Abstract
Atherosclerosis is characterized by lipid accumulation and chronic inflammation of the arterial wall, and its main complications—myocardial infarction and ischemic stroke—together constitute the first cause of death worldwide. Accumulation of lipid-laden macrophage foam cells in the intima of inflamed arteries has long been recognized as a hallmark of atherosclerosis. However, in recent years, an unexpected complexity in the mechanisms of macrophage accumulation in lesions, in the protective and pathogenic functions performed by macrophages and how they are regulated has been uncovered. Here, we provide an overview of the latest developments regarding the various mechanisms of macrophage accumulation in lesion, the major functional features of lesion macrophages, and how the plaque microenvironment may affect macrophage phenotype. Finally, we discuss how best to apprehend the heterogeneous ontogeny and functionality of atherosclerotic plaque macrophages and argue that moving away from a rigid nomenclature of arbitrarily defined macrophage subsets would be beneficial for research in the field.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Atherosclerosis is a chronic inflammatory disease of the vascular wall, and its complications, in particular myocardial infarction and stroke, constitute the first cause of death in the world. Macrophages are the most abundant immune cell type in atherosclerotic lesions and have an essential role during all stages of the disease, from lesion initiation to plaque rupture. Hence, thoroughly understanding macrophage biology in vascular inflammation and atherosclerosis appears as a prerequisite to develop novel therapeutic strategies aiming at taming the disease burden associated with this pathology.
A long standing paradigm regarding the major steps of macrophage-dependent vascular inflammation in atherosclerosis could be briefly described as follows: in areas of non-laminar vascular blood flow, endothelial dysfunction permits circulating low density lipoprotein (LDL) infiltration into the vascular intima, which is increased under conditions of hyperlipidemia. In the vessel wall, LDL undergoes modifications such as oxidation, and modified LDL activates endothelial cells and resident immune cells leading to expression of chemokines and adhesion molecules that attract circulating monocytes to the vessel wall and allow their adhesion, rolling and transendothelial migration, resulting in monocyte infiltration in the intima. Once in the intima, monocytes differentiate into macrophages and ingest modified lipoprotein to become foam cells. Gradually accumulating foam cells die in the intima through apoptosis and, when not promptly disposed of, become necrotic, progressively leading to the formation of a thrombogenic and proinflammatory necrotic core. Macrophages further fuel lesion inflammation through secretion of cytokines, and their proteolytic activity promotes plaque destabilization and rupture that leads to atherothrombosis and the associated ischemic events such as myocardial infarction or stroke. Although this view is still considered generally valid and an important underlying mechanism of lesion formation, recent research has revealed an unexpected complexity in the mechanisms of macrophage accumulation in atherosclerotic plaques, in the functions performed by macrophages, and in the mechanisms determining macrophage phenotype in lesions. In this review, we provide a brief overview of the origins of macrophages in the vessel wall, their fundamental function, and how these are regulated. Finally, we will discuss how to best apprehend investigations of macrophage phenotypic heterogeneity and its implications in disease, and how moving beyond the macrophage polarization paradigm might be beneficial for research in the field.
Mechanisms of macrophage accumulation in the vessel wall
Recruitment of bone marrow and spleen-derived circulating monocytes
Recruitment of circulating monocytes to the inflamed vessel wall is instrumental in the initiation and progression of atherosclerotic lesions. In mice, hypercholesterolemia induces monocytosis and more particularly a rise in levels of circulating Ly6Chi monocytes, the equivalent of human CD14+ CD16− monocytes [120, 123]. Ly6ChiCCR2+ monocytes are considered to be the major precursors of plaque macrophages, and their recruitment to plaques depends on expression of the chemokine receptors CCR2, CCR5, and CX3CR1 [120, 123] and receptors that bind various adhesion molecules expressed on endothelial cells [46]. Interestingly, the systemic control of circulating monocyte levels appears to have a crucial role in lesional macrophage accumulation as plaque macrophage content is directly correlated to circulating monocyte levels [30]. Medullary hematopoiesis has long been considered as the major—if not sole—source of monocytes in atherosclerosis, and hyperlipidemia as well as associated comorbidities, such as hyperglycemia, promote monocyte production in the bone marrow [81, 114]. Bone marrow monocytes are mobilized to the bloodstream mainly in response to chemokine/chemokine receptor signaling, in particular CCL2/CCR2 [30, 107]. However, it is now well established that also extramedullary hematopoiesis in the spleen significantly contributes to atherosclerosis-associated monocytosis and that monocytes originating from the spleen participate in macrophage accumulation within lesions [97]. Mechanistically, hematopoietic stem cells (HSCs) migrate from the bone marrow to the spleen, where the microenvironment supports their progressive differentiation towards mature monocytes [97]. Interestingly, mobilization of monocytes from the splenic reservoir appears to be independent of CCR2, but relies on angiotensin II signaling through its type 1 receptor AT1 [121], and may also depend on splenic B cells, although the underlying mechanisms are still unclear [78].
Local proliferation of macrophages
Proliferation of macrophages in atherosclerotic plaques has been observed in human and animal models more than 20 years ago [49, 95, 102], but its quantitative contribution to macrophage accumulation in lesions has long remained enigmatic. A study based on a model of parabiosis, where the blood circulation of two mice is joined, shed new light on this paradigm. By allowing a discriminating of the origin of cells in tissue from either recruited circulating or locally expanded cells, macrophage proliferation was proposed to account for ∼87% of macrophage accumulation in established lesions, while recruitment of circulating monocytes was only instrumental in the early phases of the disease [98]. However, data from the same study using a long-term bone marrow chimera model showed that all macrophages in established lesions ultimately originate from circulating precursors, and a proportional increase in monocyte recruitment with lesion progression was also reported [119]. In contrast to the work by Robbins et al. [98], a recent report suggested that macrophage proliferative activity was higher in early rather than in advanced lesions [69]. To add another layer of complexity, it was recently found that a substantial proportion of macrophages in established lesions are senescent, thus by definition incapable of proliferating, and that their clearance through pharmacological intervention significantly reduces macrophage content in established lesions [23]. The fact that highly proliferative and plastic vascular smooth muscle cells can adopt a macrophage-like phenotype and express classical macrophage markers such as Mac3 or Mac2 (Lgals3) in lesions (as discussed below) further complicates interpretation of studies investigating macrophage proliferation in atherosclerosis [20, 40, 109].
VSMC transdifferentiation into macrophage-like cells
Cells co-expressing markers of macrophages and vascular smooth muscle cells (VSMCs) have been described in human atherosclerotic lesions 20 years ago [8], and a study recently showed that up to 50% of cells identified as macrophage foam cells co-expressed smooth muscle cell markers [7]. However, these studies based on immunohistological analyses cannot determine whether these cells originate from macrophages upregulating VSMC markers or VSMCs transdifferentiating into macrophage foam cells. Convincing evidence of VSMC transdifferentiation into macrophage-like cells in lesions has been obtained from lineage fate mapping of VSMCs in murine atherosclerosis. Using pulse-labeling of VSMCs in reporter Apoe −/− -ROSA26 mice expressing the tamoxifen-inducible Cre recombinase under the control of a VSMC-specific promoter in the SM22α gene locus, Feil et al. demonstrated loss of VSMC markers and conversion to a macrophage-like cell phenotype of clonally expanded VSMC-originating cells in advanced lesions [40]. Using a similar fate mapping strategy, albeit with expression of inducible Cre under the control of another VSMC-specific promoter, Shankman et al. demonstrated that a vast proportion of pulse-labeled cells lost expression of VSMC markers and acquired expression of macrophage or mesenchymal stem cell markers. Furthermore, it was demonstrated that this phenotypic transition depended on expression of the transcription factor Klf4 [109]. A recent report using a model of stochastic multicolor fate mapping further suggested that a subset of highly proliferative plastic VSMCs can clonally expand in lesions and that progeny of a single VSMC can adopt different phenotypes, including conversion to a macrophage-like phenotype with expression of the macrophage marker Mac3 [20].
In in vivo studies, VSMC-derived macrophage-like cells could form foam cells, but these are likely functionally different from bona fide macrophages [40, 109]. This notion is corroborated by in vitro studies, where cholesterol-loading induces VSMC transdifferentiation to macrophage-like cells [101]. Indeed, these VSMC-derived macrophage-like cells have a transcriptional profile that largely differs from bone marrow-derived macrophages and perform poorly in classical macrophage function tests, such as phagocytosis and efferocytosis [131].
Resident aortic macrophages and differentiation of local precursors
In the absence of hypercholesterolemia and atherosclerotic disease, healthy arteries already contain resident macrophages and dendritic cells. This was recently demonstrated with the help of genetic fate mapping models, and it could be revealed that murine arteries contain a resident population of macrophages derived from two distinct sources, namely CX3CR1+ embryonic precursors and bone marrow-derived monocytes that colonize arterial tissue during embryonic development and in the first few days after birth, repsectively [35]. This arterial macrophage population appears to self-renew during homeostasis and after infection-induced depletion [35]. The role of this resident macrophage population in atherosclerosis remains to be determined, but it is conceivable that they may impact disease initiation in a similar fashion as vascular resident dendritic cells that take up lipids and form foam cells at very early stages of lesion formation [86]. Their proliferative ability also suggests that they may contribute to macrophage accumulation during lesion progression. In addition to resident arterial macrophages, some studies have furthermore suggested that a population of adventitial macrophage progenitor cells resides in the adult mouse aorta and may locally differentiate to contribute to macrophage accumulation in atherosclerosis [90, 91].
The notion that a large part of resident tissue macrophages arise from stem cells during embryonic development has challenged the general “monocyte-derived macrophage” paradigm [47, 87]. The exact developmental origin of these macrophages, however, is still debated [47, 87]. It appears that macrophages in any given tissue originate from various sources of precursors such as yolk sac-derived progenitors, fetal hematopoietic stem cells (HSCs), or adult HSC-derived monocytes, both during homeostasis and inflammation [47]. It remains to be determined whether ontogeny has functional implications in mature macrophages [47], and it will be interesting to investigate not only the contribution of resident arterial macrophages and macrophage progenitors to macrophage accumulation in atherosclerosis, but also the impact of macrophage origin on disease-relevant functions.
Macrophages and plaque regression
Atherosclerotic plaques—and their macrophage content—can regress, as shown in particular in murine models of hypercholesterolemia reversal [39, 42, 89]. However, the exact underlying mechanisms are still debated. Some reports based on models of atherosclerotic aortic transplantation proposed that egress of mature macrophages from lesions drove plaque regression in a process involving liver X receptor (LXR)-dependent induction of CCR7 in macrophages [38] and that this is hampered by expression of the adhesion molecule JAM-C on endothelial cells [17]. It was further proposed that lesional macrophages can express retention molecules such as netrin-1 and its receptor UNC5b, which inhibit their emigration from lesions and thereby promote plaque progression [129]. Another study proposed that regression of lesional macrophage burden was independent of CCR7-driven macrophage emigration, but rather relied on suppression of monocyte recruitment and steady local apoptosis of plaque macrophages [89].
