Abstract
Macrophages reside in a dense cellular network in the intestinal muscularis externa, and there is emerging evidence that the functionality of these cells determines the local microenvironment. Inflammatory responses during intestinal diseases change the homeostatic functionality of these cells causing inflammation and intestinal dysmotility. Such disturbances are not only induced by a change in the cellular composition in the intestinal muscularis but also by an altered crosstalk with the peripheral and central nervous system. In this review, we summarize the role of muscularis macrophages in the intestine in homeostasis and inflammation. We compare the functionality, the phenotype, and the origin of muscularis macrophages to their neighboring counterparts within the different layers of the intestine. We outline the cellular crosstalk with the enteric and the peripheral nervous system and summarize the current therapeutic approaches to modulate the functionality of these phagocytes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
General role of intestinal macrophages
Tissue macrophages perform a variety of different functions and are an essential component of the mammalian organism. These functions depend on and are instructed by factors from the local environment and executed in response to specific functional demands [40]. These functions are mainly determined by the macrophage’s ability to sense their local environment. Under healthy conditions, these functions promote tissue homeostasis, but noxious challenges, i.e., infections, trauma, ischemia and metabolic stress, rapidly change the homeostatic functionality of macrophages to immunological mechanisms ensuring host defense and inflammation [2]. Tissue macrophages from different organs, even those with different ontogeny and development, may exhibit some common “classical” functions, i.e., phagocytosis and anti-microbial activity. However, each population is individually shaped by its local microenvironment and therefore tissue macrophages are extremely heterogeneous and their functions go far beyond the classical phagocytic and anti-microbial activities.
Excellent reviews are available focusing on tissue macrophage function in several organs including the lung, the liver, the spleen, and the intestine [6, 44, 103]. Intestinal macrophages are crucial in maintaining gut homeostasis [20, 42, 73]. Furthermore, these cells are numerous in the intestinal muscularis externa to regulate intestinal motility under homeostasis and during inflammation [3, 32, 75, 106]. Here, we focus on specialized resident macrophages which inhabit the gastrointestinal (GI) tract. As these cells consist of different subpopulations significantly differing in function, we compare macrophages of the muscularis externa to their proximate neighbors in the lamina propria mucosae and illuminate their functions under homeostasis and during acute inflammation. Given that the function and the phenotype overlap to dendritic cells, this review also discusses the recent advances to differentiate these myeloid cell subsets.
Historical view on muscularis macrophages
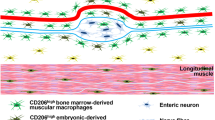
First evidence for the presence of macrophages in the muscularis externa was published by Taxi et al. who detected a macrophage-like interstitial cell type between the adjacent intestinal muscle layers that was able to take up trypan red [93]. These cells were mainly observed in three layers: in the subserosal layer, the myenteric plexus (between the circular and longitudinal muscle layer), and in association with the deep muscular plexus within the circular muscle layer (Fig. 1). Early microscopical analysis by Mikkelsen and colleagues demonstrated that cells in these three locations differ in their microanatomy, pattern formation, capability of dextran uptake, and express typical macrophage markers [69]. Subserosal macrophages showed a few longer slender processes, which are rather regularly distributed in rows. In contrast, cells of the myenteric plexus are more stellate shaped with shorter branches and are evenly distributed throughout the plexus (Fig. 1). Both cells were identified as specialized cells of the mononuclear phagocyte system [70]. Additional work from the same group demonstrated that these cells depend on the colony-stimulating factor 1 (CSF-1), because op/op mice, which are defective in the production of functional CSF-1 [113], lack resident muscularis macrophages with exception of some rare subserosal cells [68]. Further work demonstrated that these macrophages constitutively express MHC class II+ and outnumber F4/80+ cells indicating parallel presence of at least two different cell populations [72].
Neuroimmune interactions in the intestinal muscularis externa. Macrophages can be found throughout the complete GI tract. They differ in numbers, phenotypes, and functions. They are predominant in the mucosa but are also located in the submucosal plexus and form dense networks in the muscularis externa. In the latter, they appear in distinct dendritic morphologies reaching from a simple bipolar shape up to a stellate appearance with dendriform extensions connecting them with the surrounding tissue. Extrinsic and intrinsic neuronal processes innervate all intestinal layers, but intrinsic neuronal cell bodies are restricted to the submucosal and myenteric plexus. Close proximity and functional relationship between nerves and macrophages are particularly observed in the muscularis externa. Extrinsic parasympathetic (Nodose ganglion/Vagus nerve) and sympathetic (Superior/Celiac ganglia) innervation was shown to modulate macrophage immune function by acetylcholine or norepinephrine, respectively. In myenteric ganglia, macrophages also interact with enteric glia and can promote acute inflammation
Macrophage subsets in the intestinal muscularis: ontogeny and specialization in the different layers of the intestine
Macrophages are a heterogeneous population of phagocytic immune cells, which are present in almost all tissues [42]. In the dermis, macrophages are established prenatally but also after birth from blood-derived Ly6Chi monocytes. However, epidermal macrophages, also called Langerhans cells, seem to replenish independently of blood precursors [62]. Comparably, macrophages of the brain and the liver are long-living cells, which are derived from earlier yolk sac and fetal liver progenitors. These cells maintain themselves during life with little contribution of circulating Ly6C+ monocytes [1, 45, 112]. In contrast, intestinal lamina propria macrophages have an exceptionally short half-life of 3 weeks [50], which requires constant de novo generation by recruitment and differentiation of myeloid bone marrow-derived progenitors [8, 12, 101, 102, 115]. Fate mapping experiments established that short-lived Ly6C+ monocytes constitute homeostatic precursors of intestinal macrophages in the lamina propria [112]. These data demonstrated that neonatal intestinal macrophages are not maintained through adulthood [7, 8] and that lamina propria macrophages in the intestine represent a continuum of cells with an important role in regulating intestinal immunity [111]. However, these studies about lamina propria macrophages are mostly based on digests of the whole intestine, which include the population of macrophages in the intestinal muscularis. Hence, muscularis macrophages might share the functionality and the origin of lamina propria macrophages, but their specific characteristics might have also been overlooked.
Little is known about the specific characteristics of muscularis macrophages. The distribution of these cells in adult mice seems to be phenocopied in embryos, newborns and germ-free mice indicating that these cells develop independently of foreign antigens, and their turnover may be slower in comparison to lamina propria macrophages [71]. Macrophages can exhibit a high degree of specialization, and the local microenvironment shapes the functional adaptations of macrophages in space and time. This process of local tissue “imprinting” and the mechanisms that regulate the spatial functionality of macrophage have been reviewed recently [2]. Tissue-dependent adaptations might also contribute to the differential expression of several surface molecules within the different layers of the intestine [57]. A recent study demonstrated a specific functionality of two macrophage populations with distinct localization in the intestinal tissue [38]. By employing imaging and transcriptional profiling, lamina propria macrophages expressed proinflammatory genes, while muscularis macrophages showed genes encoding tissue-protective factors which resembled M2 macrophages and these intra-tissue adaptations seemed to be related to the high density of neuronal processes in the muscularis layer [38, 104]. Also, specific isolation techniques revealed that macrophages in the different intestinal layers also differ in surface molecule expression [57]. These data demonstrate unique intra-tissue adaptation of macrophages and a high degree of specialization within intestinal layers. Furthermore, these findings indicate that lamina propria and muscularis macrophages may develop independently from a common progenitor. As mentioned previously, lamina propria macrophages are constantly replaced by infiltrating Ly6C+ blood monocytes, which acquire a tolerogenic phenotype after tissue entry [12, 101, 102, 115]. In contrast, muscularis macrophages resemble a phenotype of tissue macrophages, which are non-inflammatory and protective to the tissue. Very recently, a reservoir of macrophages in the peritoneal cavity that invade visceral organs to affect tissue repair has been identified [105]. Following injury, these fully mature tissue macrophages infiltrated the liver via a non-vascular route to contribute to tissue repair [105]. These cells might also be capable to infiltrate the intestinal muscularis to populate this intestinal layer with tissue macrophages. However, further experiments are required to elucidate the origin and the functional properties of muscularis macrophages during health and disease.
