Abstract
Atherosclerosis can be regarded as chronic inflammatory disease driven by lipid accumulation in the arterial wall. Macrophages play a key role in the development of local inflammatory response and atherosclerotic lesion growth. Atherosclerotic plaque is a complex microenvironment, in which different subsets of macrophages coexist executing distinct, although in some cases overlapping functions. According to the classical simplified nomenclature, lesion macrophages can belong to pro-inflammatory or anti-inflammatory or alternatively activated types. While the former promote the inflammatory response and participate in lipid accumulation, the latter are responsible for the inflammation resolution and plaque stabilisation. Atherosclerotic lesion dynamics depends therefore on the balance between these macrophages populations. The diverse functions of macrophages make them an attractive therapeutic target for the development of novel anti-atherosclerotic treatments. In this chapter, we discuss different types of macrophages and their roles in atherosclerotic lesion dynamics and describe the results of several experiments studying macrophage polarisation in atherosclerosis.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Atherosclerosis and related cardiovascular diseases remain the leading cause of morbidity and mortality worldwide (Sanchis-Gomar et al. 2016). Despite the considerable progress during the recent years, treatment and prevention of atherosclerosis are still a challenge for the modern medicine, partly because of the complex nature of the disease pathogenesis. It is currently established that atherosclerotic lesion development is dependent on several processes. First, altered blood lipoprotein profile with prevailing low-density lipoprotein (LDL) and especially its modified forms facilitates lipid accumulation in the arterial wall (Krauss 2010). Second, endothelial dysfunction allows lipoprotein particles penetrating into the subendothelial layer of the arterial wall (intima), where the plaque development takes place (Gimbrone and García-Cardeña 2016). Third, local inflammatory response and misbalanced functioning of tissue macrophages contribute to the plaque growth and the formation of lipid core, inside which necrotic processes can take place (Libby 2013). Although growing atherosclerotic plaques may reduce the vessel volume, they often remain asymptomatic for years, which hinder the timely diagnostics of the condition. At later stages, atherosclerotic plaques can acquire a fibrous cap, which separates them from the vessel milieu and renders them stable. The primary danger comes from the so-called unstable plaques, as they are likely to induce thrombus formation on the surface (Hansson et al. 2015). This process can have a serious or fatal outcome, inducing thromboembolism of vital organs. For many patients, this serious event would be the first clinical manifestation of atherosclerosis.
Inflammation plays a central role at all stages of atherosclerosis development. In fact, atherosclerosis is currently regarded as a chronic inflammatory condition driven by misbalance of plasma lipoprotein profile and other factors (Ross 1999; Libby 2002). Importantly, inflammation and the imbalanced macrophage function are likely to play a decisive role in the formation of unstable plaques and plaque rupture followed by life-threatening thrombus formation. Therefore, studying inflammatory processes associated with atherosclerosis became a hot research topic during recent years.
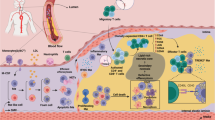
The development of atherosclerotic lesion is associated with a local inflammatory response with activation of various cell types, including monocytes/macrophages (Moore and Tabas 2011). Monocytes are recruited to the lesion site, where they differentiate into macrophages and actively participate in lipoprotein particle uptake. The increase of the tissue population of macrophages can result not only from the recruitment of monocytes from circulation but also from macrophage proliferation in the tissue (Ginhoux and Jung 2014). In advanced plaques, proliferation accounts for the largest part of the macrophage population increase (Robbins et al. 2013; Orekhov et al. 2010). Intracellular lipid accumulation by macrophages leads to the formation of foam cells that have cytoplasm filled with lipid droplets. Such cells are abundant in progressing atherosclerotic plaques. Interestingly, the subendothelial layer of arterial intima contains a population of pluripotent cells also known as macrovascular pericytes, which have a capacity for phagocytosis, express macrophage marker CD68 and can also become foam cells (Orekhov et al. 2014). Foam cells secrete various signalling molecules contributing to the formation of atherosclerotic plaque microenvironment rich in pro-inflammatory cytokines and factors (Libby 2002). This promotes further recruitment of circulating monocytes, lipoprotein retention and extracellular matrix remodelling. Macrophages populating atherosclerotic plaques have a decreased ability to migrate (Randolph 2014). Failure to remove dying macrophages in the lipid core of the plaque leads to the formation of necrotic area (Seimon and Tabas 2009). In advanced plaques, where neovascularisation has taken place, macrophages are responsible for clearance of erythrocytes that enter the plaque following ruptures of blood vessels (Kockx et al. 2003). These processes further contribute to the lesion progression and the development of complicated plaques.
As can be seen from this brief overview, macrophages represent an important component of the pathogenesis of atherosclerosis and a potential point of therapeutic intervention. Several macrophage-targeting strategies have been proposed, such as inhibiting monocyte recruitment to the lesion site, stimulating cholesterol efflux and diminishing lipid storage in macrophages and modulating macrophage polarisation towards pro- or anti-inflammatory phenotypes (Moore et al. 2013). However, the development of macrophage-targeting therapy is complicated by macrophage heterogeneity and plasticity that necessitate a well-balanced approach.
2 Reticuloendothelial System
Monocytes and macrophages are regarded as a continuous system, also known as mononuclear phagocyte system, which plays a central role in the innate immune response (van Furth and Cohn 1968). Monocytes circulate in the bloodstream and can differentiate to macrophages in response to various signals, such as tissue injury and pathogen invasion that induce secretion of cytokines and chemokines by tissue cells. Monocyte-derived macrophages are capable of active phagocytosis and produce pro-inflammatory factors that orchestrate the immune response to pathogens. The results of early experiments with radiolabelled monocytes in animal models suggested that all macrophages at the lesion site derive from the recruited monocytes and represent a terminally differentiated cellular population. This view, however, has been challenged by more recent studies that have demonstrated that, at least in some cases, tissue macrophage population can expand by proliferation (Ginhoux and Jung 2014). Importantly, macrophage proliferation has been shown to contribute to atherosclerotic lesion growth (Orekhov et al. 2010).