Functions of macrophages in atherosclerosis
Production of inflammatory cytokines and proteases
Once in lesions, macrophages actively participate in vascular inflammation through secretion of proinflammatory cytokines and the production of chemokines to promote further recruitment of immune cells. Plaque macrophages express proinflammatory cytokines which have been assigned a pro-atherogenic role such as TNFα [84], IL-18 [75, 138], and IL-12 [67]. Although affecting all hematopoietic cells and not only macrophages, experimental studies of lethal irradiation and bone marrow reconstitution in atherosclerosis-prone mice have been essential in establishing the crucial role of macrophage-derived cytokines in plaque inflammation. IL-1α and IL-1β deficiency in bone marrow-derived cells was shown to reduce lesion formation and inflammation in Apoe −/− mice [59], although a subsequent study proposed that only leukocyte-derived IL-1α, but not IL-1β, has a crucial role in atherogenesis [44]. CCL2 overexpression in bone marrow cells increased macrophage recruitment to lesions, showing that leukocyte-derived chemokines can fuel plaque inflammation [3]. Plaque macrophages also produce anti-inflammatory cytokines, and leukocyte-specific deficiency in IL-10 or IL-13 increased plaque inflammation and lesion formation [19, 88].
Proteolytic activity in lesions is associated with the progression towards a vulnerable and rupture-prone phenotype [112]. Lesional macrophages are known to have a strong proteolytic activity and are thus considered to substantially participate in plaque destabilization and rupture [92]. A proof-of-concept for the critical role of macrophage-mediated proteolysis in plaque destabilization was obtained in Apoe −/− mice overexpressing active matrix metalloproteinase (MMP)-9 specifically in advanced lesion macrophages, which induced features of plaque disruption [50]. Conversely, MMP-14 deficiency in bone marrow-derived cells led to formation of stable plaques with increased collagen content in Ldlr −/− mice, suggesting a role of macrophage-derived MMP-14 in plaque destabilization [105].
Foam cell formation
Formation of macrophage foam cells in the vascular intima is a hallmark of atherosclerosis. The extent to which macrophages accumulate intracellular lipids represents the balance between extracellular lipid uptake and reverse lipid transport from the intracellular compartment to extracellular lipid acceptors. Macrophages take up modified LDL through various scavenger receptors such as CD36, scavenger receptor A1 (SR-A1, also known as MSR1), scavenger receptor B1, LDL receptor-related protein 1 (LRP-1), and lectin-like oxLDL receptor-1 (LOX-1) [122]. Experimental studies in mice deficient for scavenger receptors have investigated their role in foam cell formation and atherogenesis. Ldlr −/− mice with macrophage-specific deficiency in LRP-1 have decreased cholesterol accumulation in macrophages upon high-fat diet feeding but accumulate cholesterol and triglycerides in the bloodstream, hampering interpretation of the specific role of LRP-1 in lesion macrophages [72]. Several studies have demonstrated reduced foam cell formation in mice with total or macrophage-specific deficiency in CD36 [36, 37] and MSR1 [10, 118]. However, another report in Apoe −/− mice deficient for CD36 or MSR1 demonstrated normal lesional foam cell accumulation despite reduced cholesterol accumulation in peritoneal macrophages, suggesting that alternative pathways of lipid uptake can compensate for the loss of CD36 or MSR1 [79]. In addition to its role in lipid uptake, a critical role for MSR1 has been identified in macrophage proliferation in advanced lesions [98]. Recent research demonstrated that the adhesion receptor CD146 (also known as melanoma cell adhesion molecule, MCAM), which is expressed on human and murine plaque macrophages, promotes the accumulation of oxidized LDL in macrophages by aiding CD36 internalization. Interestingly, CD146 also appears to promote macrophage retention within lesions [73].
Intracellular cholesterol can be removed from macrophages through an active reverse cholesterol transport process from the intracellular compartment to extracellular lipid acceptors such as Apolipoprotein A-1 (ApoA-1), a major constituent of high-density lipoprotein (HDL) [141]. Macrophage cholesterol efflux depends on the ATP-binding cassette transporters (ABC) ABCA1 and ABCG1 [141]. Accordingly, hypercholesterolemic mice lacking ABCA1 and ABCG1 expression in leukocytes display enhanced atherosclerotic lesion formation, likely in part due to increased accumulation of macrophage foam cells [139]. However, the role of ABCA1 and ABCG1-dependent cholesterol efflux pathways goes beyond the regulation of foam cell formation. Indeed, ABCA1 and ABCG1 are suppressors of hematopoietic stem and progenitor cell (HSPC) proliferation and mobilization [136, 140], and Acba1 −/− Abcg1 −/− mice show uncontrolled HSPC proliferation, leukocytosis [140], and increased extramedullary hematopoiesis [136], which altogether promote immune cell accumulation within lesions [140].
Proliferation and senescence, survival, and apoptosis
Given its recently appreciated importance in lesional macrophage accumulation (see above), proliferative ability can also be considered an important functional trait of lesion macrophages. As macrophage proliferation has been proposed to be preferentially activated in advanced lesions [10], deciphering the mechanism underlying the transition from a non-proliferative to a proliferative state would be of interest. In contrast to proliferating macrophages, lesions also contain cells with terminal loss of proliferative ability, namely senescent cells. Although a general pathogenic role of senescence in atherosclerosis was already suspected [22, 134], a recent report proposed that senescent macrophage foam cells within the vascular intima were pathogenic during the whole atherogenic process [23]. Intimal macrophage foam cells expressing senescent markers were abundantly found in the intima of the lesion-prone lesser curvature of the aortic arch in Ldlr −/− mice after 9 days of atherogenic diet feeding, and their pharmacological clearance reduced mRNA expression of Tnfa, Vcam1, and Mcp1, suggesting a role of these cells in lesion initiation. Senescent markers were also found in ∼20% of macrophage foam cells in established lesions, and sorted senescent macrophages had increased mRNA expression of some proinflammatory cytokines (Il1α, Mcp1) and metalloproteases (Mmp12, Mmp13). Accordingly, clearance of senescent cells from established lesions halted lesion progression and was associated with features of plaque stability [23]. Thus, senescence can be considered as a novel deleterious dysfunctional state of macrophages in lesions.
Survival and apoptosis in plaques represent critical aspects of macrophage biology in atherosclerosis as they dictate not only formation of the necrotic core, but can also underlie plaque regression and resolution of inflammation upon normalization of blood lipid levels [89]. In line, it is still unclear how modulation of macrophage survival impacts atherosclerosis, as contrasting results have been reported in various genetic mouse models that showed that increased macrophage survival appeared either beneficial [16, 104] or deleterious [11, 12, 48, 143]. Whether increased macrophage survival or apoptosis ultimately determines plaque progression or regression most likely depends on the plaque stage and total efferocytotic ability of surrounding viable macrophages.
Efferocytosis and authophagy
Efferocytosis corresponds to the immunologically silent clearance of apoptotic cells by phagocytes and is essential in the maintenance of self-tolerance [51]. Several lines of evidence indicate that macrophage efferocytotic ability has a critical impact on atherosclerotic lesion formation and progression [130]. Evidence supporting this concept mostly stems from genetic mouse models in which major pathways of apoptotic cell recognition by phagocytes have been invalidated. MFG-E8 bridges apoptotic cells to macrophages through binding of exposed phosphatidilyserine (PS) on the membrane of apoptotic cells and αvβ3/β5 integrins on macrophages, and Ldlr −/− mice with immune cell-specific deficiency in MFG-E8 displayed increased atherosclerosis and necrotic core formation [4]. Apoptotic cells can also be recognized by the PS-binding protein GAS6, which binds the receptor MER-TK on macrophages. Increased atherosclerosis, accumulation of apoptotic cells, and plaque necrosis were observed in Apoe −/− Mertk −/− mice [126] and in Ldlr −/− mice [5] with immune cell MER-TK deficiency. In line, it was recently proposed that proteolytic cleavage of MER-TK from the macrophage surface triggers defective efferocytosis in advanced lesions, as Ldlr −/− mice expressing a cleavage-resistant MER-TK showed improved efferocytosis and decreased necrosis in lesions [18]. Blockade of TIM-1 or TIM-4, which are expressed by macrophages and recognize PS on apoptotic cells [43], or genetic deficiency in the complement C1q, known to be involved in apoptotic cell clearance, also increased lesion formation in Ldlr −/− mice [14]. Apoptotic cells can also express “do not eat me” signals such as CD47, which inhibit efferocytosis upon binding to its receptor SIRPα on macrophages, and therapeutic neutralization of CD47 via monoclonal antibodies was recently shown to promote efferocytosis and reduce lesion formation in murine models of atherosclerosis [63]. This further shows that efferocytosis is a crucial and therapeutically targetable function of atherosclerotic lesion macrophages. Beyond its role in preventing accumulation of secondary necrotic cells, efferocytosis also has a profound impact on macrophage phenotype. Notably, phagocytosis of apoptotic cells reprograms macrophages towards an anti-inflammatory profile, with increased expression of anti-inflammatory IL-10 and TGFβ, and decreased expression of proinflammatory IL-12 and TNFα [25]. Furthermore, apoptotic cell phagocytosis triggers LXR-dependent expression of MER-TK by phagocytes, further promoting efferocytosis [83]. Given the multifaceted anti-atherogenic functions of LXR-mediated gene expression in macrophages [38, 57, 115], LXR activation through efferocytosis may further participate in a beneficial macrophage phenotype.
Autophagy can be induced in response to oxidative and endoplasmic reticulum (ER) stress in plaque macrophages, and disruption of this process through genetic deficiency in the autophagy-related gene 5 (Atg5) increased plaque macrophage apoptosis and necrosis. Additionally, apoptotic Atg5-deficient macrophages were less efficiently efferocytosed by viable macrophages [71]. These findings suggest that macrophage autophagy plays a protective role in advanced atherosclerosis.
Microenvironmental determinants of macrophage function in atherosclerosis
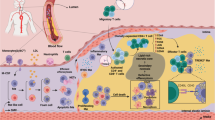
In lesions, macrophages are exposed to a vast variety of stimuli that can profoundly impact on their phenotype. In response to microenvironmental factors, several intracellular pathways involving signaling hubs such as, e.g., nuclear factor “kappa-light-chain-enhancer” of activated B cells (NF-κB), various signal transducer and activation of transcription (STAT) transcription factors or nuclear receptors, notably of the peroxisome-proliferator activating receptor (PPAR) family, are activated and control macrophage phenotype [29]. To illustrate the complexity of the plaque microenvironment and of the determinants of lesional macrophage phenotype, we here provide a brief and non-exhaustive overview of some of the major cues governing macrophage function in atherosclerosis (Fig. 1 ).