Demarcation of muscularis macrophages to dendritic cells
In the intestinal lamina propria, macrophages and dendritic cells (DCs) form a continuous network of phagocytic cells [20]. Early studies demonstrated the presence of a phagocyte network also in the intestinal muscularis, and these cells were initially termed muscularis macrophages [69]. These cells are the main phagocyte subset in the healthy intestinal muscularis [57], which express low levels of CD11c but high levels of MHC class II and the CSF-1 receptor (CSF-1R) [75]. The important role of the CSF-1R is supported by the finding that CSF-1R-deficient mice showed dramatically reduced numbers of the resident muscularis CD11clow MHCIIhi macrophages [57]. It remains to be determined whether muscularis macrophages originate from early progenitors or whether there is constant replacement by the hematopoietic compartment as seen in the lamina propria [8].
Some of these muscularis phagocytes expressed high levels of DEC205 and were equipped with the activation markers CD80, CD86, and CD95 to function as antigen-presenting cells suggesting that some phagocytes in the intestinal muscularis resemble a DC-like phenotype [37]. These DCs in the intestinal muscularis were also able to activate Th1 memory cells in a murine model of postoperative ileus, a frequent disease after intestinal surgery [32]. However, further studies are required to better characterize these cells during homeostasis and inflammation.
In the lamina propria, DCs can be distinguished from macrophages by the differential expression of CX3CR1, F4/80, CD64, and CD103 [20, 44]. Several studies have demonstrated that although DCs express CD103, macrophages are devoid of CD103 expression but express high levels of CX3CR1, F4/80, and CD64 [7, 19, 85]. CD103+CD11b− DCs depend on the transcription factors Batf3 and Irf8 [31, 46, 56], cross-present antigens to CD8+ T cells [60], induce regulatory T cells and modulate T cell responses [35, 43, 44]. In contrast, CD103+CD11b− DCs develop from the common Flt3L-dependent pre-cDC progenitor [12, 102] by mechanisms involving the transcription factors Irf4 [80, 83]. They are unique to the intestinal tract and contribute to intestinal immunity [12, 19, 86]. However, little is known about these DC subtypes in the intestinal muscularis and further studies are required to describe the precise role of these DCs and to clearly differentiate these cells from muscularis macrophages.
Functionality of intestinal macrophages in health and disease
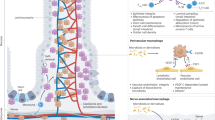
Under healthy conditions, blood-derived Ly6C+ monocytes are constantly recruited to populate the intestine with macrophages [8]. After tissue entry, these Ly6C+ monocytes possess a tolerogenic signature, which maintains homeostasis [12, 101, 102, 115]. Exposure to the luminal microbiota does not induce proinflammatory responses by these cells [7, 75]. Accordingly, muscularis macrophage polarization has also been shown to be affected by luminal bacterial infection and attributed to norepinephrine signaling to ß2 adrenergic receptors [38]. Hence, extrinsic signals by sympathetic neurons can skew muscularis macrophages to a tissue-protective phenotype identifying a protective neuroimmune communication between neurons and macrophages [38]. This neuroimmune axis also contributes to maintenance of homeostasis by regulating intestinal peristalsis [75]. A distinct macrophage subset in the intestinal muscularis regulates peristaltic activity in the colon and secreting BMP2, which activates the corresponding receptor expressed on enteric neurons [75]. However, this tolerogenic program is impaired in acute inflammation so that these cells acquire a proinflammatory signature and induce intestinal diseases [7, 32, 96, 106, 116].
A major reason for the activation of lamina propria macrophages is the continuous presence of foreign antigens, which prevents tolerance induction in macrophages during inflammation and tissue damage. The activation of muscularis macrophages under inflammatory circumstances might also depend on pathogen-associated molecular patterns (PAMPs), because translocation of bacteria from the luminal site has been observed in the murine model of postoperative Ileus and after surgical procedures in human subjects [54, 90]. However, it was recently shown that the induction of postoperative ileus was independent of Toll-like receptor signaling [92]. Instead, the upstream receptor for IL-1 (IL-1R1) was identified as a critical component for the development of postoperative ileus (POI) [92]. Bone marrow chimeric animals identified the important role of IL-1R1 expression on enteric glia cells, and these cells secreted the proinflammatory molecules MCP-1 and IL-6 after stimulation with IL1α or IL1β. Antagonism of IL-1R1 by anakinra reduced the inflammatory response and prevented POI [92]. Monocytes and macrophages are also required to maintain IFNγ and IL-17-dependent T cell responses in the mucosa during Citrobacter rodentium infection [84]. Also, in the intestinal muscularis, IL-17A induces inducible nitric oxide synthase (iNOS) expression in muscularis macrophages, which reduced intestinal motility [74]. The finding of hypomotility of the intestine is surprising, because IL-17 responses are mostly related with acute bacterial gastroenteritis, which cause hypermotility and diarrhea. Although the causative agent can be identified in some cases (e.g., bacterial gastroenteritis), non-specific viral agents and/or psychogenic factors can also induce hypermotility of the gastrointestinal tract. In rodent models of IBD, there is emerging evidence that pathogenic macrophages—from an unknown origin—can be found within the myenteric plexus [55, 59]. These cells communicate with the nervous system to induce this inflammatory disease [10, 28]. However, studies about muscularis macrophages in regulating intestinal hypermotility are limited and their role in the induction of this disease is largely unknown. In contrast, their role in inducing hypomotility has been demonstrated in several rodent models, particularly in postoperative ileus.
Specific focus on macrophages and DCs in postoperative ileus
A first interpretation of resident muscularis macrophage exhibiting immunogenic functions came from Mikkelsen and colleagues in 1995 [66]. The authors demonstrated that these cells were devoid of several enzymes that were described as macrophage activation markers indicating that these cells are not capable in activating an immunogenic cascade. However, Bauer identified resident muscularis macrophages as immunocompetent cells that become rapidly activated by a surgical trauma, by an extra-abdominal surgery and during sepsis [33, 51]. The resulting consequence is a POI or—in case of bacterial translocation—a septic ileus, both describing a motility disturbance of the GI tract. Clinical symptoms are nausea, vomiting as well as abdominal distension and pain. In consequence, aspiration of stomach contents into the lung, pneumonia, and even bowel perforation and sepsis can occur. The medico-economic burden of POI outnumbers 1.4 billion dollars annually, alone in the USA [49].
Research in the twentieth century primarily focused on investigating the neuronal mechanisms causing POI [11]. First evidence for an inflammatory impact in POI development was shown in an experimental rat model by showing increased expression of the lymphocyte-associated antigen-1 in resident muscularis macrophages after surgical manipulation of the GI tract [51]. Several subsequent studies demonstrated that resident macrophages release cytokines and chemokines during POI which in turn recruited further leukocytes from the blood circulation [11, 110]. Strong evidence for their role in the pathogenesis of POI came from depletion studies showing that either a pharmacological or a genetic depletion approach of resident macrophages prevented POI [106, 107, 109]. Importantly, some resident muscularis macrophages express markers of dendritic cells (see previous section), which complicate the specific conclusions about macrophages and DCs in regulating POI. As only a few studies distinguish between both cell types in the pathogenesis of POI, the reader should be aware that the term “macrophage” may also describe functions of DC.
