Abstract
Neurons of the enteric nervous system (ENS) are the primary controllers of gastrointestinal functions. Although the ENS has been the central focus of research areas such as motility, this has now expanded to include the modulatory roles that non-neuronal cells have on neuronal function. This review discusses how enteric glia (EGC) and resident muscularis macrophages (mMacs) influence ENS communication. It highlights how the understanding of neuroglia interactions has extended beyond EGCs responding to exogenously applied neurotransmitters. Proposed mechanisms for neuron-EGC and glio-glia communication are discussed. The significance of these interactions is evidenced by gut functions that rely on these processes. mMacs are commonly known for their roles as immune cells which sample and respond to changes in the tissue environment. However, a more recent theory suggests that mMacs and enteric neurons are mutually dependent for their maintenance and function. This review summarizes the supportive and contradictory evidence for this theory, including potential mechanisms for mMac-neuron interaction. The need for a more thorough classification scheme to define how the “state” of mMacs relates to neuron loss or impaired function in disease is discussed. Despite the growing literature suggesting EGCs and mMacs have supportive or modulatory roles in ENS communication and gut function, conflicting evidence from different groups suggests more investigation is required. A broader understanding of why enteric neurons may need assistance from EGCs and mMacs in neurotransmission is still missing.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
- Enteric neuron
- Enteric glial cells (EGCs)
- Muscularis macrophages (mMacs)
- Intercellular communication
- Calcium (Ca2+) imaging
- Immunohistochemistry
24.1 Introduction
Traditionally, enteric neurons were considered to be the only active cells in the enteric nervous system (ENS). However, with improved sensitivity of imaging technologies and advances in the genetic tools available, this paradigm has recently shifted. The idea that non-neuronal cells can assist or modulate the functions of enteric neurons is an exciting new aspect for neurogastroenterology. The interstitial cells of Cajal (ICC) and telocytes such as fibroblast-like cells (also known as PDGFRα+ cells) are established examples of non-neuronal cells that act as an intermediary, relaying the synaptic inputs from enteric motor neurons to the target smooth muscle. However, are there networks of cells in the gut wall that can directly interact with the ENS or can communicate with each other in a manner comparable to enteric neurons?
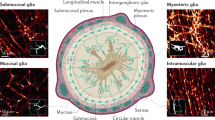
Interspersed among the enteric neurons are the enteric glial cells (EGCs) originally thought to be little more than structural support cells that hold the ENS in place. EGCs are present at similar or greater ratios than the neurons themselves (1:1), and growing evidence indicates these cells regulate enteric neural transmission. The emerging roles of tissue-resident immune cells known as muscularis macrophages (mMacs) in intercellular communication with the ENS have been similarly explored. While mMacs are generally dispersed throughout the external muscle layer of the gut wall, a subset is closely associated with the enteric ganglia and may communicate directly with the ENS [16]. Conceptual advances largely borrowed from studies of the central nervous system (CNS) have triggered an interest in considering EGCs and mMacs as more than support and immune cells [13, 26]. Astrocytes and microglia are important regulators of CNS interactions in health and disease. There are similarities in the gene expression profiles and morphologies of EGCs and mMacs with CNS astrocytes and microglia, respectively. Similar cells to EGCs and mMacs in the CNS have associations with neurons. This has led researchers to investigate whether EGC and mMacs in the ENS have analogous roles to astrocytes and microglia in the CNS. There is already evidence that both cell types can influence essential functions of the gut such as motility, secretion, and inflammation. However, mechanistic understanding of how these cells contribute to these events is limited. This review will summarize the current understanding of the functions of EGCs and mMacs in communication with the ENS network.
24.2 The Role of EGCs in ENS Communication
EGCs are considered to be electrically “silent” cells as they fail to demonstrate a response to current pulses, electrical stimulation, or pharmacological agents in electrophysiological recordings (human-Carbone unpublished data, [33]). This is in contrast to neurons which routinely generate an action potential when depolarized by current pulses. It is well documented that EGC signal via the release of intracellular Ca2+ stores [6, 34, 38], or through cAMP production [10]. Standard methods to demonstrate the roles of EGCs in gastrointestinal physiology have employed either Ca2+ indicator dyes (e.g., Fluo-4) or mice that selectively express genetically encoded calcium indicators (GECIs) in EGC [34]. Exogenously applied transmitters such as ATP and serotonin (5-HT) trigger an elevation in intracellular Ca2+ in EGCs [6]. This provides indirect evidence of the mechanisms by which enteric neurons regulate EGC functions. Specialized contact sites between enteric neurons and glial termed neuro-glial junctions have been identified in rodent intestine by electron microscopy [22, 23]. However, what evidence is there that enteric neurons release these neurotransmitters to directly activate EGCs?