2.1 Macrophage Heterogeneity
The population of macrophages is characterised by heterogeneity: several macrophage types can be distinguished based on the gene expression pattern and main functions. Studying of macrophage differentiation and heterogeneity is challenging because activation of cells can occur during the isolation process, and this can influence the obtained macrophage population properties. Moreover, macrophage subtypes described in animal models, such as mice, do not coincide fully with the subtypes present in humans, which makes the research even more complicated. In the classical model of macrophage activation, two main phenotypes have been defined mirroring the two types of T helper cells (Th1 and Th2): pro-inflammatory classically activated (M1) and alternatively activated (M2) (Mantovani et al. 2002). Later, however, the accumulating knowledge made it evident that the classical model should be revised to describe the macrophage complexity more accurately (Murray et al. 2014; Martinez and Gordon 2015). For accurate study of macrophage heterogeneity, it is currently recommended to define the macrophage subtypes based on several established markers and also the activation stimuli that triggered their differentiation. For experimental purposes, the classical model still can be used as a tool, since it is relatively simple and allows distinguishing and modelling major imbalances between pro- and anti-inflammatory macrophages in pathological conditions (de Gaetano et al. 2016; Novoselov et al. 2015).
2.2 A Simplified Classification: Pro- and Anti-inflammatory Macrophages
Pro-inflammatory M1 macrophages can be induced in response to Th1 cytokine interferon (IFN)-γ, as well as pathogen-associated molecular complexes (PAMPs), lipopolysaccharides and lipoproteins. In the context of atherosclerotic plaque development, M1 macrophage polarisation involves NF-κB and NLRP3 inflammasome pathways (Duewell et al. 2010). M1 macrophages produce pro-inflammatory factors, such as tumour necrosis factor (TNF)-α, interleukin-1β (IL-1β), IL-6, IL-12, IL-23 and Th1 cell-attracting chemokines CXCL9, CXCL10 and CXCL11, as well as reactive oxygen species (ROS) and nitric oxide (NO). Therefore, M1 macrophages stimulate and maintain the inflammatory response.
Alternatively activated M2 macrophages are induced in response to Th2-type cytokines IL-4, IL-33 and IL-13. They secrete anti-inflammatory factors, such as IL1 receptor agonist (IL-1RA) receptor agonist and IL-10 and chemokines CCL17, CCL22 and CCL24 (Martinez et al. 2006). M2 macrophages are responsible for inflammation resolution, tissue repair and remodelling. These cells are characterised by high phagocytic activity and expression of a variety of scavenger receptors. Within the group of alternatively activated macrophage, several subtypes can be distinguished based on the activation stimuli and gene expression pattern. For instance, M2a macrophages can be induced by IL-4 and IL-13 and express high levels of CD206 and IL-1 receptor agonist. M2b macrophages are induced by TLR signalling, immune complexes and IL-1R ligands and produce both pro- and anti-inflammatory cytokines, such as IL-10, IL-6 and TNF-α (Martinez et al. 2008). M2c macrophages can be induced by IL-10, transforming growth factor-β (TGF-β) and glucocorticosteroids and have strong anti-inflammatory properties, producing pentraxin-3 (PTX3), TGF-β and IL-10 (Zizzo et al. 2012). M2d macrophages are induced by TLR signalling and have angiogenic properties, playing a role in tumour progression and atherosclerotic plaque growth (Ferrante et al. 2013).
More macrophage varieties have been described in experimental conditions and discovered in vivo. The picture becomes even more complex after taking into account macrophage varieties that are induced in some pathological conditions and are clearly distinct from the classical M1 and M2 macrophages. For instance, activation with oxidised phospholipids can result in the formation of distinct macro phage phenotype Mox expressing redox regulatory genes that can be found in large quantities in atherosclerotic lesions in mouse models (Kadl et al. 2010). A shift in macrophage phenotypes and formation of mixed phenotypes has been observed in obesity, cancer and other pathological conditions (Biswas and Mantovani 2010).
3 Macrophages and Atherosclerosis
3.1 Adhesion and Penetration of Monocytes into the Arterial Wall
According to the current understanding, circulating monocytes belong to one of the several distinct subtypes described in humans and mice. These subtypes are characterised by the expression of certain surface markers and chemokine receptors (Geissmann et al. 2003). In humans, monocytes that express CD14 and CC-chemokine receptor 2 (CCR2) and are negative for CD16 surface antigen are the most prevalent and referred to as classical monocytes (Ziegler-Heitbrock 2007; Ziegler-Heitbrock et al. 2010). Monocytes that are positive for CD16 can be further divided into two subsets: CD14+CD16++ (non-classical) monocytes that perform patrolling and CD14++CD16+ (intermediate) that have pro-inflammatory properties (Cros et al. 2010; Belge et al. 2002). Interestingly, increased numbers of pro-inflammatory monocytes have been demonstrated in animal models of atherosclerosis (Apoe –/– mice) (Swirski et al. 2007). The relationship between monocyte predisposition to the inflammatory response and other cardiovascular risks remains to be elucidated in full detail. However, it has been demonstrated that hypercholesterolaemia resulted in enhanced proliferation of haematopoietic stem cells and their sensitivity to granulocyte-macrophage colony-stimulating factor (GM-CSF), which triggers macrophage polarisation towards the pro-inflammatory phenotype. On the other hand, the expression of high-density lipoprotein (HDL), which has protective properties against atherosclerosis development, reversed this phenotype (Yvan-Charvet et al. 2010).
Atherosclerotic lesion formation begins with local endothelial dysfunction with increased permeability of the endothelial lining and establishment of pro-inflammatory microenvironment. This process is known as endothelial activation (Pober and Sessa 2007). This process can be induced by a number of factors, including modified LDL, lipopolysaccharides and cytokines, such as TNF-α and interleukin (IL)-β. Endothelial activation has been demonstrated to be mediated by nuclear factor-κB (NF-κB) signalling (Collins and Cybulsky 2001). This induces the expression of cell adhesion molecules on the endothelium that facilitate the recruitment of circulating immune cells: intercellular adhesion molecule-1 (ICAM1), vascular cell adhesion molecule-1 (VCAM1), E-selectin, such as MCP1, platelet-derived growth factor (PDGF). In animal models, it has been demonstrated that both pro-inflammatory and patrolling monocytes can be recruited to growing atherosclerotic lesions by P- and E-selectin-dependent rolling followed by ICAM1- and VCAM1-dependent adhesion (Galkina and Ley 2007). Migration of pro-inflammatory monocytes into the arterial wall is dependent on CCR2, CCR5 and CX3C-chemokine receptor 1 (CX3CR1) signalling, and inhibition of these pathways had a protective effect in Apoe –/– mice reducing atherosclerotic plaque growth (Combadiere et al. 2008).