The first evidence that muscularis “macrophages” also express markers and exhibit functions of dendritic cells was shown in colonic resections from human pediatric patients [88]. The authors identified increased presence of CD11c+ CD83+, and CD11c+ CD83− DCs in the muscularis externa of patients exhibiting a severe inflammation. This increase was accompanied by a reduction in CD11c+ CD83+ lamina propria DCs indicating relocation of DCs within the intestinal layers. One year later, dendritic cells were also described in the intestinal muscularis externa in mice and these cells responded to microbial stimuli, such as LPS and oral live bacteria, by upregulation of CD80, CD86, and DC-205 [37]. However, some of these F4/80+ cells did not express DC-specific surface molecules suggesting that both macrophages and DCs coexist in the intestinal muscularis externa [66, 79, 106]. Such coexistence is further supported by findings from our group demonstrating that DC-derived IL-12 subsequently activates macrophages to induce the expression of the iNOS [32]. This in turn leads to NO release from the stellate-shaped resident macrophages which directly inhibit smooth muscle cells [99]. Together, these findings established a molecular cascade linking intestinal DCs—which sense local injury—to intestinal macrophages, which can modulate intestinal peristalsis.
Besides its effects on smooth muscle cells itself, NO can also act on other surrounding cells, i.e., intrinsic or extrinsic neurons. We recently demonstrated that enteric glial cells (EGC), another cell type of the enteric nervous system (ENS), become activated and release IL-6 and MCP-1 upon IL-1 binding during POI [92]. Although the initial activation of the resident macrophage during POI is still a conundrum, IL-1 might be derived from these cells. Previous work indicated that activation of macrophages is a very immediate and transient step in the POI cascade and involves activation of p38 MAP kinase and JNK kinase [107]. Given that some of the kinases respond in minutes after onset of the surgical trauma, one could postulate that a quick, but active release of prestored mediators, i.e., neurotransmitters, may be responsible for the immediate activation. Indeed, we demonstrated that calcitonin gene-related peptide (CGRP)+ nerve endings within the muscularis release CGRP in response to the surgical trauma and CGRP in turn activated the resident muscularis macrophages to transcribe IL-1 [41], which in turn may activate EGC. Further experiments are required to elucidate the crosstalk between these cells in POI.
Crosstalk of resident muscularis macrophages with neuronal cells
Tissue-associated neurons are classified as intrinsic or extrinsic neurons. The extrinsic innervation of the gut includes the sympathetic or parasympathetic autonomous nervous system. The intrinsic innervation consists of the ENS which contains enteric neurons and EGC. The different neuronal cell types fulfill several functions by use of different neurotransmitters and are predominantly organized within the intestinal plexus. The most prominent plexus of the muscularis externa are the myenteric and the deep myenteric plexus wherein the majority of intestinal muscularis macrophages are embedded as a regularly distributed network (Fig. 1). A recent immunohistochemical study confirmed interdigitating connections between macrophage processes and dendrites of enteric neurons [81], which enable the communication between the macrophages and the enteric nerves, predominantly those located in the myenteric ganglia [69]. Another work demonstrated that macrophages in the muscularis externa are CX3CR1+ cells that are in close proximity to βIII tubulin+ nerves [75]. In general, those nerve-macrophage associations were observed in the small intestine, the colon and the stomach of rodents and humans [9]. Additionally, resident macrophages are also distributed in the serosal plexus (containing rather fine nerve bundles than ganglia) and in the deep myenteric plexus. Of note, macrophages within the different plexuses significantly differ in morphology [65].
A general association of nerves and macrophages is known for a long time, but little is known whether muscularis macrophages interact with special subtypes of neurons. However, myenteric neurons, which consist of cholinergic and nitrergic neurons, regularly appeared to be contacted to macrophages [81]. Interestingly, reduced ChAT and nNOS expression in myenteric neurons were observed in a murine model of POI [36] and op/op mice, which lack resident macrophages due to a mutation in the CSF-1 gene [68], exhibit more myenteric neurons than wild-type mice [75]. This indicates that the neuronal environment shapes macrophage function (and vice versa). In the following paragraphs, we give further insight into nerve-macrophage interactions and speculate about other putative communications by other neurotransmitters.
Neuroimmunological features of the extrinsic parasympathetic innervation
A role of the extrinsic parasympathetic innervation in regulating peripheral immune modulation has been known for decades. More recent studies provided further mechanistic insights into the mechanisms that regulate the neuroimmune axis. Tracey and colleagues demonstrated that the vagus nerve is able to dampen inflammation by the release of acetylcholine (ACh), the principal parasympathetic neurotransmitter [13]. In a series of subsequent experiments, the concept of the so-called “cholinergic anti-inflammatory pathway” was developed. The pathways are the efferent part of a reflex which is triggered by sensory afferents in the periphery, i.e., at a site of trauma or infection [97]. In response to a systemic inflammation, this pathway turned out to be more complex and involves ACh-producing ChAT+ splenic T cells which become activated by the vagus nerve and in turn dampen macrophages via cholinergic and or adrenergic pathways [63]. In the intestinal muscularis, the anti-inflammatory effects of the vagus nerve are independent of the spleen [23]. However, vagally released ACh does not act directly on the resident macrophages but intermediately targets cholinergic enteric neurons which in turn release ACh [17, 64]. The close proximity of cholinergic nerves (that have not been further identified as extrinsic or intrinsic nerves) was also shown before [25]. The responsible cholinergic receptor that mediated macrophage suppression was identified as the α7 nicotinic ACh receptor [25, 64]. Although ACh is the principal vagal neurotransmitter, there may be other mediators coreleased from vagal inputs that can also modulate macrophage immune function. Furthermore, other nicotinic or muscularis ACh receptors could also be involved in macrophage silencing.
Neuroimmunological features of the extrinsic sympathetic innervation
Tissue macrophages were also found in close proximity to tyrosinhydroxylase+ noradrenergic nerve fibers, which are mainly of extrinsic sympathetic nature. These nerves originate from the superior mesenteric and celiac ganglia and are present in the myenteric and deep muscular plexus but not in the serosa. Muscularis macrophages highly express the β2 adregnergic receptor, and its coding gene ADRB2 is among the most differentially expressed genes between lamina propria macrophages and muscularis macrophages [38]. Importantly, sympathetic neurons cover—together with the macrophages—the outer perimeter of blood vessels in the smooth muscle wall of the GI tract [81] indicating that this neuroimmune interaction may also affect the blood vessel function and its barrier integrity. Functionally, β2 adrenergic signaling seems to be involved in macrophage polarization in vitro and in vivo and drives alternative macrophage polarization [38]. Given that recent work showed numerous functional states of macrophages beyond the M1/M2 classification [76], the β-adrenergic stimulation of macrophages likely results in a unique β-adrenergic-induced status whose in vivo function remains to be determined. On the other hand, α2-adrenoceptors were shown to induce classical macrophage activation. The same CX3CR1+ muscularis macrophages that showed high levels of β2-adrenoceptor mRNA also transcribe the α2-adrenoceptor [38]. However, compared to the β2-adrenoceptor, the α2-adrenoceptor was not upregulated compared to CX3CR1+ laminal propria macrophages [38] (see also GEO Nr.:GSE74131). In contrast, in a murine model of postoperative ileus, resident muscularis macrophages failed to show α2-adrenergic receptors expression whereas infiltrating monocytes expresses high levels of this receptor [58]. Thus, the role of α2-adrenoceptor in shaping muscularis macrophages remains unclear.
Neuroimmunological features of the intrinsic innervation
As described previously, muscularis macrophages are not only associated with extrinsic nerve fibers but are also in close proximity to cells of the ENS. Although it was suggested that muscularis macrophages may interact with special subtypes of enteric neurons [81], strong evidence for this hypothesis is missing. However, muscularis macrophages were described to respond to several neurotransmitters that are release from enteric nerves. Some of these responses are described in the following section. However, these mediators are not exclusively produced by enteric neurons. Indeed, some are co-released by extrinsic parasympathetic and sympathetic nerve fibers besides the principal neurotransmitters ACh and NE, respectively.
The list of enteric neurotransmitters with proven peripheral immune modulatory actions is long and include vasoactive intestinal peptide (VIP), serotonin, NO, adenosine, ATP, ACh, NE, and many more (Table 1). Principally, the underlying mechanism of enteric neuron-derived neurotransmitters may not differ from extrinsically delivered neurotransmitters. There may be differences in the location, concentration, and dynamics of release though. Thus, the effects of ACh and NE, which are the primary neurotransmitters of the parasympathetic and sympathetic nervous system, respectively, will not be reviewed again.