EGCs express several receptors for neurotransmitters released by enteric neurons including purinergic, adrenergic, and metabotropic glutamate receptors [29]. Ca2+ imaging studies have provided examples where pharmacological or electrical activation of enteric neurons leads to subsequent responses in EGCs. Purinergic signaling is a major mechanism by which enteric neurons can activate EGCs [25, 28]. An important consideration for neuron-glia communication is that not all responses are inhibited by the voltage-sensitive Na+ channel inhibitor, tetrodotoxin (TTX) [7, 21]. While these channels are important for initiating the pathways that lead to typical synaptic transmission, this observation suggests that other mechanisms are involved. Enteric neurons can release ATP via channels formed by the protein subunits called pannexins. The release of ATP enteric neurons through pannexin channels has been shown to mediate neuronal death [31]. However, a recent study from Boesmans et al. [7] provided evidence that pannexin channels provide a “communicating junction” between enteric neurons and EGCs. Boesmans et al. used photolytic uncaging of cytosolic calcium to activate individual enteric neurons. Their results showed calcium transients in ~2 neighboring glial cells per single neuron stimulated. Only ~0.5 EGCs responded in the presence of either purinergic P2 receptor antagonist suramin or the nonselective pannexin inhibitor probenecid. The mean amplitude of responses was also attenuated. This is not surprising as it is likely that other neurotransmitters have roles in neuron-glia communication. EGCs respond to the application of other agonists for receptors such as nicotinic acetylcholine and neurokinin 2 (NK2) receptors. Furthermore, calcium is only one measure of cell activation, and signaling through cAMP in response to various stimuli has rarely been investigated. There is evidence that neuron-glia interactions occur during physiological events, particularly during motility patterns such as colonic motor complexes (CMCs) [8, 34]. Evidence from these studies indicates that only subpopulations of EGCs are activated during this physiological event and that this activation is secondary to enteric neuron stimulation. While this suggests potential roles for EGCs in modulating GI motility, research from other groups indicates that EGCs may not have a major contribution to this process [42].
It is worth noting that EGCs can also receive input from the autonomic nervous system and extrinsic sensory neurons [15, 30]. Gulbransen et al. in 2010 demonstrated that electrical field stimulation of nerve fiber tracts in the myenteric plexus of the guinea pig colon typically elicited robust Ca2+ transients in EGCs. However, the amplitude of these responses was significantly reduced in tissues where extrinsic nerves were chemically or surgically removed. The authors suggested that the primary neurotransmitter involved may be ATP since EGCs failed to respond to exogenous application of norepinephrine [30]. While EGCs are closely associated with TRPV1 expressing neuronal varicosities [15], stimulation of primary afferent nerves with capsaicin does not elicit a Ca2+ response in these cells [30]. EGCs are also closely associated with tachykinin immunoreactive varicosities, which include both extrinsic and intrinsic nerve fibers. Given that EGCs robustly respond to exogenous application of NK2R agonists, it is likely that this provides a mechanism for interaction with extrinsic sensory fibers. While the mechanisms for transmission between extrinsic sensory nerve fibers and EGC are not clear, they may be important for the development of visceral pain following bowel inflammation [27].
24.3 Communication Between Neighboring EGCs
Functional coupling of EGCs is an important mechanism for how this network of cells interacts. While electron microscopy studies fail to demonstrate the formation of typical gap junctions between many EGCs in the myenteric plexus of the rodent intestine [23], dye filling experiments provide evidence to support functional coupling within this network of cells [32]. Gap junctions are made up of hemichannels formed from connexin protein subunits. Ca2+ imaging experiments have demonstrated the importance of this functional coupling, as responses to exogenously applied ADP are attenuated with inhibition or deletion of these hemichannels [38]. In these experiments, hemichannels were either inhibited pharmacologically in tissues from wild-type animals or deleted by glia-specific disruption of the gene encoding connexin-43. In this same study, tissue from the modified mice failed to generate contractions in response to electrical stimulation of neurons, and GI motility in vivo was generally delayed. These results highlight that coupling between EGCs is important for functional interactions within this network of cells and the functional output of the ENS in physiological processes more broadly.