At the lesion site, monocyte-derived macrophages can take part in different pro cesses: pro-inflammatory cells contribute to the inflammatory response and lesion development, while patrolling monocytes may participate in phagocytosis or differentiate into dendritic cells (Swirski et al. 2007, 2009). Dendritic cells play a prominent role in atherosclerotic plaque development, and their functions overlap to some extent with those of macrophages (Cybulsky et al. 2016). Differentiation of macrophages is accompanied by morphological changes, such as enlargement, increase of the number of organelles and alteration of gene expression patterns, which increases their sensitivity to signalling molecules. Intensification of the lyso somal enzyme activity prepares the cells to active phagocytosis (Novoselov et al. 2015). Importantly, differentiated macrophages in atherosclerotic plaques have a decreased ability to migrate, which hinders the inflammation resolution and favours plaque growth.
3.2 Pro- and Anti-inflammatory Macrophages in Atherosclerotic Lesions
Developing atherosclerotic lesion is a specific environment, which is enriched in activated cells, pro-inflammatory factors and modified lipoproteins. At later stages, plaques contain large quantities of dying and apoptotic cells that are subject to macrophage-mediated clearance. Both pro- and anti-inflammatory phenotypes have been found in atherosclerotic lesions at different stages (De Paoli et al. 2014; Bouhlel et al. 2007), and their role in the disease pathogenesis appears to be complex (Fig. 11.1). On one hand, pro-inflammatory (M1) macrophages have long been known as a major factor promoting the local inflammation and plaque growth (Smith et al. 1995). On the other hand, alternatively activated macrophages are also found at the lesion sites and are important for inflammation resolution and atherosclerotic plaque regression (Nathan and Ding 2010). Both pro- and anti-inflammatory macrophage populations increase in course of atherosclerotic lesion progression, but their distribution within the plaque is not identical. Immunocytochemistry studies demonstrated that cells positive for M1 markers are preferentially found in the plaque shoulder regions, and cells, positive for M2 markers—in the adventitia (Stoger et al. 2012). Interestingly, prevalence of M2 or M1 macrophages was demonstrated to be a hallmark of plaque stability or instability correspondingly. Macrophages positive for M2 markers surface mannose receptor (MR) and CD68 were found in more stable regions of plaques that were also enriched with IL-4. These cells were apparently more resistant to foam cell formation, as MR-positive (M2) macrophages contained fewer and smaller lipid droplets compared to MR-negative macrophages (Chinetti-Gbaguidi et al. 2011). Studies evaluating macrophage population in symptomatic versus asymptomatic plaque specimens obtained in course of carotid endarterectomy demonstrated an increased content of M1 marker-expressing and decreased content of M2 marker-expressing macrophages in symptomatic plaques (Cho et al. 2013). In contrast, asymptomatic lesions predominantly contained cells positive for M2 markers, such as CD163. This observation was further confirmed in a more recent study employing a panel of specific M1 and M2 markers (de Gaetano et al. 2016).
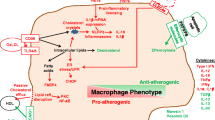
Simplified scheme of the roles of pro- and anti-inflammatory macrophages in atherosclerotic lesion progression. Pro-inflammatory conditions are associated with high plasma concentrations of atherogenic lipoprotein and local endothelial dysfunction and activation. Monocyte recruitment and infiltration into arterial wall is facilitated in such conditions, and pro-inflammatory (M1) macrophage polarisation is stimulated. M1 macrophages participate in lipid accumulation, maintain pro-inflammatory environment and promote plaque destabilisation. Alternatively activated M2 macrophages contribute to plaque stabilisation via inflammation resolution, efferocytosis of dying cells and stimulation of extracellular matrix synthesis
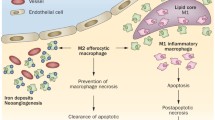
Haemorrhagic lesions develop at later stages of atherosclerotic plaque progression. Erythrocyte contamination is cleared by macrophages that are characterised by a distinct gene expression pattern. So-called HA-mac population expresses high levels of CD163 and low levels of human leukocyte antigen-DR and is resistant to oxidative stress, which allows these cells to clear iron-containing haem more efficiently (Boyle et al. 2009). These macrophages are likely to play a protective role reducing oxidative stress. Haem-induced macrophage phenotype (also called Mhem) was demonstrated to be resistant to lipid accumulation and foam cell formation through activating transcription factor 1 (ATF-1) signalling, which induces liver X receptor-β (LXR-β) leading to the induction of genes coordinating cholesterol efflux, such as LXR-α and ABCA1 (Boyle et al. 2012). This has also been demonstrated on cultured human monocytes exposed to haemoglobin:haptoglobin complexes, which led to formation of a distinct M(Hb) macrophage phenotype positive for MR and CD163 and resistant to foam cell formation (Finn et al. 2012).
Another macrophage phenotype present in atherosclerotic lesions is M4, a distinct phenotype, which can be induced by CXCL-4 chemokine. M4 macrophages are characterised by simultaneous expression of matrix metalloproteinase (MMP)7 and calcium-binding protein S100A8. They express pro-inflammatory cytokines IL-6 and TNF-α and are negative for CD163 (Erbel et al. 2015). These macrophages can be considered pro-atherogenic, since they may promote destabilisation of the plaque fibrous cap (Chistiakov et al. 2015).