VIP is a pleiotropic neuropeptide [29] known to exhibit anti-inflammatory effects in macrophages via its receptors VPAC-1 and VPAC-2 [18]. In mice, VIP-positive nerve fibers are found in close proximity to resident macrophages. VPAC-1 receptors have been identified on muscularis macrophages [17], but it is unknown if they mediate immunomodulatory signals.
Serotonin (5-hydroxytryptamin, 5-HT) is also known to trigger inflammation, but the expression of its receptors on muscularis macrophages is not well characterized. However, 5-HT seems to indirectly act on muscularis macrophages by triggering the release of ACh from cholinergic nerves via 5-HT4 receptors. In turn, this triggers an anti-inflammatory cascade in macrophages via α7-nACh receptors [98]. Existence of other 5-HT receptors that trigger alternative activation polarization of macrophages, namely 5-HT2 and 5-HT7, was recently shown in human peripheral blood-derived monocytes that underwent either GM-CSF or M-CSF stimulation to induce M1 or M2 macrophage polarization, respectively [27]. Nevertheless, existence of other 5-HT receptors and their function in muscularis macrophages remains so far elusive.
Another neurotransmitter, γ-amino butyric acid (GABA), has also been studied for its immune modulatory functions. GABA receptors are found throughout the GI tract, predominantly in the ENS and enteroendocrine cells [5]. Mikkelsen et al. found GABA immunoreactivity in the resident macrophages of different mammals [66]. Although it remains elusive if GABA signaling affects muscularis macrophages, the expression of the GABA receptors has been shown in microglia, the resident macrophages of the CNS, and GABA receptor agonisms exerted an immunosuppressive function [61].
Nitric oxide (NO) is an inhibitory neurotransmitter that suppresses excitability in neurons [21] and is continuously produced by nNOS in neurons. However, NO is also well known for its immunological and anti-microbial functions. The extent to which NO production from inhibitory motor neurons and interneurons affects local immunity is unclear. However, NO release significantly increases after iNOS activation which is a hallmark of muscularis macrophages activation during inflammation [32, 34, 47, 52]. The tremendous amounts of NO that can be released from leukocytes during inflammation exert profound changes on the ENS and on intestinal function.
The enteric nervous system also harbors a network of EGC which outnumbers enteric neurons [39]. EGC are found in all plexus and in interganglionic regions in the smooth muscle layers and the lamina propria with extensions projecting up to the tips of the villi. The immunological importance of EGC has been shown in two independent studies, which observed a fulminant jejunoileitis and enterocolitis after a severe intestinal barrier dysintegrity [16, 22]. However, the mechanisms remain elusive. Surprisingly, although the nerve-macrophage interactions are in focus of a current investigations, little is known about macrophage-EGC communication. A recent study showed that EGC of the muscularis externa responds to IL-1, which is released from resident macrophages and infiltrating monocytes during POI [92]. Whether EGC also affect muscularis macrophage function remains unclear, but their immunomodulatory role and the variety of factors released by EGC during inflammation [78] strongly indicates that they may affect muscularis macrophage function.
Crosstalk of resident muscularis macrophages with other non-immune cells
In humans and rodents, muscularis macrophages are also in close contact to smooth muscle cells and to interstitial cell of Cajal (ICC) in the myenteric and deep myenteric plexus [48]. ICCs are the pacemakers of the gut [67] and share a common precursor with smooth muscle cells [82]. While macrophage-nerve interactions are well known (see previous section), interactions of macrophages and ICC and smooth muscle cells are not well described. However, iNOS-dependent NO release by resident macrophages during LPS-induced endotoxemia diminished the amount of ICC and disturbed their tone-giving function and iNOS antagonism or macrophage inhibition by gadolinium chloride prevented this detrimental effect [100]. With regard to a smooth muscle cell-macrophage interaction, peritoneal macrophages were shown to release mediators that in turn activate smooth muscle cells and vice versa in responses a non-physiological mechanical stretch stimulus [108], which could occur also in the bowel wall. Nevertheless, interactions of muscularis macrophages with intestinal smooth muscle cells or ICC remain so far elusive.
Resident macrophages as therapeutic targets
A large number of publication demonstrated that POI originates from the activation of the resident macrophages or DCs, and this activation was also observed in surgical specimens in the human GI tract [53, 95]. These studies suggested that targeting macrophages and/or DCs might be a promising strategy for POI prevention. Targeting cytokines and chemokines contributing to POI pathogenesis may also be an effective therapy, but inappropriate pharmacokinetics and high treatment costs have limited further clinical development. A rather specific approach was achieved by administration of semapimod, a tetravalent guanylhydrazone that prevents activation of the c-Raf/p38 MAPK kinase pathway in macrophages and subsequently POI [107]. Importantly, higher peripheral doses of semapimod reached brain levels sufficiently to centrally trigger cholinergic anti-inflammatory pathways (described previously) and this may result in a cumulative inhibitory effect on muscularis macrophage [26]. Although this compound was not further developed, these data clearly indicate that selective immune modulatory drugs are effective approaches to target muscularis macrophages during inflammation.
Innervation in the cholinergic anti-inflammatory pathway also seems to be a promising target in dampening intestinal macrophage activation during inflammation, and muscularis macrophages were shown to express the target receptor of this pathway, the α7-nACh receptor [64]. Selective α7-nACh receptor agonists were developed and have been tested in experimental models of acute and chronic intestinal inflammation. One of these agonists, AR-R17779, was shown to reduce POI [94] but was also shown to worsen experimental colitis [89] Another α7-nACh receptor agonist, GTS-21, was recently shown to prevent septic ileus, but its selectivity for the α7-nACh receptor remains questionable [77]. Finally, clinical trials with use of α7-nACh agonists for treatment of immunological disorders have not pursued anymore. To this end, an electrical stimulation of the vagus nerve appears to be another promising approach [25, 64] to trigger the cholinergic anti-inflammatory pathway and data confirming effectiveness are expected from ongoing clinical trials. The electrical stimulation may have less side effects than pharmaceutical, but it is so far performed by invasive vagus nerve stimulation. Invasive treatment may not be an appropriate therapy in common clinical routine option, particularly in acute diseases like postoperative or septic ileus. However, non-invasive techniques are under development and results are expected in the near future.
In addition to the well-described vagal anti-inflammatory effects, immune modulation by sympathetic nerves is an equivalent in the (neuro)immune system. However, catecholamines, particularly NE released from sympathetic nerves, may have opposing roles. While α-adrenergic signaling is thought to boost inflammation (and this was also confirmed in the POI model [58]), β-adrenergic pathway suppresses innate and adaptive immunity. Recently, it was shown that β2-adrenergic signaling affects resident macrophage/DC polarization [38]. β2-adrenergic agonism by salbutamol increased M2 marker expression in a murine peritoneal macrophage cell line and in primary muscularis macrophages while β2-antagonism reduced it. A comparable effect was observed in an acute experimental lung injury model, and this indicates a broader anti-inflammatory effect on resident macrophages by β2-agonists [14]. Indeed, β-adrenergic innervation is disturbed during POI and also chronic inflammatory bowel diseases [30, 114]. Nevertheless, it remains unclear if β2-adrenoceptor agonism in muscularis macrophage affects outcome of intestinal diseases, but selective activation of β2-adrenergic signaling may help to counterregulate inflammation or accelerated resolution programs via resident macrophages.
Following the theory that a stimulation of endogenous immunomodulatory pathways or use of endogenous mediators may be less harmful than administration of pharmaceuticals, further research should pay special attention to those mechanisms. Besides the immunoregulatory neuronal pathways, several other endogenous mechanisms are able to effectively shut down inflammation. A new class of endogenous immune modulating molecules are poly-unsaturated-derived lipid mediators. These mediators are derived from cyclooxygenase and/or lipoxygenase metabolism and have been shown to exert powerful anti-inflammatory actions [15, 87], i.e., enhancement of efferocytosis in macrophages. During POI, docosahexaenoic acid (DHA)-derived metabolites, namely protectin DX and resolvin D2, were highly induced in the postoperative muscularis. However, only protectin DX prevented POI [91] indicating the high selectivity and specificity of these mediators. Although it remains unclear whether protectin DX directly targets an unknown protectin DX receptor on resident macrophages, resident macrophages in other tissues were shown to be sensitive to a variety of proresolving DHA-derived lipid mediators [4, 24, 87].