Nonexhaustive overview of microenvironmental and intrinsic modulators of atherosclerotic plaque macrophage phenotype. In lesions, macrophages are exposed to a variety of microenvironmental signals that either promote a pathogenic phenotype (in red), or in contrast attenuate pro-atherogenic features (in green). The exact influence of some microenvironmental signals (e.g., hypoxia) is still poorly defined. Abbreviations: ABCA1/G1 ATP binding cassette transporter A1/G1, ATP adenosine triphosphate, CHOP C/EBP homologous protein, CLEC4e C type lectin domain family 4 member E, dsDNA double-stranded deoxyribonucleic acid, FABP4 fatty acid binding protein 4, Neutro neutrophils, NETs neutrophil extracellular traps, eRNA extracellular ribonucleic acid, HDL high-density lipoprotein, HSPs heat-shock proteins, IFN interferon, IL interleukin, NF-ϰB nuclear factor “kappa-light-chain-enhancer” of activated B cells, NLRP3, NACHT, LRR and PYD domain-containing protein 3, OxLDL oxidized low-density lipoprotein, PKC protein kinase C, PS phosphatidylserine, ROS reactive oxygen species, TGFβ transforming growth factor-β, TLR Toll-like receptor, ER stress endoplasmic reticulum stress, TNFα tumor necrosis factor-α, UPR unfolded protein response
Lipids
Within lesions, macrophages are exposed to native and modified lipoproteins and accumulate intracellular lipids. Efferocytosis of apoptotic cells can also contribute to macrophage accumulation of intracellular lipids. How this affects macrophage phenotype is still unclear [122]. Lipid loading itself was unexpectedly shown to inhibit, rather than to activate inflammatory gene expression in peritoneal macrophage foam cells of Ldlr −/− mice fed a high-fat diet, a process that depended on accumulation of the lipid intermediate desmosterol and activation of LXR target gene expression [115]. This suggests that the plaque microenvironment, rather than lipid loading itself, controls proinflammatory functions of lesional macrophages. On the other hand, excess intracellular cholesterol accumulation has toxic effects in macrophages through activation of endoplasmic reticulum (ER) stress, which also has proinflammatory effects (see below) [41]. Fatty acid accumulation in macrophages can also promote a proinflammatory macrophage phenotype through activation of the fatty acid binding protein (FABP)-4 [74].
Oxidized phospholipids and lipoproteins (ox-LDL) promote macrophage inflammation [68], notably through the scavenger receptor CD36, which induces proinflammatory effects through promotion of the formation of a Toll-like receptor (TLR)-4/TLR-6 heterodimer upon ox-LDL binding [116].
Recently, it was proposed that HDL, which is essential in macrophage reverse cholesterol transport and generally considered atheroprotective, could induce a proinflammatory phenotype in macrophages, with increased secretion of IL-12, IL-1β, and TNFα and reduced IL-10 production. This study proposed that HDL-dependent passive cholesterol efflux led to depletion of membrane cholesterol and disruption of lipid rafts, leading to activation of several protein kinase C isoforms and proinflammatory effects mediated by NF-κB and STAT-1 signaling [128]. In contrast, ABCA1- and ABCG1-dependent active cholesterol efflux to HDL is thought to have anti-inflammatory effects in macrophages, as ABCA1/G1 double-deficient lesional macrophages display a clearly proinflammatory profile [137].
A family of anti-inflammatory, pro-resolving lipid mediators has a crucial role in the resolution of inflammation particularly through promotion of macrophage efferocytosis, suppression of proinflammatory, and activation of anti-inflammatory gene expression [108]. The concentration of two of these pro-resolving mediators, Maresin 1 and Resolvin D2, was recently found to decrease in vascular tissue during plaque progression, and their therapeutic delivery favored an antiatherogenic macrophage phenotype and decreased lesion formation [132].
Cholesterol crystals
In lesions, cholesterol crystals can form both intra- and extracellularly, and cholesterol crystal deposits can be detected even in early atherosclerotic lesions [33]. Importantly, cholesterol crystals can activate the NLRP-3 inflammasome, which converts the proinflammatory cytokines IL-1β and IL-18 to their mature and secretable form, and cholesterol crystal-mediated activation of the NLRP-3 inflammasome has been suggested to be essential in atherogenesis [33]. Formation of cholesterol crystals in macrophages and subsequent NLRP-3 inflammasome activation depends on CD36 [110]. Besides their direct proinflammatory effects in macrophages, cholesterol crystals also induce formation of extracellular traps (NETs) by neutrophils. NETs, in turn, prime macrophages for proinflammatory cytokine production, notably IL-1α and IL-1β, at the transcriptional level, disclosing cholesterol crystals not only as a direct inducer of macrophage inflammation but also as an indirect priming signal through NETosis [135].
Hypoxia
In advanced lesions, growing distance from the vessel lumen to the plaque core and increased metabolic demand creates areas of hypoxia. Hypoxia induces stabilization of the oxygen-sensing transcription factor hypoxia-inducible factor-1α (HIF1α), a process that can be further substantiated by inflammatory signaling pathways such as NF-κB [85, 96]. Although the critical role of HIF1α in macrophage function is well established [31, 85], contradictory findings on its effects in atherosclerosis have been obtained. Reduced atherosclerosis in LysM cre Hif1a flox mice with myeloid cell-specific HIF1α deficiency was reported after 16 weeks of high-cholesterol diet, and HIF1α-deficient macrophages showed decreased apoptosis and inflammatory cytokine expression in vitro [1]. Another study showed no differences in early lesion formation in the same model at 6 weeks of high-fat diet feeding [21]. In contrast, increased early lesion formation and IL-12 expression by antigen-presenting cells were evidenced in CD11c cre Hif1a flox mice where Cre-mediated recombination occurs not only in dendritic cells but also in some macrophages and T cells [2, 21]. Given that Cre expression driven by the LysM promoter is not macrophage specific, and that not all macrophages may be affected in CD11c cre Hif1a flox mice, these results are altogether difficult to interpret. One could speculate that HIF1α differentially affects distinct macrophage subsets and has disease stage-specific functions. Hif1α activation can also induce expression of netrin-1 and Unc5b in lesional macrophages [94], a process that could promote their retention in lesions [129], although this has yet to be addressed experimentally.
Endoplasmic reticulum stress and the unfolded protein response
In lesions, several factors (e.g., lipid loading, metabolic stress, inflammatory signaling) can induce an impairment of the endoplasmic reticulum (ER) function in macrophages, leading to activation of the unfolded protein response (UPR) [56]. The UPR/ER stress response appears to have a crucial role in the control of macrophage apoptosis in lesions (for detailed review about the role of ER stress and UPR in atherosclerosis, we refer the reader to references [56, 58]). Indeed, macrophage cholesterol loading induces ER stress and triggers cell death through a mechanism depending on the transcription factor CHOP, a major effector of the UPR [41]. CHOP deficiency drastically reduced apoptotic cell accumulation and necrotic core formation in advanced lesions in Ldlr −/− and Apoe −/− mice [127]. Other effector proteins of the UPR such as XBP1 or IRE1α may also have a substantial role in the control of lesional macrophage phenotype [26, 56].
Necrotic cells
During atherosclerotic plaque progression towards advanced and unstable phenotypes, accumulation of apoptotic cells is thought to eventually overcome macrophage efferocytic ability, leading to local secondary necrosis of plaque cells and formation of the necrotic core. In contrast to apoptotic cells, recognition and engulfment of necrotic cells elicit proinflammatory responses in macrophages [99]. Loss of cell membrane integrity allows leakage of intracellular molecules that are recognized by macrophages as damage-associated molecular patterns (DAMPs) such as double-stranded DNA (dsDNA), RNA, heat shock proteins (HSPs), or nucleotides (e.g., ATP) [100]. Notably, HSPs and dsDNA promote macrophage inflammation via Toll-like receptors (TLRs) [142], and extracellular RNA was recently shown to promote atherosclerosis, presumably in part through induction of proinflammatory cytokine secretion (e.g., TNFα, IL-1β) by macrophages [113]. A recent study demonstrated a critical role of the necrotic cell sensor C type lectin receptor 4e (CLEC4e) in the control of lesional macrophage phenotype [26]. In vitro, activation of CLEC4e increased macrophage lipid accumulation by reducing Abca1 and Abcg1 expression and inhibiting reverse cholesterol transport, without affecting ox-LDL uptake. CLEC4e signaling also activated the UPR in macrophages in vitro, with increased expression of the UPR/ER stress-associated proteins inositol-requiring enzyme-1α (Ire1α) and Chop and splicing of Xbp1 mRNA. Although the role of the UPR/ER stress response and particularly of CHOP in lesional macrophage apoptosis is well established [41, 127], as previously mentioned, Clec4e signaling was not associated with increased macrophage cell death, but instead promoted a proinflammatory macrophage phenotype and macrophage proliferation through upregulation of macrophage colony-stimulating factor (CSF1). Accordingly, Ldlr −/− mice with CLEC4e-deficient bone marrow cells showed decreased atherosclerosis associated with reduced lesional macrophage content and proliferation, lower plaque lipid accumulation, and diminished inflammatory cytokine expression [26].
Growth factors, proinflammatory and anti-inflammatory cytokines, and interaction with other immune cells
In lesions, macrophages are exposed to growth factors such as granulocyte-macrophage colony-stimulating factor (CSF2) or CSF1 that control macrophage survival, proliferation, and various aspects of their function [26, 32]. CSF2 was also shown to indirectly induce plaque macrophage apoptosis through upregulation of IL-23 expression [117]. The plaque microenvironment also contains a plethora of pro- and anti-inflammatory cytokines that impact macrophage phenotype [6, 124]. Cytokines such as IL-1β, TNFα, or IFNγ promote acquisition of a pro-atherogenic macrophage phenotype with increased expression of proinflammatory cytokines and chemokines, proteases, and can in addition also affect macrophage lipid metabolism [93]. Type I interferons seem to particularly influence foam cell formation, as IFN-α promotes lipid uptake [70] while IFN-β both increases uptake and reduces efflux of cholesterol in macrophages [15]. In contrast, IL-10 confers anti-atherogenic properties to macrophage by reducing their expression of inflammatory cytokines and proteases and a modulation of the lipid metabolism [54]. The anti-atherogenic cytokine IL-33 limits foam cell formation by reducing macrophage lipid uptake and increasing reverse cholesterol transport [77]. Likewise, IL-13 has been shown to promote an anti-atherogenic plaque phenotype [19].
In line, interaction with other cytokine-producing immune cell types shapes lesional macrophage phenotype. CD4+ T cells primed by antigen presenting cells in atherosclerotic vessels express IFNγ and TNFα and promote modified LDL uptake by lesional macrophages, as demonstrated in an ex vivo aortic explant model [64]. Co-culture with activated CD8+ T cells promotes secretion of CCL2 and CXCL1 by macrophages, and aortas from atherosclerotic mice display reduced Ccl2 and Cxcl1 mRNA levels after CD8+ T cell depletion [28]. CD8+ T cell-derived TNFα was also proposed to potentiate macrophage-mediated inflammation in lesions, and CD8+ T cell production of cytotoxic enzymes granzyme-B and perforin trigger macrophage cell death in lesions [66], indicating a potential role of CD8+ T cells in the control of plaque macrophage phenotype.