24.4 The Role of mMacs in ENS Communication
mMacs are the tissue-resident macrophages of the external muscle of the gastrointestinal tract. They have diverse and dynamic morphologies which facilitate their ability to constantly sample and respond to the local tissue environment [41]. Evidence suggests that mMacs have bidirectional relationships with the ENS that are important for the maintenance and function of both cell types. One arm of this interaction centers on the secretion of colony-stimulating factor 1 (CSF-1) by enteric neurons. CSF-1, also known as the macrophage colony-stimulating factor (M-CSF), is required for the continual maintenance and survival of mMacs [20, 40, 43]. mMacs fail to develop in the intestine of the osteopetrotic (op/op) transgenic mice, which express an inactivating mutation to the CSF-1 gene [39]. Furthermore, pharmacological or antibody inhibition of the receptor for CSF-1 (CSF-1R) leads to depletion of mMacs [3, 14]. Although enteric neurons are not the sole source of CSF-1 in the gut wall [1], it provides a hypothesized mechanism for ENS and mMacs association. The importance of these interactions is questioned by immunohistochemical studies in tissues from Hirschsprung Disease patients or from animal models of the disease, where the ENS fails to develop in the distal portion of the gastrointestinal tract (Ret knock-out) [1]. In both groups, mMacs continued to develop and colonize the GI tract despite the absence of neurons. The authors conclude that the enteric neurons may be necessary for the continual maintenance of mMac rather than the initial patterning of the cells. This was supported by their evidence that the colonization and development of mMacs precede that of enteric neurons.
The second arm of this bidirectional relationship is centered on the release of bone morphogenetic proteins (BMPs), for which there are receptors on enteric neurons (BMPR). During development, BMPs are required for the colonization of neural crest cells in the embryonic gut and the development of a functional ENS in the fetal gut [9, 20, 24]. mMacs highly express bone morphogenetic protein 2 (BMP2) [40]; therefore it is hypothesized that secretion of this soluble factor provides a mechanism for mMacs to interact with enteric neurons. In intestinal and stomach tissues from op/op mice [11, 12], the continual absence of mMacs is associated with the development of significantly greater numbers of enteric neurons. Immunohistochemistry data from Cipriani et al. suggests, that in the stomach of op/op mice, the proportion of nitrergic neurons in the myenteric plexus is more affected by the absence of mMacs than the proportion of cholinergic neurons [11, 12]. The overall increase of enteric neuron number is consistent with findings from tissues in mice that globally overexpress the BMP antagonist, noggin [9]. However, the timing of mMac depletion may have important implications for how their potential roles in ENS maintenance and function are interpreted. The use of tissues from inducible knockout models has shown that chronic mMac depletion leads to significant reductions in enteric neuron number (Cx3cr1CreERT2.Rosa26-iDTR) [14]. Muller et al. employed a different strategy to deplete macrophages by administering monoclonal antibodies directed at the CSF-1 receptor to mice [40]. They found no significant effects on enteric neuron number following acute depletion of macrophages, although functional effects were noted and will be discussed later in this review. How the changes in enteric neuron number then alter the output of the ENS is a necessary aspect to validate the impact of these changes in gastrointestinal processes.