Conclusion
Taken together, macrophages in the intestinal muscularis externa share functional and phenotypical similarities to long-living tissue macrophages, which contrast macrophages in the neighboring lamina propria. The functionality of macrophages at both sites is shaped by the local microenvironment and muscularis macrophages in particular, which seem to tightly interact with the dense nervous system. Multi-parametric state of the art imaging and isolation techniques have allowed further insights into the localization and the different phenotypes of intestinal macrophages in health and disease. Given that the phenotype and the functionality drastically change under disease conditions, a more thorough analysis is required to delineate the role of these cells in the inflamed muscularis externa. Conclusively, open questions remain with regard to the localization, origin, and functionality of macrophages in the muscularis externa and the regulatory crosstalk with the enteric and the peripheral nervous system in inflammatory settings in particular. Future studies are required to understand the crosstalk between these functional units in the different intestinal muscle layers. Unraveling the cellular and molecular mechanisms underlying the tissue-specific control of macrophage development and activation in the muscularis externa will be crucial to understand disease-specific adaptations in muscularis macrophages and the disturbance of intestinal motility to develop novel therapeutic approaches regulating the functionality of macrophages in the intestinal muscularis externa.
References
Ajami B, Bennett JL, Krieger C, Tetzlaff W, Rossi FM (2007) Local self-renewal can sustain CNS microglia maintenance and function throughout adult life. Nat Neurosci 10:1538–1543
Amit I, Winter DR, Jung S (2016) The role of the local environment and epigenetics in shaping macrophage identity and their effect on tissue homeostasis. Nat Immunol 17:18–25
Anitha M, Vijay-Kumar M, Sitaraman SV, Gewirtz AT, Srinivasan S (2012) Gut microbial products regulate murine gastrointestinal motility via Toll-like receptor 4 signaling. Gastroenterology 143:1006–1016 e1004
Aursnes M, Tungen JE, Colas RA, Vlasakov I, Dalli J, Serhan CN, Hansen TV (2015) Synthesis of the 16S,17S-Epoxyprotectin intermediate in the biosynthesis of protectins by human macrophages. J Nat Prod 78:2924–2931
Auteri M, Zizzo MG, Serio R (2015) GABA and GABA receptors in the gastrointestinal tract: from motility to inflammation. Pharmacol Res 93:11–21
Bain CC, Mowat AM (2014) Macrophages in intestinal homeostasis and inflammation. Immunol Rev 260:102–117
Bain CC, Scott CL, Uronen-Hansson H, Gudjonsson S, Jansson O, Grip O, Guilliams M, Malissen B, Agace WW, Mowat AM (2013) Resident and pro-inflammatory macrophages in the colon represent alternative context-dependent fates of the same Ly6Chi monocyte precursors. Mucosal Immunol 6:498–510
Bain CC, Bravo-Blas A, Scott CL, Gomez Perdiguero E, Geissmann F, Henri S, Malissen B, Osborne LC, Artis D, Mowat AM (2014) Constant replenishment from circulating monocytes maintains the macrophage pool in the intestine of adult mice. Nat Immunol 15:929–937
Bernard CE, Gibbons SJ, Mann IS, Froschauer L, Parkman HP, Harbison S, Abell TL, Snape WJ, Hasler WL, McCallum RW, Sarosiek I, Nguyen LA, Koch KL, Tonascia J, Hamilton FA, Kendrick ML, Shen KR, Pasricha PJ, Farrugia G, Consortium NGCR (2014) Association of low numbers of CD206-positive cells with loss of ICC in the gastric body of patients with diabetic gastroparesis. Neurogastroenterology and motility: the official journal of the European Gastrointestinal Motility Society 26:1275–1284
Bernardazzi C, Pego B, de Souza HS (2016) Neuroimmunomodulation in the gut: focus on inflammatory bowel disease. Mediat Inflamm 2016:1363818
Boeckxstaens GE, de Jonge WJ (2009) Neuroimmune mechanisms in postoperative ileus. Gut 58:1300–1311
Bogunovic M, Ginhoux F, Helft J, Shang L, Hashimoto D, Greter M, Liu K, Jakubzick C, Ingersoll MA, Leboeuf M, Stanley ER, Nussenzweig M, Lira SA, Randolph GJ, Merad M (2009) Origin of the lamina propria dendritic cell network. Immunity 31:513–525
Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, Wang H, Abumrad N, Eaton JW, Tracey KJ (2000) Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 405:458–462
Bosmann M, Grailer JJ, Zhu K, Matthay MA, Sarma JV, Zetoune FS, Ward PA (2012) Anti-inflammatory effects of beta2 adrenergic receptor agonists in experimental acute lung injury. FASEB J 26:2137–2144
Buckley CD, Gilroy DW, Serhan CN (2014) Proresolving lipid mediators and mechanisms in the resolution of acute inflammation. Immunity 40:315–327
Bush TG, Savidge TC, Freeman TC, Cox HJ, Campbell EA, Mucke L, Johnson MH, Sofroniew MV (1998) Fulminant jejuno-ileitis following ablation of enteric glia in adult transgenic mice. Cell 93:189–201
Cailotto C, Gomez-Pinilla PJ, Costes LM, van der Vliet J, Di Giovangiulio M, Nemethova A, Matteoli G, Boeckxstaens GE (2014) Neuro-anatomical evidence indicating indirect modulation of macrophages by vagal efferents in the intestine but not in the spleen. PLoS One 9:e87785
Carrion M, Perez-Garcia S, Martinez C, Juarranz Y, Estrada-Capetillo L, Puig-Kroger A, Gomariz RP, Gutierrez-Canas I (2016) VIP impairs acquisition of the macrophage proinflammatory polarization profile. J Leukoc Biol
Cerovic V, Houston SA, Scott CL, Aumeunier A, Yrlid U, Mowat AM, Milling SW (2013) Intestinal CD103(−) dendritic cells migrate in lymph and prime effector T cells. Mucosal Immunol 6:104–113
Cerovic V, Bain CC, Mowat AM, Milling SW (2014) Intestinal macrophages and dendritic cells: what's the difference? Trends Immunol 35:270–277
Cipriani G, Gibbons SJ, Kashyap PC, Farrugia G (2016) Intrinsic gastrointestinal macrophages: their phenotype and role in gastrointestinal motility. Cell Mol Gastroenterol Hepatol 2:120–130 e121
Cornet A, Savidge TC, Cabarrocas J, Deng WL, Colombel JF, Lassmann H, Desreumaux P, Liblau RS (2001) Enterocolitis induced by autoimmune targeting of enteric glial cells: a possible mechanism in Crohn's disease? Proc Natl Acad Sci U S A 98:13306–13311
Costes LM, van der Vliet J, van Bree SH, Boeckxstaens GE, Cailotto C (2014) Endogenous vagal activation dampens intestinal inflammation independently of splenic innervation in postoperative ileus. Autonomic neuroscience: basic & clinical 185:76–82
Dalli J, Serhan C (2016) Macrophage proresolving mediators-the when and where. Microbiol Spectr 4
de Jonge WJ, van der Zanden EP, The FO, Bijlsma MF, van Westerloo DJ, Bennink RJ, Berthoud HR, Uematsu S, Akira S, van den Wijngaard RM, Boeckxstaens GE (2005) Stimulation of the vagus nerve attenuates macrophage activation by activating the Jak2-STAT3 signaling pathway. NatImmunol 6:844–851