3.3 Lipid Metabolism and Accumulation by Macrophages
According to the current understanding, LDL serves as the primary source of lipid accumulation in the arterial wall during atherosclerotic plaque development. However, the accumulating evidence demonstrates that only certain types of LDL that underwent atherogenic modification are associated with the increased risk of atherosclerosis. Native (non-modified) LDL particles are recognised by LDL receptor (LDLR) and internalised by cells via receptor-mediated endocytosis, which is a highly regulated process. Particles internalised following this pathway are transported to lysosomes and degraded by lysosomal acid hydrolases. In this process, cholesterol esters are transformed to free cholesterol, which is transported to the endoplasmic reticulum and esterified by cholesterol acyltransferase (ACAT) (Brown and Goldstein 1983). High amounts of free cholesterol in the endoplasmic reticulum initiate a signalling cascade that decreases the expression of LDLR and subsequent lipid uptake. This regulation prevents lipid overload and foam cell formation. ApoB-containing lipoproteins that also contain ApoE can cause cholesterol accumulation through interaction with ApoE receptors, such as LRP1 and VLDL receptors that are not regulated by the amount of intracellular cholesterol. Uptake of native LDL via pinocytosis is also possible and can lead to foam cell formation, as this process is less strictly regulated (Kruth 2011). However, in atherosclerotic lesions, massive lipid uptake is likely to occur in process of uncontrolled phagocytosis (Torzewski and Lackner 2006; Torzewski et al. 2004). This internalisation pathway is taken by various LDL-containing aggregates that are especially atherogenic. Interestingly, modified LDL, such as desialylated LDL, is susceptible to aggregation and can also induce formation of autoantibodies followed by LDL-containing immune complexes (Sobenin et al. 2014).
Modified LDL, such as oxLDL, can be internalised by cells via scavenger receptors that are not regulated by intracellular cholesterol levels, including CD36, scavenger receptor A (SRA), lectin-like receptors (LOX) and toll-like receptors (TLRs) (Moore and Freeman 2006; Younis et al. 2008). The role of modified LDL (mLDL) in the pathogenesis of atherosclerosis has been widely studied during the last years. Converging evidence indicates that LDL particles undergo multiple modifications in the bloodstream, including desialylation, decrease of particle size and increase of density, acquisition of negative charge and oxidation (Tertov et al. 1998). Desialylated LDL could be isolated from blood plasma of patients with confirmed atherosclerosis and used for modelling lipid accumulation in human arterial wall cells in primary culture. Experiments on cultured macrophages demonstrated that atherogenic mLDL caused a substantial increase of the expression of both pro-inflammatory marker TNF-α and anti-inflammatory CCL18, while native, unmodified LDL did not possess such activity (Tables 11.1 and 11.2). These results indicate that cholesterol accumulation can result in significant alteration of macrophage gene expression involving pro- and anti-inflammatory factors, which has also been demonstrated in previous studies (De Paoli et al. 2014; Hägg et al. 2009).
At the immunohistochemical level of investigation, CD68 which is mainly present in macrophage lysosomes represents a reliable marker for the identification of macrophages and macrophage origin foam cells in atherosclerotic lesions (Bobryshev et al. 2013) (Fig. 11.2a). Ultrastructural analysis has shown that the formation of foam cells occurs as a result of the accumulation of a large number of lipid inclusions (so-called “lipid droplets”) in the cytoplasm of macrophages (Bobryshev 2006) (Fig. 11.2b). In vitro experiments, in which macrophages were incubated with modified low-density lipoproteins (LDL), showed that modified lipoproteins are captured by macrophages (Nagornev et al. 1985, 1991) (Fig. 11.3). It is commonly accepted that similar unregulated capture of modified LDL occurs in atherosclerotic lesions in situ as well and that this process is responsible for the formation of foam cells in the arterial wall (Nagornev et al. 1991; Ross 1999). The process of the formation of foam cells is tightly associated with functioning of lysosomes in macrophages, more exactly to say—with the inability of lysosomes to completely catabolise modified LDL that are captured by macrophages from the extracellular space (Nagornev et al. 1991; Bobryshev et al. 2013). As a morphological evidence of such incomplete catabolism of lipids in lysosomes, the appearance of secondary lysosomes/autophagosomes containing lipid inclusions can be considered (Figs. 11.4 and 11.5).
Formation of foam cells in atherosclerotic lesions (a, b). (a) Macrophages and foam cells of macrophage origin located in an atherosclerotic lesion, identified using anti-CD68 antibody. Immunohistochemistry; peroxidase–anti-peroxidase technique. (b) Accumulation of a large number of lipid inclusion (“lipid droplets”) in the cytoplasm of a macrophage, leading to the formation of a foam cell. Transmission Electron Microscopy. Scale bars = 25 μm (a) and 2 μm (b)
Structural appearance of lysosomes in intimal cells containing “lipid droplets” in intimal cells in fatty streaks (Type II lesions) (a–c). Note that, while few lysosomes are characterised by the presence of homogenous material of middle-high electron density, the majority of lysosomes are represented by secondary lysosomes and autophagosomes, containing lipid inclusions. (a–c): Transmission Electron Microscopy. Scale bars = 200 nm (a–c). (Reproduced from Bobryshev et al. 2013; with permission from Wiley) (Bobryshev YV, Shchelkunova TA, Morozov IA, Rubtsov PM, Sobenin IA, Orekhov AN, Smirnov AN. Changes of lysosomes in the earliest stages of the development of atherosclerosis. J Cell Mol Med 2013;17(5):626–35)
A high-resolution micrograph showing the distribution of CD68 antigen in an autophagosome in an intimal cell in a fatty streak of the human aorta. Electron microscopic immunocytochemistry; immunogold technique. Scale bar = 200 nm. (Reproduced from Bobrsyhev et al. 2013; with permission from Wiley) (Bobryshev YV, Shchelkunova TA, Morozov IA, Rubtsov PM, Sobenin IA, Orekhov AN, Smirnov AN. Changes of lysosomes in the earliest stages of the development of atherosclerosis. J Cell Mol Med 2013;17(5):626–35).
From a biochemical point of view, inside the cell, cholesteryl esters accumulate in cytoplasmic droplets, where neutral cholesterol esterase hydrolyses them to free cholesterol that, in turn, is esterified by ACAT. Accumulation of free cholesterol induces pro-inflammatory activation of macrophages resulting in the endoplasmic reticulum stress (Li et al. 2005) and calcium leak into the cytosol (Lim et al. 2008). Accumulation of lipid droplets in the cytoplasm was demonstrated to cause activation of TLR4, which increases lipid uptake and further promotes foam cell formation, while accumulation of cholesterol crystals induces inflammasome activation (Choi et al. 2009; Duewell et al. 2010; Tall and Yvan-Charvet 2015). TLR activation also promotes the production of pro-inflammatory factors by macrophages, including IL-1β and chemokine (C-C motif) ligand 5 (CCL5) (Bae et al. 2009). Cholesteryl esters can promote pro-inflammatory responses via different signalling pathways, including 7-ketocholesteryl-9-carboxynonanoate that was demonstrated to activate NF-κB pathway and cholesteryl linoleate–MAP kinase signalling (Huang et al. 2010; Huber et al. 2002). Comparison of macrophages laden with cholesterol with control cells demonstrated that that cholesterol accumulation can alter macrophage metabolism and response to the external stimuli. Cholesterol-laden macrophages exposed to pro-inflammatory stimulators expressed a lower level of inflammation markers than in control, but no difference could be seen in anti-inflammatory response of these cells (da Silva et al. 2016).