Other microenvironmental and intrinsic factors
In lesions, macrophages are exposed to reactive oxygen species (ROS) produced by other vascular cells such as endothelial cells and VSMCs that may impact their phenotype. ROS production can be activated in macrophages in response to modified LDL and is thought to have proinflammatory effects, as demonstrated in macrophages deficient for the NADPH-oxidase component Nox2 [13]. ROS also promote cellular senescence [134]. Exposure to hemostatic factors in lesions can also impact macrophage phenotype [62], and we could for example recently demonstrate that coagulation factor XII induced a proinflammatory macrophage phenotype and promoted lesion formation [133]. Epigenetic regulation of gene expression plays a role in defining macrophage phenotype in lesions, and changes in acetylation or methylation of specific histones associated with plaque progression have been observed in human lesion macrophages [52]. How these epigenetic marks are acquired and how they influence gene expression and macrophage function remain to be determined. Non-coding RNAs such as microRNAs [60] or long-noncoding RNAs [55] may also affect gene expression and macrophage function in atherosclerosis.
Some studies indicate that macrophage phenotype could be in part predetermined before their precursors reach atherosclerotic lesions. In particular, it has been demonstrated that hypercholesterolemia leads to hematopoietic stem cell priming in the bone marrow towards a proinflammatory phenotype [106]. In a model of gastrointestinal infection, resident bone marrow natural killer cells were shown to educate monocytes during medullar development and to prime them towards regulatory functions [9]. As developing and mature monocytes are in contact with a plethora of other immune cells in the bone marrow and spleen, one could speculate that this concept of monocyte pre-education at their production sites may be relevant in atherosclerosis.
Atherosclerotic lesion macrophage identity: moving beyond the polarization paradigm
Limits of the M1/M2 paradigm and its variations
It has long been recognized that macrophages can adopt a wide range of phenotypes in response to specific stimuli. A commonly used classification of macrophage phenotypes relies on the in vitro defined M1/M2 paradigm and its variants. Macrophages stimulated with IFNγ and LPS adopt a M1 phenotype, characterized by high expression of proinflammatory cytokines and chemokines, expression of inducible nitric oxide synthase (iNOS), and production of ROS, associated with pathogen destruction [76, 80, 111]. In contrast, macrophages exposed to the Th2 cytokines IL-4 and IL-13 adopt an anti-inflammatory M2 phenotype and secrete IL-10 and TGFβ [76, 80, 111]. Using various stimulation cocktails, variants of the M2 phenotype (M2a, M2b, M2c) have further been described [76, 80, 111].
The M1/M2 polarization states were defined in specific conditions in vitro and represent extreme, artificial ends of a spectrum of macrophage phenotypes. However, the M1/M2 classification is commonly used to attempt defining macrophage phenotype in in vivo contexts. For example, expression of genes associated with M1/M2 polarization (e.g., Arginase-1, CD206, iNOS) is employed to infer macrophage function in disease models in vivo. This represents at best an approximation but could potentially be misleading: recently, in an obesity model, adipose tissue macrophages expressing the M2 marker CD301b were shown to induce weight gain and glucose intolerance, which is in contrast to the predicted beneficial role of M2-polarized macrophages in this setting [61, 65]. Although prototypical M1/M2 phenotypes associated with the expression of respective markers can be faithfully recapitulated in specific contexts in vivo (e.g., allergy or parasitic infection), the M1/M2 paradigm cannot be readily transposed to any disease setting where macrophages are exposed to a more complex microenvironment [76, 111]. In light of the complexity of the atherosclerotic plaque environment (Fig. 1 ), the M1/M2 paradigm is unlikely to embrace the complexity of macrophage phenotypic heterogeneity in atherosclerosis.
Despite these widely recognized limitations, the M1/M2 paradigm is nevertheless ubiquitously used in the atherosclerosis research literature, and analysis of M1/M2-associated gene expression is a request that often arises during manuscript peer review. Researchers attempting to decipher macrophage functional heterogeneity in atherosclerosis and apprehend its role in disease have elaborated on the M1/M2 paradigm and proposed several macrophage polarization states induced by specific stimuli (e.g., M4, induced by CXCL4; Mheme, induced by Heme; Mox, induced by oxidized phospholipids) presumably associated with particular functions relevant to atherosclerosis [24, 27]. Although this approach has some merits as it attempts to integrate the role of plaque-specific stimuli in the control of lesional macrophage function, it still appears drastically reductive when considering the variety and complexity of plaque macrophage phenotypic determinants.
In line, recent articles have questioned the use of a rigid nomenclature to classify macrophage subsets in the context of cardiovascular diseases and atherosclerosis. Tabas and Bornfeldt proposed “that the phenotype of lesional macrophages cannot be classified into predetermined subsets but rather is a consequence of the lesional microenvironment and the activation of specific intracellular signaling pathways” [122]. Nahrendorf and Swirski argued that the M1/M2 paradigm constitutes a hurdle in deepening our understanding of macrophage function and should be abandoned in favor of a function-focused identification of macrophage phenotypes [82]. As true functional features of macrophages dictated by the plaque environment, rather than their affiliation to arbitrarily defined subsets, determines their contribution to atherosclerosis, we fully concur with these views. For a better understanding of their role in various stages of atherosclerosis, defining macrophages based on their origin and a set of disease-relevant functions would be much more informative than assigning them a putative polarized state based on very limited information (e.g., expression of a set of predefined markers). Efforts aiming at establishing a unifying nomenclature for macrophage activation and polarization have been made, and it was proposed to define macrophages based on their source, activators, and a consensus collection of markers [80]. Although this is convenient to implement in in vitro experiments, such an approach may not be readily applicable to lesional macrophages in vivo, as the definition of micro-environmental activators may be close to impossible and no consensus has yet been established for plaque macrophage subsets and their markers. Nevertheless, it now appears technically feasible to elaborate on a similar idea and to attempt defining lesional macrophages based on their origin, activation of intracellular signaling pathways, and functional features.
Methodological limitations and future avenues
To date, the most commonly used methodological tools unfortunately fail to embrace the full spectrum of macrophage functional heterogeneity in vivo. Use of arbitrarily defined sets of markers associated with in vitro macrophage-polarized states in flow cytometric or immunohistochemical analyses of lesion macrophages does not allow deducing functional features. Transcriptomics analyses of bulk lesion macrophage populations, even if presorted according to putative subset markers, produce averaging effects that likely mask discrete, intermediate phenotypes, and it is likely that proposed lesional macrophage subsets such as M4 or Mox actually encompass macrophages with diverse phenotypes. Recent technological advances may provide researchers with better tools to investigate macrophage functional heterogeneity in atherosclerosis and its impact on disease. For example, recent genetic fate-mapping or parabiosis models allow discriminating lesion macrophages and macrophage-like cells of different origin [35, 40, 98, 109]. High-dimensional flow cytometry analyses and mass cytometry could be used to simultaneously analyze an increasingly growing number of cell surface and intracellular markers in lesion macrophages [53, 103]. This would for example allow analyzing expression of functionally relevant cell surface proteins such as ABCA1, MER-TK, SIRPα, CD36, MSR1, and others in flow cytometric analyses of macrophages directly extracted from the lesion environment, and could help identify and sort more precisely defined macrophage subsets using a wide array of markers, some of which likely remain to be identified. Single-cell transcriptomics approaches such as single-cell RNA-sequencing are able to identify cells endowed with distinct phenotypes within seemingly homogenous immune cell populations and have for example revealed phenotypes in Th17 T cells ranging from regulatory to highly pathogenic in experimental autoimmune encephalomyelitis [45]. This individual cell approach could represent a powerful tool to analyze macrophage phenotype in atherosclerosis while avoiding bias associated with arbitrary predefinition of cell subsets. Although these methods are still costly and require a significant level of technical expertise that may preclude their routine use in many laboratories, the higher resolution that they offer may be a way to dwell deeper into lesional macrophage biology in the future.
Understanding the complex biology and function of macrophages in vascular inflammation and atherosclerosis constitutes as prerequisite for the development of novel therapeutic approaches. Precisely identifying the mechanisms of macrophage accumulation and functional regulation in lesions could pave the way towards the development of original strategies targeting these processes and limiting lesion formation or progression towards a vulnerable phenotype. This could also facilitate the development of novel imaging strategies [34] or the emergence of new biomarkers [125] to help evaluate plaque phenotype and the associated atherothrombotic risk in patients.
Conclusion
Recent research has revealed an unexpected complexity in the mechanisms of macrophage accumulation in atherosclerosis, protective and pathogenic functions performed by macrophages in lesions, and how these functions are regulated. Major recent conceptual shifts and challenges to long-standing paradigms highlight the need to constantly reevaluate how we apprehend research on the role of macrophages in atherosclerotic disease. In the near future, higher resolution analysis of macrophage phenotype in atherosclerotic lesions through novel methods will allow researchers in the field to establish more precise macrophage “identity cards” taking into account their origin and disease-relevant functional features. This will hopefully pave the way for the development of therapeutic strategies targeting macrophages to reduce the disease burden caused by atherosclerosis and associated pathologies.