de Jonge WJ, The FO, Lowenberg M, Boeckxstaens GE (2009) P38 MAPK inhibitor semapimod reduces postoperative ileus via peripheral and central mechanisms. Gastroenterology 136:1841–1842
de las Casas-Engel M, Dominguez-Soto A, Sierra-Filardi E, Bragado R, Nieto C, Puig-Kroger A, Samaniego R, Loza M, Corcuera MT, Gomez-Aguado F, Bustos M, Sanchez-Mateos P, Corbi AL (2013) Serotonin skews human macrophage polarization through HTR2B and HTR7. J Immunol 190:2301–2310
de Souza HS, Fiocchi C (2016) Immunopathogenesis of IBD: current state of the art. Nat Rev Gastroenterol Hepatol 13:13–27
Delgado M, Ganea D (2013) Vasoactive intestinal peptide: a neuropeptide with pleiotropic immune functions. Amino Acids 45:25–39
Deng Q, Chen H, Liu Y, Xiao F, Guo L, Liu D, Cheng X, Zhao M, Wang X, Xie S, Qi S, Yin Z, Gao J, Chen X, Wang J, Guo N, Ma Y, Shi M (2016) Psychological stress promotes neutrophil infiltration in colon tissue through adrenergic signaling in DSS-induced colitis model. Brain Behav Immun 57:243–254
Edelson BT, Kc W, Juang R, Kohyama M, Benoit LA, Klekotka PA, Moon C, Albring JC, Ise W, Michael DG, Bhattacharya D, Stappenbeck TS, Holtzman MJ, Sung SS, Murphy TL, Hildner K, Murphy KM (2010) Peripheral CD103+ dendritic cells form a unified subset developmentally related to CD8alpha+ conventional dendritic cells. J Exp Med 207:823–836
Engel DR, Koscielny A, Wehner S, Maurer J, Schiwon M, Franken L, Schumak B, Limmer A, Sparwasser T, Hirner A, Knolle PA, Kalff JC, Kurts C (2010) T helper type 1 memory cells disseminate postoperative ileus over the entire intestinal tract. Nat Med 16:1407–1413
Eskandari MK, Kalff JC, Billiar TR, Lee KK, Bauer AJ (1997) Lipopolysaccharide activates the muscularis macrophage network and suppresses circular smooth muscle activity. Am J Phys 273:G727–G734
Eskandari MK, Kalff JC, Billiar TR, Lee KK, Bauer AJ (1999) LPS-induced muscularis macrophage nitric oxide suppresses rat jejunal circular muscle activity. AmJPhysiol 277:G478–G486
Evers BD, Engel DR, Bohner AM, Tittel AP, Krause TA, Heuser C, Garbi N, Kastenmuller W, Mack M, Tiegs G, Panzer U, Boor P, Ludwig-Portugall I, Kurts C (2016) CD103+ kidney dendritic cells protect against crescentic GN by maintaining IL-10-producing regulatory T cells. J Am Soc Nephrol
Farro G, Gomez-Pinilla PJ, Di Giovangiulio M, Stakenborg N, Auteri M, Thijs T, Depoortere I, Matteoli G, Boeckxstaens GE (2016) Smooth muscle and neural dysfunction contribute to different phases of murine postoperative ileus. Neurogastroenterol Motil Off J Eur Gastrointest Motil Soc
Flores-Langarica A, Meza-Perez S, Calderon-Amador J, Estrada-Garcia T, Macpherson G, Lebecque S, Saeland S, Steinman RM, Flores-Romo L (2005) Network of dendritic cells within the muscular layer of the mouse intestine. ProcNatlAcadSciUSA 102:19039–19044
Gabanyi I, Muller PA, Feighery L, Oliveira TY, Costa-Pinto FA, Mucida D (2016) Neuro-immune interactions drive tissue programming in intestinal macrophages. Cell 164:378–391
Gabella G (1981) Ultrastructure of the nerve plexuses of the mammalian intestine: the enteric glial cells. Neuroscience 6:425–436
Ginhoux F, Jung S (2014) Monocytes and macrophages: developmental pathways and tissue homeostasis. Nat Rev Immunol 14:392–404
Glowka TR, Steinebach A, Stein K, Schwandt T, Lysson M, Holzmann B, Tsujikawa K, de Jonge WJ, Kalff JC, Wehner S (2015) The novel CGRP receptor antagonist BIBN4096BS alleviates a postoperative intestinal inflammation and prevents postoperative ileus. Neurogastroenterology and motility: the official journal of the European Gastrointestinal Motility Society 27:1038–1049
Gordon S, Taylor PR (2005) Monocyte and macrophage heterogeneity. Nat Rev Immunol 5:953–964
Grainger JR, Askenase MH, Guimont-Desrochers F, da Fonseca DM, Belkaid Y (2014) Contextual functions of antigen-presenting cells in the gastrointestinal tract. Immunol Rev 259:75–87
Gross M, Salame TM, Jung S (2015) Guardians of the gut-murine intestinal macrophages and dendritic cells. Front Immunol 6:254
Hashimoto D, Chow A, Noizat C, Teo P, Beasley MB, Leboeuf M, Becker CD, See P, Price J, Lucas D, Greter M, Mortha A, Boyer SW, Forsberg EC, Tanaka M, van Rooijen N, Garcia-Sastre A, Stanley ER, Ginhoux F, Frenette PS, Merad M (2013) Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity 38:792–804
Hildner K, Edelson BT, Purtha WE, Diamond M, Matsushita H, Kohyama M, Calderon B, Schraml BU, Unanue ER, Diamond MS, Schreiber RD, Murphy TL, Murphy KM (2008) Batf3 deficiency reveals a critical role for CD8alpha+ dendritic cells in cytotoxic T cell immunity. Science 322:1097–1100
Hori M, Kita M, Torihashi S, Miyamoto S, Won KJ, Sato K, Ozaki H, Karaki H (2001) Upregulation of iNOS by COX-2 in muscularis resident macrophage of rat intestine stimulated with LPS. AmJPhysiol Gastrointest Liver Physiol 280:G930–G938
Iino S, Horiguchi K (2006) Interstitial cells of cajal are involved in neurotransmission in the gastrointestinal tract. Acta Histochem Cytochem 39:145–153
Iyer S, Saunders WB, Stemkowski S (2009) Economic burden of postoperative ileus associated with colectomy in the United States. JManagCare Pharm 15:485–494
Jaensson E, Uronen-Hansson H, Pabst O, Eksteen B, Tian J, Coombes JL, Berg PL, Davidsson T, Powrie F, Johansson-Lindbom B, Agace WW (2008) Small intestinal CD103+ dendritic cells display unique functional properties that are conserved between mice and humans. J Exp Med 205:2139–2149
Kalff JC, Schraut WH, Simmons RL, Bauer AJ (1998) Surgical manipulation of the gut elicits an intestinal muscularis inflammatory response resulting in postsurgical ileus. Ann Surg 228:652–663
Kalff JC, Schraut WH, Billiar TR, Simmons RL, Bauer AJ (2000) Role of inducible nitric oxide synthase in postoperative intestinal smooth muscle dysfunction in rodents. Gastroenterology 118:316–327
Kalff JC, Turler A, Schwarz NT, Schraut WH, Lee KK, Tweardy DJ, Billiar TR, Simmons RL, Bauer AJ (2003) Intra-abdominal activation of a local inflammatory response within the human muscularis externa during laparotomy. Ann Surg 237:301–315
Kim OY, Monsel A, Bertrand M, Cavaillon JM, Coriat P, Adib-Conquy M (2009) Translocation of bacterial NOD2 agonist and its link with inflammation. Crit Care 13:R124
Kinoshita K, Horiguchi K, Fujisawa M, Kobirumaki F, Yamato S, Hori M, Ozaki H (2007) Possible involvement of muscularis resident macrophages in impairment of interstitial cells of Cajal and myenteric nerve systems in rat models of TNBS-induced colitis. Histochem Cell Biol 127:41–53