Oxysterol is another pro-inflammatory cholesterol derivative, which is present in atherosclerotic plaques. It has been demonstrated that oxysterol induced the expression of monocyte chemoattractant-1 (MCP-1) in macrophages (Leonarduzzi et al. 2010). In addition, exposure to oxidised cholesterol esters also enhances the expression of scavenger receptor CD36 (Jedidi et al. 2006). Therefore, exposure to atherogenic lipoprotein particles can induce pro-inflammatory phenotypes in monocytes/macrophages. Moreover, it has been demonstrated that oxLDL could shift the phenotype of alternatively activated M2 macrophages towards the pro-inflammatory through changes in gene expression (van Tits et al. 2011).
Within the acidic microenvironment of lipid plaques, phospholipase-mediated hydrolysis of lipoproteins can result in the formation of products that greatly contribute to the lipid accumulation in the arterial wall. For instance, phospholipase A-treated LDL increase the secretion of TNF-α and IL-6 by macrophages and promoted foam cell formation (Boyanovsky et al. 2010).
It has long been known that some classes of lipids, such as high-density lipoprotein (HDL) and polyunsaturated fatty acids (PUFA), have atheroprotective properties. These properties may partly be explained by their effect on macrophages. For instance, experiments on mice demonstrated that conjugated linoleic acid reduced the expression of pro-inflammatory genes, such as NF-κB, CCL2, MMP-9, phospholipase 2 and cyclooxygenase 2 in macrophages via the peroxisome proliferator-activated receptor γ (PPARγ) signalling pathway. This resulted in the inhibition of atherosclerosis progression. PUFA can also counterpart the pro-atherosclerotic effects of saturated fatty acids reducing the expression of LOX1 and fatty acid binding protein (Ishiyama et al. 2010). Eicosapentaenoic and dehydroascorbic acid (DHA) have anti-inflammatory properties and can be beneficial in atherosclerosis (Merched et al. 2008). Nitro-fatty acids are formed in oxidative stress conditions as a result of interaction of fatty acids with reactive nitrogen species (Khoo and Freeman 2010). They have been demonstrated to possess anti-inflammatory and atheroprotective properties mediated by Nrf2 and PPARγ signalling (Schopfer et al. 2010). In mouse model of atherosclerosis, treatment with nitro-fatty acids resulted in plaque stabilisation due to increased collagen deposition (Bonacci et al. 2011). While high level of LDL is associated with increased risk of atherosclerosis, HDL is known to have atheroprotective functions, improving cholesterol efflux and metabolism (Rader 2006). These protective effects are partly mediated by the anti-inflammatory activity of HDL. In mouse model of atherosclerosis, normalisation of HDL serum levels resulted in a decrease of pro-inflammatory macrophage numbers in the lesions and to an increase of M2 macrophage markers CD163, Arg-1 and transcription factor FIZZ1 (Feig et al. 2011). The expression of Arg-1 and FIZZ1 was dependent on STAT6 (Sanson et al. 2013). In general, there is a complex interplay between various types of native and modified lipoprotein particles and macrophage subtypes initiated by them, and more studies are needed to investigate these relationships in detail (Getz and Reardon 2015).
It is well known that the aggregation of foam cells accompanied by the destruction of foam cells in early atherosclerotic lesions eventually leads to the formation of a necrotic core in a growing atherosclerotic lesion (Bobryshev 2006). In advanced atherosclerotic plaques, some macrophage origin foam cells can be formed directly in the subendothelial space in close proximity to the luminal endo thelium (Fig. 11.6a). The formation of foam cells in such close proximity to the luminal endothelium can lead to disintegration of luminal endothelial monolayer (Fig. 11.6b) and thus can be considered as one of reasons of plaque rupture (Nagornev et al. 1991).
Macrophage foam cells in the intima of the aorta. (a) Formation of a foam cell in the subendothelial space in close proximity to the luminal endothelial cells (E). Transmission Electron Microscopy. (b) Rupture of the luminal endothelial monolayer accompanied by the exit of a degenerating foam cell to the blood circulation. Scanning Electron Microscopy. Scale bars = 2 μm (a, b). [Reproduced from Bobryshev 1983 (Bobryshev YV. Morpho-functional characterization of the endothelium of the aorta of rabbits at experimental hypercholesterolemia. Thesis of Candidate of Science. Leningrad, USSR, 1983, 312p)]
3.4 Macrophage Activation as Therapeutic Target in Atherosclerosis
The prominent role of macrophages phenotypic changes in atherosclerotic lesions indicates the importance of exploring the possibilities of immunocorrective therapy for the treatment of atherosclerotic patients. Monocytes/macrophages isolated from human blood have been used as a model for testing macrophage activation in atherosclerotic patients in comparison with healthy individuals (Orekhov et al. 2015). It has been demonstrated that monocytes/macrophages from atherosclerotic patients had a decreased ability to polarise towards pro- or anti-inflammatory phenotype in response to IFN-γ or IL-4, respectively, as well as a high degree of individual difference in macrophage ability for polarisation between the studied subjects.
Macrophage-based assay has also been successfully used for the evaluation of beneficial effects of potential anti-atherosclerotic substances. Isolated human monocytes/macrophages stimulated with IFN-γ or IL-4 were incubated with extracts of various botanicals: hawthorn flowers (Crataegus sp.), elderberry (Sambucus nigra), calendula (Calendula officinalis), St. John’s wort (Hypericum perforatum) and violet (Viola sp.). Polarisation towards pro- or anti-inflammatory phenotype was assessed by measuring the production of TNF-α and CCL18. It was demonstrated that extracts of hawthorn and St. John’s wort caused macrophage depolarisation, reducing the production of both markers, which may be exploited for therapeutic purposes (Orekhov et al. 2015).