mMacs have dynamic motile processes that are constantly changing to adapt to their cellular environment [41]. Flow cytometry and transcriptional information are available to define the various mMac subtypes in the individual layers of the gut wall [14, 36]. However, the field is lacking a more thorough classification of the “when,” “where,” and “how” the various subtypes contribute to ENS communication. Basic immunohistochemistry studies have attempted to quantify the broad groups of cells based on their density and location within the gastrointestinal tract. However, this sort of profiling does not take into account differences in cellular morphology and the number and length of processes, as has been demonstrated by studies such as De Schepper et al. [14]. These studies have aligned the location of the cells to a tissue layer, but the proximity of these subsets to the myenteric plexus and whether their processes directly contact enteric neurons or non-neural cells such as EGCs and ICCs has yet to be included in this classification. A detailed analysis of the anatomical locations of mMacs was very recently published by Dora et al., and their findings lead the authors to speculate whether a subset of mMac may function as a “barrier” around the myenteric ganglia much like the blood-brain barrier in the CNS [16]. Morphological analysis of CNS microglia is routinely used to assess their activation state [2, 44]. These cells normally have a hyper-ramified morphology during physiological conditions (anti-inflammatory state) but lose this complex branching in disease (pro-inflammatory state) [5]. There are examples of studies that have associated the activation states or morphology of mMacs, with ENS damage and changes to GI function. Kinoshita et al. compared tissues from control versus TNBS-colitis mice [35]. Using immunohistochemistry, they demonstrated that mMacs were ramified in the control intestine but were non-ramified in the colitis tissues. Damage to the ENS and ICC networks and a reduction in the contractility of tissues were also associated with these changes in mMac morphology in the inflamed intestine [35]. Immunohistochemistry and flow cytometry analyses were used by Becker et al. to demonstrate similar shifts in macrophage profiles in aging from an “anti-inflammatory” profile in intestinal samples from younger mice to a pro-inflammatory state in samples from older mice. This was associated with an overall reduction in enteric neuron density and a reduction in gastrointestinal motility measured in vivo [4]. More knowledge is needed to relate why these changes occur and the mechanisms that connect these changes with associated damage to the ENS and other non-neural cells.
24.5 How Do mMac Interactions with Enteric Neurons Relate to Changes to Gastrointestinal Function?
Several studies have investigated the potential involvement of mMac in gastrointestinal motility and secretion. In a study by De Schepper et al., depletion of mMacs inhibited the ability for ileal segments to generate neurally evoked contractions and limited neurally mediated increases in short circuit current (a measure of secretion). This was supported by in vivo analysis showing that mMac depletion reduced small intestinal transit and increased transit times in treated mice [14]. The hypothesis from this paper centered on the idea that mMacs are necessary for the continual maintenance of the enteric neurons. While Muller et al. showed that fecal pellet output was delayed in mice treated with αCSF-1 to deplete mMacs, they identified that this delay was due to “dysmotility” [40]. In contrast to findings by De Schepper et al., contractility increased in small colonic segments from mMac-depleted mice, and this increase was perturbed by stretching the tissue. Colonic motility returns to normal in mMac-depleted tissues treated with exogenously applied BMP2, as mMacs typically release BMP2 for which there are receptors on enteric neurons. This led the authors to postulate that BMP2 is an important signaling molecule for the direct activation of enteric neurons during physiological processes. Observations by Luo et al. challenge the relative involvement of enteric neurons in mMac-mediated contractions [37]. Using optogenetics and genetic approaches (DREADD mice, Designer Receptors Exclusively Activated by Designer Drugs), they were able to selectively activate mMacs and demonstrate a corresponding contraction in segments of the colon. These contractions were TTX-insensitive suggesting mMacs directly stimulated the smooth muscle cells. This study provided evidence that the mechanisms for this interaction involved the release of prostaglandin E2 (PGE2) from mMac acting on the prostaglandin E receptors on smooth muscle cells. However, others have shown that activation of mMac with the bacterially derived endotoxin lipopolysaccharide (LPS) inhibits circular muscle contractility, through an iNOS-dependent mechanism [18, 19]. Unpublished evidence from our laboratory questions whether mMacs have a clear role in controlling motility under physiological conditions, and we postulate whether these roles may be more pronounced in pathophysiology. The conflicting findings across the literature certainly demonstrate that our understanding of the functions of mMacs in GI motility and physiological functions more broadly is still being defined.
24.6 Evidence for EGC and mMac Interactions in the Gut Wall
The fact that mMacs still populate and develop within the intestine, despite the absence of enteric neurons, highlights that there must be other sources of CSF-1 in the gut wall. Grubisic et al. recently demonstrated using immunohistochemistry that around 10% of myenteric neurons in the mouse colon express the membrane-bound form of CSF-1, versus 60% of enteric glia. Using 3D analysis, they also showed that mMacs within the ganglia have processes that physically interact with EGCs [27]. They provided evidence that colonic inflammation stimulated CSF-1 release by EGCs, which in turn was associated with activation of mMacs toward a pro-inflammatory phenotype. This signaling may drive visceral pain in colitis.