Klebanoff CA, Spencer SP, Torabi-Parizi P, Grainger JR, Roychoudhuri R, Ji Y, Sukumar M, Muranski P, Scott CD, Hall JA, Ferreyra GA, Leonardi AJ, Borman ZA, Wang J, Palmer DC, Wilhelm C, Cai R, Sun J, Napoli JL, Danner RL, Gattinoni L, Belkaid Y, Restifo NP (2013) Retinoic acid controls the homeostasis of pre-cDC-derived splenic and intestinal dendritic cells. J Exp Med 210:1961–1976
Koscso B, Gowda K, Schell TD, Bogunovic M (2015) Purification of dendritic cell and macrophage subsets from the normal mouse small intestine. J Immunol Methods 421:1–13
Kreiss C, Toegel S, Bauer AJ (2004) Alpha2-adrenergic regulation of NO production alters postoperative intestinal smooth muscle dysfunction in rodents. AmJPhysiol Gastrointest Liver Physiol 287:G658–G666
Kuhl AA, Erben U, Kredel LI, Siegmund B (2015) Diversity of intestinal macrophages in inflammatory bowel diseases. Front Immunol 6:613
Kurts C, Robinson BW, Knolle PA (2010) Cross-priming in health and disease. Nat Rev Immunol 10:403–414
Lee M, Schwab C, McGeer PL (2011) Astrocytes are GABAergic cells that modulate microglial activity. Glia 59:152–165
Malissen B, Tamoutounour S, Henri S (2014) The origins and functions of dendritic cells and macrophages in the skin. Nat Rev Immunol 14:417–428
Martelli D, McKinley MJ, McAllen RM (2014) The cholinergic anti-inflammatory pathway: a critical review. Autonomic neuroscience: basic & clinical 182:65–69
Matteoli G, Gomez-Pinilla PJ, Nemethova A, Di Giovangiulio M, Cailotto C, van Bree SH, Michel K, Tracey KJ, Schemann M, Boesmans W, Vanden Berghe P, Boeckxstaens GE (2014) A distinct vagal anti-inflammatory pathway modulates intestinal muscularis resident macrophages independent of the spleen. Gut 63:938–948
Mikkelsen HB (1995a) Macrophages in the external muscle layers of mammalian intestines. Histol Histopathol 10:719–736
Mikkelsen HB (1995b) Macrophages in the external muscle layers of mammalian intestines. Histol Histopathol 10:719–736
Mikkelsen HB (2010) Interstitial cells of Cajal, macrophages and mast cells in the gut musculature: morphology, distribution, spatial and possible functional interactions. J Cell Mol Med 14:818–832
Mikkelsen HB, Thuneberg L (1999) Op/op mice defective in production of functional colony-stimulating factor-1 lack macrophages in muscularis externa of the small intestine. Cell Tissue Res 295:485–493
Mikkelsen HB, Thuneberg L, Rumessen JJ, Thorball N (1985) Macrophage-like cells in the muscularis externa of mouse small intestine. Anat Rec 213:77–86
Mikkelsen HB, Mirsky R, Jessen KR, Thuneberg L (1988) Macrophage-like cells in muscularis externa of mouse small intestine: immunohistochemical localization of F4/80, M1/70, and Ia-antigen. Cell Tissue Res 252:301–306
Mikkelsen HB, Garbarsch C, Tranum-Jensen J, Thuneberg L (2004) Macrophages in the small intestinal muscularis externa of embryos, newborn and adult germ-free mice. J Mol Histol 35:377–387
Mikkelsen HB, Larsen JO, Hadberg H (2008) The macrophage system in the intestinal muscularis externa during inflammation: an immunohistochemical and quantitative study of osteopetrotic mice. Histochem Cell Biol 130:363–373
Mildner A, Jung S (2014) Development and function of dendritic cell subsets. Immunity 40:642–656
Mori D, Watanabe N, Kaminuma O, Murata T, Hiroi T, Ozaki H, Hori M (2014) IL-17A induces hypo-contraction of intestinal smooth muscle via induction of iNOS in muscularis macrophages. J Pharmacol Sci 125:394–405
Muller PA, Koscso B, Rajani GM, Stevanovic K, Berres ML, Hashimoto D, Mortha A, Leboeuf M, Li XM, Mucida D, Stanley ER, Dahan S, Margolis KG, Gershon MD, Merad M, Bogunovic M (2014) Crosstalk between muscularis macrophages and enteric neurons regulates gastrointestinal motility. Cell 158:300–313
Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, Gordon S, Hamilton JA, Ivashkiv LB, Lawrence T, Locati M, Mantovani A, Martinez FO, Mege JL, Mosser DM, Natoli G, Saeij JP, Schultze JL, Shirey KA, Sica A, Suttles J, Udalova I, van Ginderachter JA, Vogel SN, Wynn TA (2014) Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity 41:14–20
Nullens S, Staessens M, Peleman C, Schrijvers DM, Malhotra-Kumar S, Francque S, Matteoli G, Boeckxstaens GE, De Man JG, De Winter BY (2016) Effect of Gts-21, an alpha7 nicotinic acetylcholine receptor agonist, on Clp-induced inflammatory, gastrointestinal motility, and colonic permeability changes in mice. Shock 45:450–459
Ochoa-Cortes F, Turco F, Linan-Rico A, Soghomonyan S, Whitaker E, Wehner S, Cuomo R, Christofi FL (2016) Enteric glial cells: a new frontier in neurogastroenterology and clinical target for inflammatory bowel diseases. Inflamm Bowel Dis 22:433–449
Ozaki H, Kawai T, Shuttleworth CW, Won KJ, Suzuki T, Sato K, Horiguchi H, Hori M, Karaki H, Torihashi S, Ward SM, Sanders KM (2004) Isolation and characterization of resident macrophages from the smooth muscle layers of murine small intestine. Neurogastroenterology and motility: the official journal of the European Gastrointestinal Motility Society 16:39–51
Persson EK, Uronen-Hansson H, Semmrich M, Rivollier A, Hagerbrand K, Marsal J, Gudjonsson S, Hakansson U, Reizis B, Kotarsky K, Agace WW (2013) IRF4 transcription-factor-dependent CD103(+)CD11b(+) dendritic cells drive mucosal T helper 17 cell differentiation. Immunity 38:958–969
Phillips RJ, Powley TL (2012) Macrophages associated with the intrinsic and extrinsic autonomic innervation of the rat gastrointestinal tract. Autonomic neuroscience: basic & clinical 169:12–27
Sanders KM, Ordog T, Koh SD, Torihashi S, Ward SM (1999) Development and plasticity of interstitial cells of Cajal. Neurogastroenterology and motility: the official journal of the European Gastrointestinal Motility Society 11:311–338
Schlitzer A, McGovern N, Teo P, Zelante T, Atarashi K, Low D, Ho AW, See P, Shin A, Wasan PS, Hoeffel G, Malleret B, Heiseke A, Chew S, Jardine L, Purvis HA, Hilkens CM, Tam J, Poidinger M, Stanley ER, Krug AB, Renia L, Sivasankar B, Ng LG, Collin M, Ricciardi-Castagnoli P, Honda K, Haniffa M, Ginhoux F (2013) IRF4 transcription factor-dependent CD11b+ dendritic cells in human and mouse control mucosal IL-17 cytokine responses. Immunity 38:970–983
Schreiber HA, Loschko J, Karssemeijer RA, Escolano A, Meredith MM, Mucida D, Guermonprez P, Nussenzweig MC (2013) Intestinal monocytes and macrophages are required for T cell polarization in response to Citrobacter rodentium. J Exp Med 210:2025–2039
Schulz O, Jaensson E, Persson EK, Liu X, Worbs T, Agace WW, Pabst O (2009) Intestinal CD103+, but not CX3CR1+, antigen sampling cells migrate in lymph and serve classical dendritic cell functions. J Exp Med 206:3101–3114
Scott CL, Bain CC, Wright PB, Sichien D, Kotarsky K, Persson EK, Luda K, Guilliams M, Lambrecht BN, Agace WW, Milling SW, Mowat AM (2015) CCR2(+) CD103(−) intestinal dendritic cells develop from DC-committed precursors and induce interleukin-17 production by T cells. Mucosal Immunol 8:327–339