The ability of several anti-atherogenic drugs to influence macrophage phenotype was tested on primary monocyte-derived macrophages stimulated or not with IFN-γ for 7 days. Allicor is a garlic powder preparation manufactured by Inat-Pharma (Russia), which possesses the hypocholesterolaemic and anti-atherosclerotic activities (Orekhov et al. 2013). SkQ1 is an antioxidant manufactured by Lomonosov Moscow State University (Russia). Vezugen is a peptide complex (lysine, glutamic acid, aspartic acid) manufactured by JSC Pharm-Sintez (Russia), which improves metabolism in vascular wall cells. Cellex is another polypeptide drug based on pig brain extract manufactured by JSC Pharm-Sintez (Russia), which is aimed to improve cerebral functions. CardioHealth is a plant complex with antihypertensive and moderate hypoglycaemic and hypolipidaemic effects (Ter-Grigoryan et al. 2003; Khavinson et al. 2014; Kulesh and Shestakov 2016). Vezugen, Allikor, Cellex, CardioHealth and SkQ1 (see Appendix) were added to macrophages at the concentrations ranging from 10−5 to 10−2 μg/ml and the cells were incubated for 24 h. We found that Vezugen, Allikor and CardioHealth had no effect, while Tsellex at concentration 10−3 μg/ml caused a decrease of TNF-α expression level in M0 macrophages (Table 11.3). At the same time, SkQ1 at concentration 10−4 μg/ml caused a decrease of TNF-α expression level in both of M0 and M1 macrophages.
Macrophage-based model was used for studying anti-atherogenic effects of the previously described experimental drugs with regard to reducing intracellular cholesterol accumulation. Blood sampling was performed prior to the drug administration, as well as in 2 and 4 h after administration. The obtained serum was evaluated for the ability to induce intracellular cholesterol accumulation in primary cultures of macrophages and was isolated from a healthy donor. Macrophages were incubated with patient serum samples (10%) for 24 h, and intracellular cholesterol concentration was measured. The experimental drugs were demonstrated to reduce cholesterol accumulation in the macrophage-based cellular model (Table 11.4). The description of the experimental drugs is provided in Appendix.
4 Conclusion
Macrophages represent a cell type, importantly involved in the development of local inflammatory response and atherosclerotic lesion growth. Macrophages that are present in atherosclerotic lesions belong to either pro-inflammatory phenotype or anti-inflammatory phenotype or alternatively activated phenotype. While pro-inflammatory macrophages promote the inflammatory response and participate in lipid accumulation, anti-inflammatory macrophages are responsible for the inflammation resolution and plaque stabilisation. The growth of atherosclerotic lesions notably depends on the balance between different macrophage phenotype populations. The properties of macrophages relevant to diverse functional predisposition make macrophages to be an attractive therapeutic target in search of novel anti-atherosclerotic treatments.
References
Bae YS, Lee JH, Choi SH, Kim S, Almazan F, Witztum JL, Miller YI (2009) Macrophages generate reactive oxygen species in response to minimally oxidized low-density lipoprotein: Toll-like receptor 4- and spleen tyrosine kinase-dependent activation of nadph oxidase 2. Circ Res 104:210–218, 221p following 218
Belge KU, Dayyani F, Horelt A, Siedlar M, Frankenberger M, Frankenberger B, Espevik T, Ziegler-Heitbrock L (2002) The proinflammatory cd14+cd16+dr++ monocytes are a major source of tnf. J Immunol 168:3536–3542
Biswas SK, Mantovani A (2010) Macrophage plasticity and interaction with lymphocyte susbets: cancer as a paradigm. Nat Immunol 11:889–896
Bobryshev YV (1983) Morpho-functional characterization of the endothelium of the aorta of rabbits at experimental hypercholesterolemia. Thesis of Candidate of Science. Leningrad, USSR, 312p
Bobryshev YV (2006) Monocyte recruitment and foam cell formation in atherosclerosis. Micron 37:208–222
Bobryshev YV, Shchelkunova TA, Morozov IA, Rubtsov PM, Sobenin IA, Orekhov AN, Smirnov AN (2013) Changes of lysosomes in the earliest stages of the development of atherosclerosis. J Cell Mol Med 17:626–635
Bonacci G, Schopfer FJ, Batthyany CI, Rudolph TK, Rudolph V, Khoo NK, Kelley EE, Freeman BA (2011) Electrophilic fatty acids regulate matrix metalloproteinase activity and expression. J Biol Chem 286:16074–16081
Bouhlel MA, Derudas B, Rigamonti E, Dièvart R, Brozek J, Haulon S, Zawadzki C, Jude B, Torpier G, Marx N, Staels B, Chinetti-Gbaguidi G (2007) Ppar gamma activation primes human monocytes into alternative m2 macrophages with anti-inflammatory properties. Cell Metab 6:137–143
Boyanovsky BB, Li X, Shridas P, Sunkara M, Morris AJ, Webb NR (2010) Bioactive products generated by group v spla(2) hydrolysis of ldl activate macrophages to secrete pro-inflammatory cytokines. Cytokine 50:50–57
Boyle JJ, Harrington HA, Piper E, Elderfield K, Stark J, Landis RC, Haskard DO (2009) Coronary intraplaque hemorrhage evokes a novel atheroprotective macrophage phenotype. Am J Pathol 174:1097–1108
Boyle JJ, Johns M, Kampfer T, Nguyen AT, Schaer DJ, Mason JC, Haskard DO (2012) Activating transcription factor 1 directs Mhem atheroprotective macrophages through coordinated iron handling and foam cell protection. Circ Res 110:20–33
Brown MS, Goldstein JL (1983) Lipoprotein metabolism in the macrophage: implications for cholseterol deposition in atherosclerosis. Annu Rev Biochem 52:223–261
