plant Physiol 121:1217–1226
Rock CO, Garwin JL (1979) Preparative enzymatic synthesis and hydrophobic chromatography of acyl-acyl carrier protein. J Biol Chem 254:7123–7128
Roughan PG, Slack CR (1982) Cellular organization of glycerolipid metabolism. Annu Rev Plant Physiol 33:97–132
Ruuska SA, Girke T, Benning C, Ohlrogge JB (2002) Contrapuntal networks of gene expression during Arabidopsis seed filling. Plant Cell 14:1191–1206
Salas JJ, Ohlrogge JB (2002) Characterization of substrate specificity of plant FatA and FatB acyl-ACP thioesterases. Arch Biochem Biophys 403:25–34
Sánchez-García A, Moreno-Pérez AJ, Muro-Pastor AM, Salas JJ, Garcés R, Martínez-Force E (2010) Acyl–ACP thioesterases from castor (Ricinus communis L.): an enzymatic system appropriate for high rates of oil synthesis and accumulation. Phytochemistry 71:860–869
Schmid M, Davison TS, Henz SR, Pape UJ, Demar M, Vingron M, Schölkopf B, Weigel D, Lohmann JU (2005) A gene expression map of Arabidopsis thaliana development. Nat Genet 37:501–506
Serrano-Vega MJ, Garcés R, Martínez-Force E (2005) Cloning, characterization and structural model of a FatA-type thioesterase from sunflower seeds (Helianthus annuus L.). Planta 221:868–880
Thelen JJ, Ohlrogge JB (2002) Both antisense and sense expression of biotin carboxyl carrier protein isoform 2 inactivates the plastid acetyl-coenzyme A carboxylase in Arabidopsis thaliana. Plant J 32:419–431
Thomas DR, Jalil MNH, Ariffin A, Cooke RJ, McLaren I, Yong BCS, Wood C (1983) The synthesis of short- and long-chain acylcarnitine by etioplasts of greening barley leaves. Planta 158:259–263
Voelker TA, Worrell AC, Anderson L, Bleibaum J, Fan C, Hawkins DJ, Radke SE, Davies HM (1992) Fatty acid biosynthesis redirected to medium chains in transgenic oilseed plants. Science 257:72–74
Voelker TA, Jones A, Cranmer AM, Davies HM, Knutzon DS (1997) Broad-range and binary-range acyl–acyl-carrier-protein thioesterases suggest an alternative mechanism for medium-chain production in seeds. Plant Physiol 114:669–677
Acknowledgments
We are grateful to Rosario Sánchez and Valeria Gazda for their technical assistance. We also thank Dr. Luisa Hernández for help with experimental approach. This work was supported by the Spanish MICINN and FEDER, Project AGL2008-01086/ALI.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Moreno-Pérez, A.J., Venegas-Calerón, M., Vaistij, F.E. et al. Reduced expression of FatA thioesterases in Arabidopsis affects the oil content and fatty acid composition of the seeds. Planta 235, 629–639 (2012). https://doi.org/10.1007/s00425-011-1534-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-011-1534-5