24.7 Conclusion
Results from various studies have shown that the absence of EGCs or mMacs disrupts the interactions between enteric neurons and the smooth muscle cells. If this outcome is true, then it suggests that EGCs and mMacs have continual roles in regulating the output of enteric neurons, and it raises several questions for the field. What benefit is there for EGCs or mMacs to regulate neuromuscular transmission and why do enteric neurons require an intermediary cell to regulate the process? At present, enteric neurons are divided into several, much more diverse subtypes based on transmitter complement and expression than EGCs and mMacs [17]. While there are subsets of EGCs and mMac, they appear to be far more similar to each other than enteric neuron subsets. EGCs and mMacs form functional networks and perhaps the more limited diversity compared to enteric neurons assists in the synchrony of the system. By firing in synchrony EGCs/mMac may amplify the outcome of neural firing. Understanding the roles of EGCs and mMacs in ENS communication is a growing area, with many questions remain to be answered. Potential mechanisms for how these cells communicate with the ENS have been identified. However, future directions should aim to seek clarification as to when these interactions are important.
References
Avetisyan M, Rood JE, Huerta Lopez S, Sengupta R, Wright-Jin E, Dougherty JD, Behrens EM, Heuckeroth RO (2018) Muscularis macrophage development in the absence of an enteric nervous system. Proc Natl Acad Sci U S A 115:4696–4701
Bachiller S, Jimenez-Ferrer I, Paulus A, Yang Y, Swanberg M, Deierborg T, Boza-Serrano A (2018) Microglia in neurological diseases: a road map to brain-disease dependent-inflammatory response. Front Cell Neurosci 12:488
Bain CC, Bravo-Blas A, Scott CL, Perdiguero EG, Geissmann F, Henri S, Malissen B, Osborne LC, Artis D, Mowat AM (2014) Constant replenishment from circulating monocytes maintains the macrophage pool in the intestine of adult mice. Nat Immunol 15:929–937
Becker L, Nguyen L, Gill J, Kulkarni S, Pasricha PJ, Habtezion A (2018) Age-dependent shift in macrophage polarisation causes inflammation-mediated degeneration of enteric nervous system. Gut 67:827–836
Beynon SB, Walker FR (2012) Microglial activation in the injured and healthy brain: what are we really talking about? Practical and theoretical issues associated with the measurement of changes in microglial morphology. Neuroscience 225:162–171
Boesmans W, Cirillo C, Van den Abbeel V, Van den Haute C, Depoortere I, Tack J, Vanden Berghe P (2013) Neurotransmitters involved in fast excitatory neurotransmission directly activate enteric glial cells. Neurogastroenterol Motil 25:e151–e160
Boesmans W, Hao MM, Fung C, Li Z, Van den Haute C, Tack J, Pachnis V, Vanden Berghe P (2019) Structurally defined signaling in neuro-glia units in the enteric nervous system. Glia 67:1167–1178
Broadhead MJ, Bayguinov PO, Okamoto T, Heredia DJ, Smith TK (2012) Ca2+ transients in myenteric glial cells during the colonic migrating motor complex in the isolated murine large intestine. J Physiol 590:335–350
Chalazonitis A, Pham TD, Li Z, Roman D, Guha U, Gomes W, Kan L, Kessler JA, Gershon MD (2008) Bone morphogenetic protein regulation of enteric neuronal phenotypic diversity: relationship to timing of cell cycle exit. J Comp Neurol 509:474–492
Christofi FL, Hanani M, Maudlej N, Wood JD (1993) Enteric glial cells are major contributors to formation of cyclic Amp in myenteric plexus cultures from adult guinea-pig small intestine. Neurosci Lett 159:107–110
Cipriani G, Gibbons SJ, Miller KE, Yang DS, Terhaar ML, Eisenman ST, Ordog T, Linden DR, Gajdos GB, Szurszewski JH, Farrugia G (2018) Change in populations of macrophages promotes development of delayed gastric emptying in mice. Gastroenterology 154:2122–2136 e12