Chen EY, Tan CM, Kou Y, Duan Q, Wang Z, Meirelles GV, Clark NR, Ma’ayan A (2013) Enrichr: interactive and collaborative HTML5 gene list enrichment analysis tool. BMC Bioinform 14:128
Chinetti-Gbaguidi G, Baron M, Bouhlel MA, Vanhoutte J, Copin C, Sebti Y, Derudas B, Mayi T, Bories G, Tailleux A, Haulon S, Zawadzki C, Jude B, Staels B (2011) Human atherosclerotic plaque alternative macrophages display low cholesterol handling but high phagocytosis because of distinct activities of the ppargamma and lxralpha pathways. Circ Res 108:985–995
Chistiakov DA, Bobryshev YV, Nikiforov NG, Elizova NV, Sobenin IA, Orekhov AN (2015) Macrophage phenotypic plasticity in atherosclerosis: the associated features and the peculiarities of the expression of inflammatory genes. Int J Cardiol 184:436–445
Cho KY, Miyoshi H, Kuroda S, Yasuda H, Kamiyama K, Nakagawara J, Takigami M, Kondo T, Atsumi T (2013) The phenotype of infiltrating macrophages influences arteriosclerotic plaque vulnerability in the carotid artery. J Stroke Cerebrovasc Dis 22:910–918
Choi SH, Harkewitz R, Lee JH, Boullier A, Almazan F, Li AC, Witzum JL, Bae YS, Miller YI (2009) Lipoprotein accumulation in macrophages via toll-like receptor-4-dependent fluid phase uptake. Circ Res 104:1355–1363
Collins T, Cybulsky MI (2001) NF-kappaB: pivotal mediator or innocent bystander in atherogenesis? J Clin Invest 107:255–264
Combadiere C, Potteaux S, Rodero M, Simon T, Pezard A, Esposito B, Merval R, Proudfoot A, Tedgui A, Mallat Z (2008) Combined inhibition of ccl2, cx3cr1, and ccr5 abrogates ly6c(hi) and ly6c(lo) monocytosis and almost abolishes atherosclerosis in hypercholesterolemic mice. Circulation 117:1649–1657
Cros J, Cagnard N, Woollard K, Patey N, Zhang SY, Senechal B, Puel A, Biswas SK, Moshous D, Picard C, Jais JP, D’Cruz D, Casanova JL, Trouillet C, Geissmann F (2010) Human cd14dim monocytes patrol and sense nucleic acids and viruses via tlr7 and tlr8 receptors. Immunity 33:375–386
Cybulsky MI, Cheong C, Robbins CS (2016) Macrophages and dendritic cells—partners in atherogenesis. Circ Res 118:637–652
da Silva RF, Lappalainen J, Lee-Rueckert M, Kovanen PT (2016) Conversion of human M-SCF macrophages into foam cells reduces their proinflammatory response to classical M1-polarizing activation. Atherosclerosis 248:170–178
de Gaetano M, Crean D, Barry M, Belton O (2016) M1- and M2-type macrophage responses are predictive of adverse outcomes in human atherosclerosis. Front Immunol 7:275
De Paoli F, Staels B, Chinetti-Gbaguidi G (2014) Macrophage phenotypes and their modulation in atherosclerosis. Circ J 78:1775–1781
Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, Abela GS, Franchi L, Nuñez G, Schnurr M, Espevik T, Lien E, Fitzgerald KA, Rock KL, Moore KJ, Wright SD, Hornung V, Latz E (2010) NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature 464:1357–1361
Erbel C, Tyka M, Helmes CM, Akhavanpoor M, Rupp G, Domschke G, Linden F, Wolf A, Doesch A, Lasitschka F, Katus HA, Gleissner CA (2015) CXCL4-induced plaque macrophages can be specifically identified by co-expression of MMP7+S100A8+ in vitro and in vivo. Innate Immun 21:255–265
Feig JE, Rong JX, Shamir R, Sanson M, Vengrenyuk Y, Liu J, Rayner K, Moore K, Garabedian M, Fisher EA (2011) Hdl promotes rapid atherosclerosis regression in mice and alters inflammatory properties of plaque monocyte-derived cells. Proc Natl Acad Sci U S A 108:7166–7171
Ferrante CJ, Pinhal-Enfield G, Elson G, Cronstein BN, Hasko G, Outram S, Leibovich SJ (2013) The adenosine-dependent angiogenic switch of macrophages to an m2-like phenotype is independent of interleukin-4 receptor alpha (il-4ralpha) signaling. Inflammation 36:921–931
Finn AV, Nakano M, Polavarapu R, Karmali V, Saeed O, Zhao X, Yazdani S, Otsuka F, Davis T, Habib A, Narula J, Kolodgie FD, Virmani R (2012) Hemoglobin directs macrophage differentiation and prevents foam cell formation in human atherosclerotic plaques. J Am Coll Cardiol 59:166–177
Galkina E, Ley K (2007) Vascular adhesion molecules in atherosclerosis. Arterioscler Thromb Vasc Biol 27:2292–2301
Geissmann F, Jung S, Littman DR (2003) Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity 19:71–82
Getz GS, Reardon CA (2015) Atherogenic lipids and macrophage subsets. Curr Opin Lipidol 26:357–361
Gimbrone MA, García-Cardeña G (2016) Endothelial cell dysfunction and the pathobiology of atherosclerosis. Circ Res 118:620–636
Ginhoux F, Jung S (2014) Monocytes and macrophages: developmental pathways and tissue homeostasis. Nat Rev Immunol 14:392–404
Hägg DA, Olson FJ, Kjelldahl J, Jernås M, Thelle DS, Carlsson LM, Fagerberg B, Svensson PA (2009) Expression of chemokine (C-C motif) ligand 18 in human macrophages and atherosclerotic plaques. Atherosclerosis 204:e15–e20
Hansson GK, Libby P, Tabas I (2015) Inflammation and plaque vulnerability. J Intern Med 278:483–493
Huang Z, Li W, Wang R, Zhang F, Chi Y, Wang D, Liu Z, Zhang Y, Matsuura E, Liu Q (2010) 7-ketocholesteryl-9-carboxynonanoate induced nuclear factor-kappa b activation in j774a.1 macrophages. Life Sci 87:651–657
Huber J, Boechzelt H, Karten B, Surboeck M, Bochkov VN, Binder BR, Sattler W, Leitinger N (2002) Oxidized cholesteryl linoleates stimulate endothelial cells to bind monocytes via the extracellular signal-regulated kinase 1/2 pathway. Arterioscler Thromb Vasc Biol 22:581–586