References
Aarup A, Pedersen TX, Junker N, Christoffersen C, Bartels ED, Madsen M, Nielsen CH, Nielsen LB (2016) Hypoxia-inducible factor-1alpha expression in macrophages promotes development of atherosclerosis. Arterioscler Thromb Vasc Biol 36:1782–1790. doi:10.1161/ATVBAHA.116.307830
Abram CL, Roberge GL, Hu Y, Lowell CA (2014) Comparative analysis of the efficiency and specificity of myeloid-Cre deleting strains using ROSA-EYFP reporter mice. J Immunol Methods 408:89–100. doi:10.1016/j.jim.2014.05.009
Aiello RJ, Bourassa PA, Lindsey S, Weng W, Natoli E, Rollins BJ, Milos PM (1999) Monocyte chemoattractant protein-1 accelerates atherosclerosis in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol 19:1518–1525
Ait-Oufella H, Kinugawa K, Zoll J, Simon T, Boddaert J, Heeneman S, Blanc-Brude O, Barateau V, Potteaux S, Merval R, Esposito B, Teissier E, Daemen MJ, Leseche G, Boulanger C, Tedgui A, Mallat Z (2007) Lactadherin deficiency leads to apoptotic cell accumulation and accelerated atherosclerosis in mice. Circulation 115:2168–2177. doi:10.1161/CIRCULATIONAHA.106.662080
Ait-Oufella H, Pouresmail V, Simon T, Blanc-Brude O, Kinugawa K, Merval R, Offenstadt G, Leseche G, Cohen PL, Tedgui A, Mallat Z (2008) Defective mer receptor tyrosine kinase signaling in bone marrow cells promotes apoptotic cell accumulation and accelerates atherosclerosis. Arterioscler Thromb Vasc Biol 28:1429–1431. doi:10.1161/ATVBAHA.108.169078
Ait-Oufella H, Taleb S, Mallat Z, Tedgui A (2011) Recent advances on the role of cytokines in atherosclerosis. Arterioscler Thromb Vasc Biol 31:969–979. doi:10.1161/ATVBAHA.110.207415
Allahverdian S, Chehroudi AC, McManus BM, Abraham T, Francis GA (2014) Contribution of intimal smooth muscle cells to cholesterol accumulation and macrophage-like cells in human atherosclerosis. Circulation 129:1551–1559. doi:10.1161/CIRCULATIONAHA.113.005015
Andreeva ER, Pugach IM, Orekhov AN (1997) Subendothelial smooth muscle cells of human aorta express macrophage antigen in situ and in vitro. Atherosclerosis 135:19–27
Askenase MH, Han SJ, Byrd AL, Morais da Fonseca D, Bouladoux N, Wilhelm C, Konkel JE, Hand TW, Lacerda-Queiroz N, Su XZ, Trinchieri G, Grainger JR, Belkaid Y (2015) Bone-marrow-resident NK cells prime monocytes for regulatory function during infection. Immunity 42:1130–1142. doi:10.1016/j.immuni.2015.05.011
Babaev VR, Gleaves LA, Carter KJ, Suzuki H, Kodama T, Fazio S, Linton MF (2000) Reduced atherosclerotic lesions in mice deficient for total or macrophage-specific expression of scavenger receptor-a. Arterioscler Thromb Vasc Biol 20:2593–2599
Babaev VR, Ding L, Zhang Y, May JM, Lin PC, Fazio S, Linton MF (2016a) Macrophage IKKalpha deficiency suppresses Akt phosphorylation, reduces cell survival, and decreases early atherosclerosis. Arterioscler Thromb Vasc Biol 36:598–607. doi:10.1161/ATVBAHA.115.306931
Babaev VR, Yeung M, Erbay E, Ding L, Zhang Y, May JM, Fazio S, Hotamisligil GS, Linton MF (2016b) Jnk1 deficiency in hematopoietic cells suppresses macrophage apoptosis and increases atherosclerosis in low-density lipoprotein receptor null mice. Arterioscler Thromb Vasc Biol 36:1122–1131. doi:10.1161/ATVBAHA.116.307580
Bae YS, Lee JH, Choi SH, Kim S, Almazan F, Witztum JL, Miller YI (2009) Macrophages generate reactive oxygen species in response to minimally oxidized low-density lipoprotein: toll-like receptor 4- and spleen tyrosine kinase-dependent activation of NADPH oxidase 2. Circ Res 104:210–218 . doi:10.1161/CIRCRESAHA.108.181040221p following 218
Bhatia VK, Yun S, Leung V, Grimsditch DC, Benson GM, Botto MB, Boyle JJ, Haskard DO (2007) Complement C1q reduces early atherosclerosis in low-density lipoprotein receptor-deficient mice. Am J Pathol 170:416–426. doi:10.2353/ajpath.2007.060406
Boshuizen MC, Hoeksema MA, Neele AE, van der Velden S, Hamers AA, Van den Bossche J, Lutgens E, de Winther MP (2016) Interferon-beta promotes macrophage foam cell formation by altering both cholesterol influx and efflux mechanisms. Cytokine 77:220–226. doi:10.1016/j.cyto.2015.09.016
Bouchareychas L, Pirault J, Saint-Charles F, Deswaerte V, Le Roy T, Jessup W, Giral P, Le Goff W, Huby T, Gautier EL, Lesnik P (2015) Promoting macrophage survival delays progression of pre-existing atherosclerotic lesions through macrophage-derived apoE. Cardiovasc Res 108:111–123. doi:10.1093/cvr/cvv177
Bradfield PF, Menon A, Miljkovic-Licina M, Lee BP, Fischer N, Fish RJ, Kwak B, Fisher EA, Imhof BA (2016) Divergent JAM-C expression accelerates monocyte-derived cell exit from atherosclerotic plaques. PLoS One 11:e0159679. doi:10.1371/journal.pone.0159679
Cai B, Thorp EB, Doran AC, Sansbury BE, Daemen MJ, Dorweiler B, Spite M, Fredman G, Tabas I (2017) MerTK receptor cleavage promotes plaque necrosis and defective resolution in atherosclerosis. J Clin Invest. doi:10.1172/JCI90520
Cardilo-Reis L, Gruber S, Schreier SM, Drechsler M, Papac-Milicevic N, Weber C, Wagner O, Stangl H, Soehnlein O, Binder CJ (2012) Interleukin-13 protects from atherosclerosis and modulates plaque composition by skewing the macrophage phenotype. EMBO Mol Med 4:1072–1086. doi:10.1002/emmm.201201374
Chappell J, Harman JL, Narasimhan VM, Yu H, Foote K, Simons BD, Bennett MR, Jorgensen HF (2016) Extensive proliferation of a subset of differentiated, yet plastic, medial vascular smooth muscle cells contribute to Neointimal formation in mouse injury and atherosclerosis models. Circ Res. doi:10.1161/CIRCRESAHA.116.309799
Chaudhari SM, Sluimer JC, Koch M, Theelen TL, Manthey HD, Busch M, Caballero-Franco C, Vogel F, Cochain C, Pelisek J, Daemen MJ, Lutz MB, Gorlach A, Kissler S, Hermanns HM, Zernecke A (2015) Deficiency of HIF1alpha in antigen-presenting cells aggravates atherosclerosis and type 1 T-helper cell responses in mice. Arterioscler Thromb Vasc Biol 35:2316–2325. doi:10.1161/ATVBAHA.115.306171
Childs BG, Durik M, Baker DJ, van Deursen JM (2015) Cellular senescence in aging and age-related disease: from mechanisms to therapy. Nat Med 21:1424–1435. doi:10.1038/nm.4000
Childs BG, Baker DJ, Wijshake T, Conover CA, Campisi J, van Deursen JM (2016) Senescent intimal foam cells are deleterious at all stages of atherosclerosis. Science 354:472–477. doi:10.1126/science.aaf6659
Chinetti-Gbaguidi G, Colin S, Staels B (2015) Macrophage subsets in atherosclerosis. Nat Rev Cardiol 12:10–17. doi:10.1038/nrcardio.2014.173
Chung EY, Kim SJ, Ma XJ (2006) Regulation of cytokine production during phagocytosis of apoptotic cells. Cell Res 16:154–161. doi:10.1038/sj.cr.7310021
Clement M, Basatemur G, Masters L, Baker L, Bruneval P, Iwawaki T, Kneilling M, Yamasaki S, Goodall J, Mallat Z (2016) Necrotic cell sensor Clec4e promotes a Proatherogenic macrophage phenotype through activation of the unfolded protein response. Circulation 134:1039–1051. doi:10.1161/CIRCULATIONAHA.116.022668
Cochain C, Zernecke A (2015) Macrophages and immune cells in atherosclerosis: recent advances and novel concepts. Basic Res Cardiol 110:34. doi:10.1007/s00395-015-0491-8
Cochain C, Koch M, Chaudhari SM, Busch M, Pelisek J, Boon L, Zernecke A (2015) CD8+ T cells regulate Monopoiesis and circulating Ly6C-high monocyte levels in atherosclerosis in mice. Circ Res 117:244–253. doi:10.1161/CIRCRESAHA.117.304611
Colin S, Chinetti-Gbaguidi G, Staels B (2014) Macrophage phenotypes in atherosclerosis. Immunol Rev 262:153–166. doi:10.1111/imr.12218
Combadiere C, Potteaux S, Rodero M, Simon T, Pezard A, Esposito B, Merval R, Proudfoot A, Tedgui A, Mallat Z (2008) Combined inhibition of CCL2, CX3CR1, and CCR5 abrogates Ly6C(hi) and Ly6C(lo) monocytosis and almost abolishes atherosclerosis in hypercholesterolemic mice. Circulation 117:1649–1657. doi:10.1161/CIRCULATIONAHA.107.745091
Cramer T, Yamanishi Y, Clausen BE, Forster I, Pawlinski R, Mackman N, Haase VH, Jaenisch R, Corr M, Nizet V, Firestein GS, Gerber HP, Ferrara N, Johnson RS (2003) HIF-1alpha is essential for myeloid cell-mediated inflammation. Cell 112:645–657
Di Gregoli K, Johnson JL (2012) Role of colony-stimulating factors in atherosclerosis. Curr Opin Lipidol 23:412–421. doi:10.1097/MOL.0b013e328357ca6e
Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, Abela GS, Franchi L, Nunez G, Schnurr M, Espevik T, Lien E, Fitzgerald KA, Rock KL, Moore KJ, Wright SD, Hornung V, Latz E (2010) NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature 464:1357–1361. doi:10.1038/nature08938
Dweck MR, Aikawa E, Newby DE, Tarkin JM, Rudd JH, Narula J, Fayad ZA (2016) Noninvasive molecular imaging of disease activity in atherosclerosis. Circ Res 119:330–340. doi:10.1161/CIRCRESAHA.116.307971
Ensan S, Li A, Besla R, Degousee N, Cosme J, Roufaiel M, Shikatani EA, El-Maklizi M, Williams JW, Robins L, Li C, Lewis B, Yun TJ, Lee JS, Wieghofer P, Khattar R, Farrokhi K, Byrne J, Ouzounian M, Zavitz CC, Levy GA, Bauer CM, Libby P, Husain M, Swirski FK, Cheong C, Prinz M, Hilgendorf I, Randolph GJ, Epelman S, Gramolini AO, Cybulsky MI, Rubin BB, Robbins CS (2016) Self-renewing resident arterial macrophages arise from embryonic CX3CR1(+) precursors and circulating monocytes immediately after birth. Nat Immunol 17:159–168. doi:10.1038/ni.3343