Cipriani G, Terhaar ML, Eisenman ST, Ji S, Linden DR, Wright AM, Sha L, Ordog T, Szurszewski JH, Gibbons SJ, Farrugia G (2019) Muscularis propria macrophages alter the proportion of nitrergic but not cholinergic gastric myenteric neurons. Cell Mol Gastroenterol Hepatol 7:689–691 e4
De Schepper S, Stakenborg N, Matteoli G, Verheijden S, Boeckxstaens GE (2018) Muscularis macrophages: key players in intestinal homeostasis and disease. Cell Immunol 330:142–150
De Schepper S, Verheijden S, Aguilera-Lizarraga J, Viola MF, Boesmans W, Stakenborg N, Voytyuk I, Schmidt I, Boeckx B, Dierckx de Casterle I, Baekelandt V, Gonzalez Dominguez E, Mack M, Depoortere I, de Strooper B, Sprangers B, Himmelreich U, Soenen S, Guilliams M, Vanden Berghe P, Jones E, Lambrechts D, Boeckxstaens G (2018) Self-maintaining gut macrophages are essential for intestinal homeostasis. Cell 175:400–415 e13
Delvalle NM, Dharshika C, Morales-Soto W, Fried DE, Gaudette L, Gulbransen BD (2018) Communication between enteric neurons, glia, and nociceptors underlies the effects of tachykinins on neuroinflammation. Cell Mol Gastroenterol Hepatol 6:321–344
Dora D, Ferenczi S, Stavely R, Toth VE, Varga ZV, Kovacs T, Bodi I, Hotta R, Kovacs KJ, Goldstein AM, Nagy N (2021) Evidence of a myenteric plexus barrier and its macrophage-dependent degradation during murine colitis: implications in enteric neuroinflammation. Cell Mol Gastroenterol Hepatol 12:1617
Drokhlyansky E, Smillie CS, Van Wittenberghe N, Ericsson M, Griffin GK, Eraslan G, Dionne D, Cuoco MS, Goder-Reiser MN, Sharova T, Kuksenko O, Aguirre AJ, Boland GM, Graham D, Rozenblatt-Rosen O, Xavier RJ, Regev A (2020) The human and mouse enteric nervous system at single-cell resolution. Cell 182:1606–1622 e23
Eskandari MK, Kalff JC, Billiar TR, Lee KK, Bauer AJ (1997) Lipopolysaccharide activates the muscularis macrophage network and suppresses circular smooth muscle activity. Am J Phys 273:G727–G734
Eskandari MK, Kalff JC, Billiar TR, Lee KK, Bauer AJ (1999) Lps-induced muscularis macrophage nitric oxide suppresses rat jejunal circular muscle activity. Am J Phys 277:G478–G486
Fu M, Vohra BP, Wind D, Heuckeroth RO (2006) Bmp signaling regulates murine enteric nervous system precursor migration, neurite fasciculation, and patterning via altered Ncam1 polysialic acid addition. Dev Biol 299:137–150
Fung C, Boesmans W, Cirillo C, Foong JPP, Bornstein JC, Vanden Berghe P (2017) Vpac receptor subtypes tune purinergic neuron-to-glia communication in the murine submucosal plexus. Front Cell Neurosci 11:118
Gabella G (1972) Fine structure of the myenteric plexus in the guinea-pig ileum. J Anat 111:69–97
Gabella G (1981) Ultrastructure of the nerve plexuses of the mammalian intestine: the enteric glial cells. Neuroscience 6:425–436
Goldstein AM, Brewer KC, Doyle AM, Nagy N, Roberts DJ (2005) Bmp signaling is necessary for neural crest cell migration and ganglion formation in the enteric nervous system. Mech Dev 122:821–833
Gomes P, Chevalier J, Boesmans W, Roosen L, van den Abbeel V, Neunlist M, Tack J, Vanden Berghe P (2009) ATP-dependent paracrine communication between enteric neurons and glia in a primary cell culture derived from embryonic mice. Neurogastroenterol Motil 21:870–e62
Grubisic V, Gulbransen BD (2017) Enteric glia: the most alimentary of all glia. J Physiol 595:557–570
Grubisic V, McClain JL, Fried DE, Grants I, Rajasekhar P, Csizmadia E, Ajijola OA, Watson RE, Poole DP, Robson SC, Christofi FL, Gulbransen BD (2020) Enteric glia modulate macrophage phenotype and visceral sensitivity following inflammation. Cell Rep 32:108100
Gulbransen BD, Sharkey KA (2009) Purinergic neuron-to-glia signaling in the enteric nervous system. Gastroenterology 136:1349–1358
Gulbransen BD, Sharkey KA (2012) Novel functional roles for enteric glia in the gastrointestinal tract. Nat Rev Gastroenterol Hepatol 9:625–632
Gulbransen BD, Bains JS, Sharkey KA (2010) Enteric glia are targets of the sympathetic innervation of the myenteric plexus in the guinea pig distal colon. J Neurosci 30:6801–6809