Serhan CN (2014) Pro-resolving lipid mediators are leads for resolution physiology. Nature 510:92–101
Silva MA, Lopez CB, Riverin F, Oligny L, Menezes J, Seidman EG (2004) Characterization and distribution of colonic dendritic cells in Crohn's disease. Inflamm Bowel Dis 10:504–512
Snoek SA, Verstege MI, van der Zanden EP, Deeks N, Bulmer DC, Skynner M, Lee K, Te Velde AA, Boeckxstaens GE, de Jonge WJ (2010) Selective alpha7 nicotinic acetylcholine receptor agonists worsen disease in experimental colitis. Br J Pharmacol 160:322–333
Snoek SA, Dhawan S, van Bree SH, Cailotto C, van Diest SA, Duarte JM, Stanisor OI, Hilbers FW, Nijhuis L, Koeman A, van den Wijngaard RM, Zuurbier CJ, Boeckxstaens GE, de Jonge WJ (2012) Mast cells trigger epithelial barrier dysfunction, bacterial translocation and postoperative ileus in a mouse model. Neurogastroenterol Motil 24:172–184 e191
Stein K, Stoffels M, Lysson M, Schneiker B, Dewald O, Kronke G, Kalff JC, Wehner S (2015) A role for 12/15-lipoxygenase-derived proresolving mediators in postoperative ileus: protectin DX-regulated neutrophil extravasation. J Leukoc Biol
Stoffels B, Hupa KJ, Snoek SA, van Bree S, Stein K, Schwandt T, Vilz TO, Lysson M, Veer CV, Kummer MP, Hornung V, Kalff JC, de Jonge WJ, Wehner S (2014) Postoperative ileus involves interleukin-1 receptor signaling in enteric glia. Gastroenterology 146:176–187 e171
Taxi J (1965) Contribution … ltude des connexions des neurones moteurs du systeme nerveux autonome. Annales des Naturelles-Zoologie et Biologie Animale 7:413–674
The FO, Boeckxstaens GE, Snoek SA, Cash JL, Bennink R, Larosa GJ, van den Wijngaard RM, Greaves DR, de Jonge WJ (2007) Activation of the cholinergic anti-inflammatory pathway ameliorates postoperative ileus in mice. Gastroenterology 133:1219–1228
The FO, Bennink RJ, Ankum WM, Buist MR, Busch OR, Gouma DJ, van der Heide S, van den Wijngaard RM, de Jonge WJ, Boeckxstaens GE (2008) Intestinal handling-induced mast cell activation and inflammation in human postoperative ileus. Gut 57:33–40
Tilg H, Moschen AR (2015) Food, immunity, and the microbiome. Gastroenterology 148:1107–1119
Tracey KJ (2007) Physiology and immunology of the cholinergic antiinflammatory pathway. JClinInvest 117:289–296
Tsuchida Y, Hatao F, Fujisawa M, Murata T, Kaminishi M, Seto Y, Hori M, Ozaki H (2011) Neuronal stimulation with 5-hydroxytryptamine 4 receptor induces anti-inflammatory actions via alpha7nACh receptors on muscularis macrophages associated with postoperative ileus. Gut 60:638–647
Turler A, Kalff JC, Moore BA, Hoffman RA, Billiar TR, Simmons RL, Bauer AJ (2006) Leukocyte-derived inducible nitric oxide synthase mediates murine postoperative ileus. Ann Surg 244:220–229
Ueshima S, Nishida T, Koike M, Matsuda H, Sawa Y, Uchiyama Y (2014) Nitric oxide-mediated injury of interstitial cells of cajal and intestinal dysmotility under endotoxemia of mice. Biomed Res 35:251–262
Varol C, Landsman L, Fogg DK, Greenshtein L, Gildor B, Margalit R, Kalchenko V, Geissmann F, Jung S (2007) Monocytes give rise to mucosal, but not splenic, conventional dendritic cells. J Exp Med 204:171–180
Varol C, Vallon-Eberhard A, Elinav E, Aychek T, Shapira Y, Luche H, Fehling HJ, Hardt WD, Shakhar G, Jung S (2009) Intestinal lamina propria dendritic cell subsets have different origin and functions. Immunity 31:502–512
Varol C, Mildner A, Jung S (2015) Macrophages: development and tissue specialization. Annu Rev Immunol 33:643–675
Veiga-Fernandes H, Mucida D (2016) Neuro-immune interactions at barrier surfaces. Cell 165:801–811
Wang J, Kubes P (2016) A reservoir of mature cavity macrophages that can rapidly invade visceral organs to affect tissue repair. Cell
Wehner S, Behrendt FF, Lyutenski BN, Lysson M, Bauer AJ, Hirner A, Kalff JC (2007) Inhibition of macrophage function prevents intestinal inflammation and postoperative ileus in rodents. Gut 56:176–185
Wehner S, Straesser S, Vilz TO, Pantelis D, Sielecki T, de la Cruz VF, Hirner A, Kalff JC (2009) Inhibition of p38 mitogen-activated protein kinase pathway as prophylaxis of postoperative ileus in mice. Gastroenterology 136:619–629
Wehner S, Buchholz BM, Schuchtrup S, Rocke A, Schaefer N, Lysson M, Hirner A, Kalff JC (2010) Mechanical strain and TLR4 synergistically induce cell-specific inflammatory gene expression in intestinal smooth muscle cells and peritoneal macrophages. AmJPhysiol GastrointestLiver Physiol 299:G1187–G1197
Wehner S, Vilz TO, Sommer N, Sielecki T, Hong GS, Lysson M, Stoffels B, Pantelis D, Kalff JC (2012a) The novel orally active guanylhydrazone CPSI-2364 prevents postoperative ileus in mice independently of anti-inflammatory vagus nerve signaling. Langenbeck’s archives of surgery/Deutsche Gesellschaft fur Chirurgie
Wehner S, Vilz TO, Stoffels B, Kalff JC (2012b) Immune mediators of postoperative ileus. Langenbeck's archives of surgery/Deutsche Gesellschaft fur Chirurgie 397:591–601
Wynn TA, Chawla A, Pollard JW (2013) Macrophage biology in development, homeostasis and disease. Nature 496:445–455
Yona S, Kim KW, Wolf Y, Mildner A, Varol D, Breker M, Strauss-Ayali D, Viukov S, Guilliams M, Misharin A, Hume DA, Perlman H, Malissen B, Zelzer E, Jung S (2013) Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity 38:79–91
Yoshida H, Hayashi S, Kunisada T, Ogawa M, Nishikawa S, Okamura H, Sudo T, Shultz LD (1990) The murine mutation osteopetrosis is in the coding region of the macrophage colony stimulating factor gene. Nature 345:442–444
Zhao A, Bossone C, Pineiro-Carrero V, Shea-Donohue T (2001) Colitis-induced alterations in adrenergic control of circular smooth muscle in vitro in rats. J Pharmacol Exp Ther 299:768–774
Zigmond E, Jung S (2013) Intestinal macrophages: well educated exceptions from the rule. Trends Immunol 34:162–168
Zigmond E, Varol C, Farache J, Elmaliah E, Satpathy AT, Friedlander G, Mack M, Shpigel N, Boneca IG, Murphy KM, Shakhar G, Halpern Z, Jung S (2012) Ly6C hi monocytes in the inflamed colon give rise to proinflammatory effector cells and migratory antigen-presenting cells. Immunity 37:1076–1090
Author information
Authors and Affiliations
Corresponding authors
Additional information
This article is part of the special issue on macrophages in tissue homeostasis in Pflügers Archiv – European Journal of Physiology
Rights and permissions
About this article
Cite this article
Wehner, S., Engel, D.R. Resident macrophages in the healthy and inflamed intestinal muscularis externa. Pflugers Arch - Eur J Physiol 469, 541–552 (2017). https://doi.org/10.1007/s00424-017-1948-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-017-1948-4