Febbraio M, Podrez EA, Smith JD, Hajjar DP, Hazen SL, Hoff HF, Sharma K, Silverstein RL (2000) Targeted disruption of the class B scavenger receptor CD36 protects against atherosclerotic lesion development in mice. J Clin Invest 105:1049–1056. doi:10.1172/JCI9259
Febbraio M, Guy E, Silverstein RL (2004) Stem cell transplantation reveals that absence of macrophage CD36 is protective against atherosclerosis. Arterioscler Thromb Vasc Biol 24:2333–2338. doi:10.1161/01.ATV.0000148007.06370.68
Feig JE, Pineda-Torra I, Sanson M, Bradley MN, Vengrenyuk Y, Bogunovic D, Gautier EL, Rubinstein D, Hong C, Liu J, Wu C, van Rooijen N, Bhardwaj N, Garabedian M, Tontonoz P, Fisher EA (2010) LXR promotes the maximal egress of monocyte-derived cells from mouse aortic plaques during atherosclerosis regression. J Clin Invest 120:4415–4424. doi:10.1172/JCI38911
Feig JE, Parathath S, Rong JX, Mick SL, Vengrenyuk Y, Grauer L, Young SG, Fisher EA (2011) Reversal of hyperlipidemia with a genetic switch favorably affects the content and inflammatory state of macrophages in atherosclerotic plaques. Circulation 123:989–998. doi:10.1161/CIRCULATIONAHA.110.984146
Feil S, Fehrenbacher B, Lukowski R, Essmann F, Schulze-Osthoff K, Schaller M, Feil R (2014) Transdifferentiation of vascular smooth muscle cells to macrophage-like cells during atherogenesis. Circ Res 115:662–667. doi:10.1161/CIRCRESAHA.115.304634
Feng B, Yao PM, Li Y, Devlin CM, Zhang D, Harding HP, Sweeney M, Rong JX, Kuriakose G, Fisher EA, Marks AR, Ron D, Tabas I (2003) The endoplasmic reticulum is the site of cholesterol-induced cytotoxicity in macrophages. Nat Cell Biol 5:781–792. doi:10.1038/ncb1035
Fisher EA (2016) Regression of atherosclerosis: the journey from the liver to the plaque and back. Arterioscler Thromb Vasc Biol 36:226–235. doi:10.1161/ATVBAHA.115.301926
Foks AC, Engelbertsen D, Kuperwaser F, Alberts-Grill N, Gonen A, Witztum JL, Lederer J, Jarolim P, DeKruyff RH, Freeman GJ, Lichtman AH (2016) Blockade of Tim-1 and Tim-4 enhances atherosclerosis in low-density lipoprotein receptor-deficient mice. Arterioscler Thromb Vasc Biol 36:456–465. doi:10.1161/ATVBAHA.115.306860
Freigang S, Ampenberger F, Weiss A, Kanneganti TD, Iwakura Y, Hersberger M, Kopf M (2013) Fatty acid-induced mitochondrial uncoupling elicits inflammasome-independent IL-1alpha and sterile vascular inflammation in atherosclerosis. Nat Immunol 14:1045–1053. doi:10.1038/ni.2704
Gaublomme JT, Yosef N, Lee Y, Gertner RS, Yang LV, Wu C, Pandolfi PP, Mak T, Satija R, Shalek AK, Kuchroo VK, Park H, Regev A (2015) Single-cell genomics unveils critical regulators of Th17 cell pathogenicity. Cell 163:1400–1412. doi:10.1016/j.cell.2015.11.009
Gerhardt T, Ley K (2015) Monocyte trafficking across the vessel wall. Cardiovasc Res 107:321–330. doi:10.1093/cvr/cvv147
Ginhoux F, Guilliams M (2016) Tissue-resident macrophage ontogeny and homeostasis. Immunity 44:439–449. doi:10.1016/j.immuni.2016.02.024
Gonzalez-Navarro H, Abu Nabah YN, Vinue A, Andres-Manzano MJ, Collado M, Serrano M, Andres V (2010) p19(ARF) deficiency reduces macrophage and vascular smooth muscle cell apoptosis and aggravates atherosclerosis. J Am Coll Cardiol 55:2258–2268. doi:10.1016/j.jacc.2010.01.026
Gordon D, Reidy MA, Benditt EP, Schwartz SM (1990) Cell proliferation in human coronary arteries. Proc Natl Acad Sci U S A 87:4600–4604
Gough PJ, Gomez IG, Wille PT, Raines EW (2006) Macrophage expression of active MMP-9 induces acute plaque disruption in apoE-deficient mice. J Clin Invest 116:59–69. doi:10.1172/JCI25074
Green DR, Oguin TH, Martinez J (2016) The clearance of dying cells: table for two. Cell Death Differ 23:915–926. doi:10.1038/cdd.2015.172
Greissel A, Culmes M, Burgkart R, Zimmermann A, Eckstein HH, Zernecke A, Pelisek J (2016) Histone acetylation and methylation significantly change with severity of atherosclerosis in human carotid plaques. Cardiovasc Pathol : Off J Soc Cardiovasc Pathol 25:79–86. doi:10.1016/j.carpath.2015.11.001
Guilliams M, Dutertre CA, Scott CL, McGovern N, Sichien D, Chakarov S, Van Gassen S, Chen J, Poidinger M, De Prijck S, Tavernier SJ, Low I, Irac SE, Mattar CN, Sumatoh HR, Low GH, Chung TJ, Chan DK, Tan KK, Hon TL, Fossum E, Bogen B, Choolani M, Chan JK, Larbi A, Luche H, Henri S, Saeys Y, Newell EW, Lambrecht BN, Malissen B, Ginhoux F (2016) Unsupervised high-dimensional analysis aligns dendritic cells across tissues and species. Immunity 45:669–684. doi:10.1016/j.immuni.2016.08.015
Han X, Boisvert WA (2015) Interleukin-10 protects against atherosclerosis by modulating multiple atherogenic macrophage function. Thromb Haemost 113:505–512. doi:10.1160/TH14-06-0509
Holdt LM, Stahringer A, Sass K, Pichler G, Kulak NA, Wilfert W, Kohlmaier A, Herbst A, Northoff BH, Nicolaou A, Gabel G, Beutner F, Scholz M, Thiery J, Musunuru K, Krohn K, Mann M, Teupser D (2016) Circular non-coding RNA ANRIL modulates ribosomal RNA maturation and atherosclerosis in humans. Nat Commun 7:12429. doi:10.1038/ncomms12429
Hotamisligil GS (2010) Endoplasmic reticulum stress and atherosclerosis. Nat Med 16:396–399. doi:10.1038/nm0410-396
Im SS, Osborne TF (2011) Liver x receptors in atherosclerosis and inflammation. Circ Res 108:996–1001. doi:10.1161/CIRCRESAHA.110.226878
Ivanova EA, Orekhov AN (2016) The Role of Endoplasmic Reticulum Stress and Unfolded Protein Response in Atherosclerosis. International journal of molecular sciences 17. doi:10.3390/ijms17020193
Kamari Y, Shaish A, Shemesh S, Vax E, Grosskopf I, Dotan S, White M, Voronov E, Dinarello CA, Apte RN, Harats D (2011) Reduced atherosclerosis and inflammatory cytokines in apolipoprotein-E-deficient mice lacking bone marrow-derived interleukin-1alpha. Biochem Biophys Res Commun 405:197–203. doi:10.1016/j.bbrc.2011.01.008
Karunakaran D, Rayner KJ (2016) Macrophage miRNAs in atherosclerosis. Biochim Biophys Acta 1861:2087–2093. doi:10.1016/j.bbalip.2016.02.006
Knudsen NH, Lee CH (2016) Identity crisis: CD301b(+) mononuclear phagocytes blur the M1-M2 macrophage line. Immunity 45:461–463. doi:10.1016/j.immuni.2016.09.004
Koch M, Zernecke A (2014) The hemostatic system as a regulator of inflammation in atherosclerosis. IUBMB life 66:735–744. doi:10.1002/iub.1333
Kojima Y, Volkmer JP, McKenna K, Civelek M, Lusis AJ, Miller CL, Direnzo D, Nanda V, Ye J, Connolly AJ, Schadt EE, Quertermous T, Betancur P, Maegdefessel L, Matic LP, Hedin U, Weissman IL, Leeper NJ (2016) CD47-blocking antibodies restore phagocytosis and prevent atherosclerosis. Nature 536:86–90. doi:10.1038/nature18935
Koltsova EK, Garcia Z, Chodaczek G, Landau M, McArdle S, Scott SR, von Vietinghoff S, Galkina E, Miller YI, Acton ST, Ley K (2012) Dynamic T cell-APC interactions sustain chronic inflammation in atherosclerosis. J Clin Invest 122:3114–3126. doi:10.1172/JCI61758
Kumamoto Y, Camporez JP, Jurczak MJ, Shanabrough M, Horvath T, Shulman GI, Iwasaki A (2016) CD301b(+) mononuclear phagocytes maintain positive energy balance through secretion of Resistin-like molecule alpha. Immunity 45:583–596. doi:10.1016/j.immuni.2016.08.002
Kyaw T, Winship A, Tay C, Kanellakis P, Hosseini H, Cao A, Li P, Tipping P, Bobik A, Toh BH (2013) Cytotoxic and proinflammatory CD8+ T lymphocytes promote development of vulnerable atherosclerotic plaques in apoE-deficient mice. Circulation 127:1028–1039. doi:10.1161/CIRCULATIONAHA.112.001347
Lee TS, Yen HC, Pan CC, Chau LY (1999) The role of interleukin 12 in the development of atherosclerosis in ApoE-deficient mice. Arterioscler Thromb Vasc Biol 19:734–742
Lee S, Birukov KG, Romanoski CE, Springstead JR, Lusis AJ, Berliner JA (2012) Role of phospholipid oxidation products in atherosclerosis. Circ Res 111:778–799. doi:10.1161/CIRCRESAHA.111.256859
Lhotak S, Gyulay G, Cutz JC, Al-Hashimi A, Trigatti BL, Richards CD, Igdoura SA, Steinberg GR, Bramson J, Ask K, Austin RC (2016) Characterization of proliferating lesion-resident cells during all stages of atherosclerotic growth. J Am Heart Assoc 5. doi:10.1161/JAHA.116.003945
Li J, Fu Q, Cui H, Qu B, Pan W, Shen N, Bao C (2011) Interferon-alpha priming promotes lipid uptake and macrophage-derived foam cell formation: a novel link between interferon-alpha and atherosclerosis in lupus. Arthritis Rheum 63:492–502. doi:10.1002/art.30165
Liao X, Sluimer JC, Wang Y, Subramanian M, Brown K, Pattison JS, Robbins J, Martinez J, Tabas I (2012) Macrophage autophagy plays a protective role in advanced atherosclerosis. Cell Metab 15:545–553. doi:10.1016/j.cmet.2012.01.022
Lillis AP, Muratoglu SC, Au DT, Migliorini M, Lee MJ, Fried SK, Mikhailenko I, Strickland DK (2015) LDL receptor-related protein-1 (LRP1) regulates cholesterol accumulation in macrophages. PLoS One 10:e0128903. doi:10.1371/journal.pone.0128903
Luo Y, Duan H, Qian Y, Feng L, Wu Z, Wang F, Feng J, Yang D, Qin Z, Yan X (2017) Macrophagic CD146 promotes foam cell formation and retention during atherosclerosis. Cell Res. doi:10.1038/cr.2017.8
Makowski L, Boord JB, Maeda K, Babaev VR, Uysal KT, Morgan MA, Parker RA, Suttles J, Fazio S, Hotamisligil GS, Linton MF (2001) Lack of macrophage fatty-acid-binding protein aP2 protects mice deficient in apolipoprotein E against atherosclerosis. Nat Med 7:699–705. doi:10.1038/89076