Gulbransen BD, Bashashati M, Hirota SA, Gui X, Roberts JA, MacDonald JA, Muruve DA, McKay DM, Beck PL, Mawe GM, Thompson RJ, Sharkey KA (2012) Activation of neuronal P2X7 receptor-pannexin-1 mediates death of enteric neurons during colitis. Nat Med 18:600–604
Hanani M, Zamir O, Baluk P (1989) Glial cells in the guinea pig myenteric plexus are dye coupled. Brain Res 497:245–249
Hanani M, Francke M, Hartig W, Grosche J, Reichenbach A, Pannicke T (2000) Patch-clamp study of neurons and glial cells in isolated myenteric ganglia. Am J Physiol Gastrointest Liver Physiol 278:G644–G651
Hennig GW, Gould TW, Koh SD, Corrigan RD, Heredia DJ, Shonnard MC, Smith TK (2015) Use of genetically encoded calcium indicators (Gecis) combined with advanced motion tracking techniques to examine the behavior of neurons and glia in the enteric nervous system of the intact murine colon. Front Cell Neurosci 9:436
Kinoshita K, Horiguchi K, Fujisawa M, Kobirumaki F, Yamato S, Hori M, Ozaki H (2007) Possible involvement of muscularis resident macrophages in impairment of interstitial cells of Cajal and myenteric nerve systems in rat models of TNBS-induced colitis. Histochem Cell Biol 127:41–53
Koscso B, Gowda K, Schell TD, Bogunovic M (2015) Purification of dendritic cell and macrophage subsets from the normal mouse small intestine. J Immunol Methods 421:1–13
Luo J, Qian A, Oetjen LK, Yu W, Yang P, Feng J, Xie Z, Liu S, Yin S, Dryn D, Cheng J, Riehl TE, Zholos AV, Stenson WF, Kim BS, Hu H (2018) Trpv4 channel signaling in macrophages promotes gastrointestinal motility via direct effects on smooth muscle cells. Immunity 49(107–119):e4
Mcclain J, Grubisic V, Fried D, Gomez-Suarez RA, Leinninger GM, Sevigny J, Parpura V, Gulbransen BD (2014) Ca2+ responses in enteric glia are mediated by connexin-43 hemichannels and modulate colonic transit in mice. Gastroenterology 146:497–507 e1
Mikkelsen HB, Thuneberg L (1999) Op/op mice defective in production of functional colony-stimulating factor-1 lack macrophages in muscularis externa of the small intestine. Cell Tissue Res 295:485–493
Muller PA, Koscso B, Rajani GM, Stevanovic K, Berres ML, Hashimoto D, Mortha A, Leboeuf M, Li XM, Mucida D, Stanley ER, Dahan S, Margolis KG, Gershon MD, Merad M, Bogunovic M (2014) Crosstalk between muscularis macrophages and enteric neurons regulates gastrointestinal motility. Cell 158:300–313
Phillips RJ, Powley TL (2012) Macrophages associated with the intrinsic and extrinsic autonomic innervation of the rat gastrointestinal tract. Auton Neurosci 169:12–27
Rao M, Rastelli D, Dong L, Chiu S, Setlik W, Gershon MD, Corfas G (2017) Enteric glia regulate gastrointestinal motility but are not required for maintenance of the epithelium in mice. Gastroenterology 153:1068–1081 e7
Schneider S, Wright CM, Heuckeroth RO (2019) Unexpected roles for the second brain: enteric nervous system as master regulator of bowel function. Annu Rev Physiol 81:235–259
York EM, Ledue JM, Bernier LP, MacVicar BA (2018) 3DMorph automatic analysis of microglial morphology in three dimensions from ex vivo and in vivo imaging. eNeuro 5:ENEURO.0266-18.2018
Acknowledgments
Many thanks to Daniel Poole for reading and revising this manuscript and for mentoring me to become a better scientist.
Funding
SEC is an Australian Research Council DECRA Fellow (DE200100825).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this paper
Cite this paper
Carbone, S.E. (2022). Neurons, Macrophages, and Glia: The Role of Intercellular Communication in the Enteric Nervous System. In: Spencer, N.J., Costa, M., Brierley, S.M. (eds) The Enteric Nervous System II. Advances in Experimental Medicine and Biology, vol 1383. Springer, Cham. https://doi.org/10.1007/978-3-031-05843-1_24
Download citation
DOI: https://doi.org/10.1007/978-3-031-05843-1_24
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-05842-4
Online ISBN: 978-3-031-05843-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)