Mallat Z, Corbaz A, Scoazec A, Besnard S, Leseche G, Chvatchko Y, Tedgui A (2001) Expression of interleukin-18 in human atherosclerotic plaques and relation to plaque instability. Circulation 104:1598–1603
Martinez FO, Gordon S (2014) The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000 Prime Rep 6:13. doi:10.12703/P6-13
McLaren JE, Michael DR, Salter RC, Ashlin TG, Calder CJ, Miller AM, Liew FY, Ramji DP (2010) IL-33 reduces macrophage foam cell formation. J Immunol 185:1222–1229. doi:10.4049/jimmunol.1000520
Mellak S, Ait-Oufella H, Esposito B, Loyer X, Poirier M, Tedder TF, Tedgui A, Mallat Z, Potteaux S (2015) Angiotensin II mobilizes spleen monocytes to promote the development of abdominal aortic aneurysm in apoe−/− mice. Arterioscler Thromb Vasc Biol 35:378–388. doi:10.1161/ATVBAHA.114.304389
Moore KJ, Kunjathoor VV, Koehn SL, Manning JJ, Tseng AA, Silver JM, McKee M, Freeman MW (2005) Loss of receptor-mediated lipid uptake via scavenger receptor a or CD36 pathways does not ameliorate atherosclerosis in hyperlipidemic mice. J Clin Invest 115:2192–2201. doi:10.1172/JCI24061
Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, Gordon S, Hamilton JA, Ivashkiv LB, Lawrence T, Locati M, Mantovani A, Martinez FO, Mege JL, Mosser DM, Natoli G, Saeij JP, Schultze JL, Shirey KA, Sica A, Suttles J, Udalova I, van Ginderachter JA, Vogel SN, Wynn TA (2014) Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity 41:14–20. doi:10.1016/j.immuni.2014.06.008
Nagareddy PR, Murphy AJ, Stirzaker RA, Hu Y, Yu S, Miller RG, Ramkhelawon B, Distel E, Westerterp M, Huang LS, Schmidt AM, Orchard TJ, Fisher EA, Tall AR, Goldberg IJ (2013) Hyperglycemia promotes myelopoiesis and impairs the resolution of atherosclerosis. Cell Metab 17:695–708. doi:10.1016/j.cmet.2013.04.001
Nahrendorf M, Swirski FK (2016) Abandoning M1/M2 for a network model of macrophage function. Circ Res 119:414–417. doi:10.1161/CIRCRESAHA.116.309194
Noelia AG, Bensinger SJ, Hong C, Beceiro S, Bradley MN, Zelcer N, Deniz J, Ramirez C, Diaz M, Gallardo G, de Galarreta CR, Salazar J, Lopez F, Edwards P, Parks J, Andujar M, Tontonoz P, Castrillo A (2009) Apoptotic cells promote their own clearance and immune tolerance through activation of the nuclear receptor LXR. Immunity 31:245–258. doi:10.1016/j.immuni.2009.06.018
Ohta H, Wada H, Niwa T, Kirii H, Iwamoto N, Fujii H, Saito K, Sekikawa K, Seishima M (2005) Disruption of tumor necrosis factor-alpha gene diminishes the development of atherosclerosis in ApoE-deficient mice. Atherosclerosis 180:11–17. doi:10.1016/j.atherosclerosis.2004.11.016
Palazon A, Goldrath AW, Nizet V, Johnson RS (2014) HIF transcription factors, inflammation, and immunity. Immunity 41:518–528. doi:10.1016/j.immuni.2014.09.008
Paulson KE, Zhu SN, Chen M, Nurmohamed S, Jongstra-Bilen J, Cybulsky MI (2010) Resident intimal dendritic cells accumulate lipid and contribute to the initiation of atherosclerosis. Circ Res 106:383–390. doi:10.1161/CIRCRESAHA.109.210781
Perdiguero EG, Geissmann F (2016) The development and maintenance of resident macrophages. Nat Immunol 17:2–8. doi:10.1038/ni.3341
Potteaux S, Esposito B, van Oostrom O, Brun V, Ardouin P, Groux H, Tedgui A, Mallat Z (2004) Leukocyte-derived interleukin 10 is required for protection against atherosclerosis in low-density lipoprotein receptor knockout mice. Arterioscler Thromb Vasc Biol 24:1474–1478. doi:10.1161/01.ATV.0000134378.86443.cd
Potteaux S, Gautier EL, Hutchison SB, van Rooijen N, Rader DJ, Thomas MJ, Sorci-Thomas MG, Randolph GJ (2011) Suppressed monocyte recruitment drives macrophage removal from atherosclerotic plaques of apoe−/− mice during disease regression. J Clin Invest 121:2025–2036. doi:10.1172/JCI43802
Psaltis PJ, Harbuzariu A, Delacroix S, Witt TA, Holroyd EW, Spoon DB, Hoffman SJ, Pan S, Kleppe LS, Mueske CS, Gulati R, Sandhu GS, Simari RD (2012) Identification of a monocyte-predisposed hierarchy of hematopoietic progenitor cells in the adventitia of postnatal murine aorta. Circulation 125:592–603. doi:10.1161/CIRCULATIONAHA.111.059360
Psaltis PJ, Puranik AS, Spoon DB, Chue CD, Hoffman SJ, Witt TA, Delacroix S, Kleppe LS, Mueske CS, Pan S, Gulati R, Simari RD (2014) Characterization of a resident population of adventitial macrophage progenitor cells in postnatal vasculature. Circ Res 115:364–375. doi:10.1161/CIRCRESAHA.115.303299
Quillard T, Croce K, Jaffer FA, Weissleder R, Libby P (2011) Molecular imaging of macrophage protease activity in cardiovascular inflammation in vivo. Thromb Haemost 105:828–836. doi:10.1160/TH10-09-0589
Ramji DP, Davies TS (2015) Cytokines in atherosclerosis: key players in all stages of disease and promising therapeutic targets. Cytokine Growth Factor Rev 26:673–685. doi:10.1016/j.cytogfr.2015.04.003
Ramkhelawon B, Yang Y, van Gils JM, Hewing B, Rayner KJ, Parathath S, Guo L, Oldebeken S, Feig JL, Fisher EA, Moore KJ (2013) Hypoxia induces netrin-1 and Unc5b in atherosclerotic plaques: mechanism for macrophage retention and survival. Arterioscler Thromb Vasc Biol 33:1180–1188. doi:10.1161/ATVBAHA.112.301008
Rekhter MD, Gordon D (1995) Active proliferation of different cell types, including lymphocytes, in human atherosclerotic plaques. Am J Pathol 147:668–677
Rius J, Guma M, Schachtrup C, Akassoglou K, Zinkernagel AS, Nizet V, Johnson RS, Haddad GG, Karin M (2008) NF-kappaB links innate immunity to the hypoxic response through transcriptional regulation of HIF-1alpha. Nature 453:807–811. doi:10.1038/nature06905
Robbins CS, Chudnovskiy A, Rauch PJ, Figueiredo JL, Iwamoto Y, Gorbatov R, Etzrodt M, Weber GF, Ueno T, van Rooijen N, Mulligan-Kehoe MJ, Libby P, Nahrendorf M, Pittet MJ, Weissleder R, Swirski FK (2012) Extramedullary hematopoiesis generates Ly-6C(high) monocytes that infiltrate atherosclerotic lesions. Circulation 125:364–374. doi:10.1161/CIRCULATIONAHA.111.061986
Robbins CS, Hilgendorf I, Weber GF, Theurl I, Iwamoto Y, Figueiredo JL, Gorbatov R, Sukhova GK, Gerhardt LM, Smyth D, Zavitz CC, Shikatani EA, Parsons M, van Rooijen N, Lin HY, Husain M, Libby P, Nahrendorf M, Weissleder R, Swirski FK (2013) Local proliferation dominates lesional macrophage accumulation in atherosclerosis. Nat Med 19:1166–1172. doi:10.1038/nm.3258
Rock KL, Kono H (2008) The inflammatory response to cell death. Annu Rev Pathol 3:99–126. doi:10.1146/annurev.pathmechdis.3.121806.151456
Rock KL, Lai JJ, Kono H (2011) Innate and adaptive immune responses to cell death. Immunol Rev 243:191–205. doi:10.1111/j.1600-065X.2011.01040.x
Rong JX, Shapiro M, Trogan E, Fisher EA (2003) Transdifferentiation of mouse aortic smooth muscle cells to a macrophage-like state after cholesterol loading. Proc Natl Acad Sci U S A 100:13531–13536. doi:10.1073/pnas.1735526100
Rosenfeld ME, Ross R (1990) Macrophage and smooth muscle cell proliferation in atherosclerotic lesions of WHHL and comparably hypercholesterolemic fat-fed rabbits. Arteriosclerosis 10:680–687
Saeys Y, Gassen SV, Lambrecht BN (2016) Computational flow cytometry: helping to make sense of high-dimensional immunology data. Nat Rev Immunol 16:449–462. doi:10.1038/nri.2016.56
Sarrazy V, Sore S, Viaud M, Rignol G, Westerterp M, Ceppo F, Tanti JF, Guinamard R, Gautier EL, Yvan-Charvet L (2015) Maintenance of macrophage redox status by ChREBP limits inflammation and apoptosis and protects against advanced atherosclerotic lesion formation. Cell Rep 13:132–144. doi:10.1016/j.celrep.2015.08.068
Schneider F, Sukhova GK, Aikawa M, Canner J, Gerdes N, Tang SM, Shi GP, Apte SS, Libby P (2008) Matrix-metalloproteinase-14 deficiency in bone-marrow-derived cells promotes collagen accumulation in mouse atherosclerotic plaques. Circulation 117:931–939. doi:10.1161/CIRCULATIONAHA.107.707448
Seijkens T, Hoeksema MA, Beckers L, Smeets E, Meiler S, Levels J, Tjwa M, de Winther MP, Lutgens E (2014) Hypercholesterolemia-induced priming of hematopoietic stem and progenitor cells aggravates atherosclerosis. FASEB J: Off Publ Fed Am Soc Exp Biol 28:2202–2213. doi:10.1096/fj.13-243105
Serbina NV, Pamer EG (2006) Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nat Immunol 7:311–317. doi:10.1038/ni1309
Serhan CN (2014) Pro-resolving lipid mediators are leads for resolution physiology. Nature 510:92–101. doi:10.1038/nature13479
Shankman LS, Gomez D, Cherepanova OA, Salmon M, Alencar GF, Haskins RM, Swiatlowska P, Newman AA, Greene ES, Straub AC, Isakson B, Randolph GJ, Owens GK (2015) KLF4-dependent phenotypic modulation of smooth muscle cells has a key role in atherosclerotic plaque pathogenesis. Nat Med 21:628–637. doi:10.1038/nm.3866
Sheedy FJ, Grebe A, Rayner KJ, Kalantari P, Ramkhelawon B, Carpenter SB, Becker CE, Ediriweera HN, Mullick AE, Golenbock DT, Stuart LM, Latz E, Fitzgerald KA, Moore KJ (2013) CD36 coordinates NLRP3 inflammasome activation by facilitating intracellular nucleation of soluble ligands into particulate ligands in sterile inflammation. Nat Immunol 14:812–820. doi:10.1038/ni.2639
Sica A, Mantovani A (2012) Macrophage plasticity and polarization: in vivo veritas. J Clin Invest 122:787–795. doi:10.1172/JCI59643
Silvestre-Roig C, de Winther MP, Weber C, Daemen MJ, Lutgens E, Soehnlein O (2014) Atherosclerotic plaque destabilization: mechanisms, models, and therapeutic strategies. Circ Res 114:214–226. doi:10.1161/CIRCRESAHA.114.302355
Simsekyilmaz S, Cabrera-Fuentes HA, Meiler S, Kostin S, Baumer Y, Liehn EA, Weber C, Boisvert WA, Preissner KT, Zernecke A (2014) Role of extracellular RNA in atherosclerotic plaque formation in mice. Circulation 129:598–606. doi:10.1161/CIRCULATIONAHA.113.002562
Soehnlein O, Swirski FK (2013) Hypercholesterolemia links hematopoiesis with atherosclerosis. Trends Endocrinol Metab: TEM 24:129–136. doi:10.1016/j.tem.2012.10.008
Spann NJ, Garmire LX, McDonald JG, Myers DS, Milne SB, Shibata N, Reichart D, Fox JN, Shaked I, Heudobler D, Raetz CR, Wang EW, Kelly SL, Sullards MC, Murphy RC, Merrill AH Jr, Brown HA, Dennis EA, Li AC, Ley K, Tsimikas S, Fahy E, Subramaniam S, Quehenberger O, Russell DW, Glass CK (2012) Regulated accumulation of desmosterol integrates macrophage lipid metabolism and inflammatory responses. Cell 151:138–152. doi:10.1016/j.cell.2012.06.054
Stewart CR, Stuart LM, Wilkinson K, van Gils JM, Deng J, Halle A, Rayner KJ, Boyer L, Zhong R, Frazier WA, Lacy-Hulbert A, El Khoury J, Golenbock DT, Moore KJ (2010) CD36 ligands promote sterile inflammation through assembly of a toll-like receptor 4 and 6 heterodimer. Nat Immunol 11:155–161. doi:10.1038/ni.1836
Subramanian M, Thorp E, Tabas I (2015) Identification of a non-growth factor role for GM-CSF in advanced atherosclerosis: promotion of macrophage apoptosis and plaque necrosis through IL-23 signaling. Circ Res 116:e13–e24. doi:10.1161/CIRCRESAHA.116.304794
Suzuki H, Kurihara Y, Takeya M, Kamada N, Kataoka M, Jishage K, Ueda O, Sakaguchi H, Higashi T, Suzuki T, Takashima Y, Kawabe Y, Cynshi O, Wada Y, Honda M, Kurihara H, Aburatani H, Doi T, Matsumoto A, Azuma S, Noda T, Toyoda Y, Itakura H, Yazaki Y, Kodama T et al (1997) A role for macrophage scavenger receptors in atherosclerosis and susceptibility to infection. Nature 386:292–296. doi:10.1038/386292a0
Swirski FK, Pittet MJ, Kircher MF, Aikawa E, Jaffer FA, Libby P, Weissleder R (2006) Monocyte accumulation in mouse atherogenesis is progressive and proportional to extent of disease. Proc Natl Acad Sci U S A 103:10340–10345. doi:10.1073/pnas.0604260103
Swirski FK, Libby P, Aikawa E, Alcaide P, Luscinskas FW, Weissleder R, Pittet MJ (2007) Ly-6Chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. J Clin Invest 117:195–205. doi:10.1172/JCI29950
Swirski FK, Nahrendorf M, Etzrodt M, Wildgruber M, Cortez-Retamozo V, Panizzi P, Figueiredo JL, Kohler RH, Chudnovskiy A, Waterman P, Aikawa E, Mempel TR, Libby P, Weissleder R, Pittet MJ (2009) Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science 325:612–616. doi:10.1126/science.1175202
Tabas I, Bornfeldt KE (2016) Macrophage phenotype and function in different stages of atherosclerosis. Circ Res 118:653–667. doi:10.1161/CIRCRESAHA.115.306256
Tacke F, Alvarez D, Kaplan TJ, Jakubzick C, Spanbroek R, Llodra J, Garin A, Liu J, Mack M, van Rooijen N, Lira SA, Habenicht AJ, Randolph GJ (2007) Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. J Clin Invest 117:185–194. doi:10.1172/JCI28549
Tedgui A, Mallat Z (2006) Cytokines in atherosclerosis: pathogenic and regulatory pathways. Physiol Rev 86:515–581. doi:10.1152/physrev.00024.2005
Thomas MR, Lip GY (2017) Novel risk markers and risk assessments for cardiovascular disease. Circ Res 120:133–149. doi:10.1161/CIRCRESAHA.116.309955
Thorp E, Cui D, Schrijvers DM, Kuriakose G, Tabas I (2008) Mertk receptor mutation reduces efferocytosis efficiency and promotes apoptotic cell accumulation and plaque necrosis in atherosclerotic lesions of apoe−/− mice. Arterioscler Thromb Vasc Biol 28:1421–1428. doi:10.1161/ATVBAHA.108.167197
Thorp E, Li G, Seimon TA, Kuriakose G, Ron D, Tabas I (2009) Reduced apoptosis and plaque necrosis in advanced atherosclerotic lesions of apoe−/− and Ldlr−/− mice lacking CHOP. Cell Metab 9:474–481. doi:10.1016/j.cmet.2009.03.003
van der Vorst EP, Theodorou K, Wu Y, Hoeksema MA, Goossens P, Bursill CA, Aliyev T, Huitema LF, Tas SW, Wolfs IM, Kuijpers MJ, Gijbels MJ, Schalkwijk CG, Koonen DP, Abdollahi-Roodsaz S, McDaniels K, Wang CC, Leitges M, Lawrence T, Plat J, Van Eck M, Rye KA, Touqui L, de Winther MP, Biessen EA, Donners MM (2016) High-density lipoproteins exert pro-inflammatory effects on macrophages via passive cholesterol depletion and PKC-NF-kappaB/STAT1-IRF1 signaling. Cell Metab. doi:10.1016/j.cmet.2016.10.013
van Gils JM, Derby MC, Fernandes LR, Ramkhelawon B, Ray TD, Rayner KJ, Parathath S, Distel E, Feig JL, Alvarez-Leite JI, Rayner AJ, McDonald TO, O’Brien KD, Stuart LM, Fisher EA, Lacy-Hulbert A, Moore KJ (2012) The neuroimmune guidance cue netrin-1 promotes atherosclerosis by inhibiting the emigration of macrophages from plaques. Nat Immunol 13:136–143. doi:10.1038/ni.2205
Van Vre EA, Ait-Oufella H, Tedgui A, Mallat Z (2012) Apoptotic cell death and efferocytosis in atherosclerosis. Arterioscler Thromb Vasc Biol 32:887–893. doi:10.1161/ATVBAHA.111.224873
Vengrenyuk Y, Nishi H, Long X, Ouimet M, Savji N, Martinez FO, Cassella CP, Moore KJ, Ramsey SA, Miano JM, Fisher EA (2015) Cholesterol loading reprograms the microRNA-143/145-myocardin axis to convert aortic smooth muscle cells to a dysfunctional macrophage-like phenotype. Arterioscler Thromb Vasc Biol 35:535–546. doi:10.1161/ATVBAHA.114.304029
Viola JR, Lemnitzer P, Jansen Y, Csaba G, Winter C, Neideck C, Silvestre-Roig C, Dittmar G, Doring Y, Drechsler M, Weber C, Zimmer R, Cenac N, Soehnlein O (2016) Resolving lipid mediators Maresin 1 and Resolvin D2 prevent Atheroprogression in mice. Circ Res 119:1030–1038. doi:10.1161/CIRCRESAHA.116.309492
Vorlova S, Koch M, Manthey HD, Cochain C, Busch M, Chaudhari SM, Stegner D, Yepes M, Lorenz K, Nolte MW, Nieswandt B, Zernecke A (2016) Coagulation factor XII induces pro-inflammatory cytokine responses in macrophages and promotes atherosclerosis in mice. Thromb Haemost. doi:10.1160/TH16-06-0466
Wang JC, Bennett M (2012) Aging and atherosclerosis: mechanisms, functional consequences, and potential therapeutics for cellular senescence. Circ Res 111:245–259. doi:10.1161/CIRCRESAHA.111.261388
Warnatsch A, Ioannou M, Wang Q, Papayannopoulos V (2015) Inflammation. Neutrophil extracellular traps license macrophages for cytokine production in atherosclerosis. Science 349:316–320. doi:10.1126/science.aaa8064
Westerterp M, Gourion-Arsiquaud S, Murphy AJ, Shih A, Cremers S, Levine RL, Tall AR, Yvan-Charvet L (2012) Regulation of hematopoietic stem and progenitor cell mobilization by cholesterol efflux pathways. Cell Stem Cell 11:195–206. doi:10.1016/j.stem.2012.04.024
Westerterp M, Murphy AJ, Wang M, Pagler TA, Vengrenyuk Y, Kappus MS, Gorman DJ, Nagareddy PR, Zhu X, Abramowicz S, Parks JS, Welch C, Fisher EA, Wang N, Yvan-Charvet L, Tall AR (2013) Deficiency of ATP-binding cassette transporters A1 and G1 in macrophages increases inflammation and accelerates atherosclerosis in mice. Circ Res 112:1456–1465. doi:10.1161/CIRCRESAHA.113.301086
Whitman SC, Ravisankar P, Daugherty A (2002) Interleukin-18 enhances atherosclerosis in apolipoprotein E(−/−) mice through release of interferon-gamma. Circ Res 90:E34–E38
Yvan-Charvet L, Ranalletta M, Wang N, Han S, Terasaka N, Li R, Welch C, Tall AR (2007) Combined deficiency of ABCA1 and ABCG1 promotes foam cell accumulation and accelerates atherosclerosis in mice. J Clin Invest 117:3900–3908. doi:10.1172/JCI33372
Yvan-Charvet L, Pagler T, Gautier EL, Avagyan S, Siry RL, Han S, Welch CL, Wang N, Randolph GJ, Snoeck HW, Tall AR (2010a) ATP-binding cassette transporters and HDL suppress hematopoietic stem cell proliferation. Science 328:1689–1693. doi:10.1126/science.1189731
Yvan-Charvet L, Wang N, Tall AR (2010b) Role of HDL, ABCA1, and ABCG1 transporters in cholesterol efflux and immune responses. Arterioscler Thromb Vasc Biol 30:139–143. doi:10.1161/ATVBAHA.108.179283
Zheng Y, Gardner SE, Clarke MC (2011) Cell death, damage-associated molecular patterns, and sterile inflammation in cardiovascular disease. Arterioscler Thromb Vasc Biol 31:2781–2786. doi:10.1161/ATVBAHA.111.224907
Zhong W, Pan G, Wang L, Li S, Ou J, Xu M, Li J, Zhu B, Cao X, Ma H, Li C, Xu J, Olkkonen VM, Staels B, Yan D (2016) ORP4L facilitates macrophage survival via G-protein-coupled signaling: ORP4L−/− mice display a reduction of atherosclerosis. Circ Res. doi:10.1161/CIRCRESAHA.116.309603
Acknowledgments
This work was supported by the Deutsche Forschungsgemeinschaft (SFB688 TPA22).
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the special issue on macrophages in tissue homeostasis in Pflügers Archiv – European Journal of Physiology
Rights and permissions
About this article
Cite this article
Cochain, C., Zernecke, A. Macrophages in vascular inflammation and atherosclerosis. Pflugers Arch - Eur J Physiol 469, 485–499 (2017). https://doi.org/10.1007/s00424-017-1941-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-017-1941-y