Ishiyama J, Taguchi R, Yamamoto A, Murakami K (2010) Palmitic acid enhances lectin-like oxidized ldl receptor (lox-1) expression and promotes uptake of oxidized ldl in macrophage cells. Atherosclerosis 209:118–124
Jedidi I, Couturier M, Therond P, Gardes-Albert M, Legrand A, Barouki R, Bonnefont-Rousselot D, Aggerbeck M (2006) Cholesteryl ester hydroperoxides increase macrophage cd36 gene expression via pparalpha. Biochem Biophys Res Commun 351:733–738
Kadl A, Meher AK, Sharma PR, Lee MY, Doran AC, Johnstone SR, Elliott MR, Gruber F, Han J, Chen W, Kensler T, Ravichandran KS, Isakson BE, Wamhoff BR, Leitinger N (2010) Identification of a novel macrophage phenotype that develops in response to atherogenic phospholipids via Nrf2. Circ Res 107:737–746
Khavinson VK, Lin’kova NS, Evlashkina EV, Durnova AO, Kozlov KL, Gutop OE (2014) Molecular aspects of anti-atherosclerotic effects of short peptides. Bull Exp Biol Med 158:159–163
Khoo NK, Freeman BA (2010) Electrophilic nitro-fatty acids: anti-inflammatory mediators in the vascular compartment. Curr Opin Pharmacol 10:179–184
Kockx MM, Cromheeke KM, Knaapen MW, Bosmans JM, De Meyer GR, Herman AG, Bult H (2003) Phagocytosis and macrophage activation associated with hemorrhagic microvessels in human atherosclerosis. Arterioscler Thromb Vasc Biol 23:440–446
Krauss RM (2010) Lipoprotein subfractions and cardiovascular disease risk. Curr Opin Lipidol 21:305–311
Kruth HS (2011) Receptor-independent fluid-phase pinocytosis mechanisms for induction of foam cell formation with native low-density lipoprtotein particles. Curr Opin Lipidol 22:386–393
Kulesh AA, Shestakov VV (2016) Post-stroke cognitive impairment and the possibility of treatment with cellex. Zh Nevrol Psikhiatr Im S S Korsakova 116:38–42
Kuleshov MV, Jones MR, Rouillard AD, Fernandez NF, Duan Q, Wang Z, Koplev S, Jenkins SL, Jagodnik KM, Lachmann A, McDermott MG, Monteiro CD, Gundersen GW, Ma'ayan A (2016) Enrichr: a comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res 44(W1):W90–W97
Leonarduzzi G, Gargiulo S, Gamba P, Perrelli MG, Castellano I, Sapino A, Sottero B, Poli G (2010) Molecular signaling operated by a diet-compatible mixture of oxysterols in up-regulating cd36 receptor in cd68 positive cells. Mol Nutr Food Res 54(Suppl 1):S31–S41
Li Y, Schwabe RF, DeVries-Seimon T, Yao PM, Gerbod-Giannone MC, Tall AR, Davis RJ, Flavell R, Brenner DA, Tabas I (2005) Free cholesterol-loaded macrophages are an abundant source of tumor necrosis factor-alpha and interleukin-6: model of NF-kappaB- and map kinase-dependent inflammation in advanced atherosclerosis. J Biol Chem 280:21763–21772
Libby P (2002) Inflammation in atherosclerosis. Nature 420:868–874
Libby P (2013) Mechanisms of acute coronary syndromes and their implications for therapy. N Engl J Med 368:2004–2013
Lim WS, Timmins JM, Seimon TA, Sadler A, Kolodgie FD, Virmani R, Tabas I (2008) Signal transducer and activator of transcription-1 is critical for apoptosis in macrophages subjected to endoplasmic reticulum stress in vitro and in advanced atherosclerotic lesions in vivo. Circulation 117:940–951
Mantovani A, Sozzani S, Locati M, Allavena P, Sica A (2002) Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol 23:549–555
Martinez FO, Gordon S (2015) The evolution of our understanding of macrophages and translation of findings toward the clinic. Expert Rev Clin Immunol 11:5–13
Martinez FO, Gordon S, Locati M, Mantovani A (2006) Transcriptional profiling of the human monocyte-to macrophage differentiation and polarization: new molecules and patterns of gene expression. J Immunol 177:7303–7311
Martinez FO, Sica A, Mantovani A, Locati M (2008) Macrophage activation and polarization. Front Biosci 13:453–461
Merched AJ, Ko K, Gotlinger KH, Serhan CN, Chan L (2008) Atherosclerosis: evidence for impairment of resolution of vascular inflammation governed by specific lipid mediators. FASEB J 22:3595–3606
Moore KJ, Freeman MW (2006) Scavenger receptors in atherosclerosis; beyond lipid uptake. Arterioscler Thromb Vasc Biol 26:1702–1711
Moore KJ, Tabas I (2011) Macrophages in the pathogenesis of atherosclerosis. Cell 145:341–355
Moore KJ, Sheedy FJ, Fisher EA (2013) Macrophages in atherosclerosis: a dynamic balance. Nat Rev Immunol 13:709–721
Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, Gordon S, Hamilton JA, Ivashkiv LB, Lawrence T, Locati M, Mantovani A, Martinez FO, Mege JL, Mosser DM, Natoli G, Saeij JP, Schultze JL, Shirey KA, Sica A, Suttles J, Udalova I, van Ginderachter JA, Vogel SN, Wynn TA (2014) Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity 41:14–20
Nagornev VA, Popov AV, Pleskov VM, Bobryshev IV (1985) Ultrastructural characteristics of macrophage transformation in foam cells in in vitro experiments. Biull Eksp Biol Med 99:617–619
Nagornev VA, Bobryshev IV, Ivanovskiĭ IV, Bogachev IV (1991) Role of monocytes-macrophages in atherogenesis. Arkh Patol 53:23–29
Nathan C, Ding A (2010) Nonresolving inflammation. Cell 140:871–882
Novoselov VV, Sazonova MA, Ivanova EA, Orekhov AN (2015) Study of the activated macrophage transcriptome. Exp Mol Pathol 99:575–580
Orekhov AN, Andreeva ER, Andrianova IV, Bobryshev YV (2010) Peculiarities of cell composition and cell proliferation in different type atherosclerotic lesions in carotid and coronary arteries. Atherosclerosis 212:436–443
Orekhov AN, Sobenin IA, Korneev NV, Kirichenko TV, Myasoedova VA, Melnichenko AA, Balcells M, Edelman ER, Bobryshev YV (2013) Anti-atherosclerotic therapy based on botanicals. Recent Pat Cardiovasc Drug Discov 8:56–66
Orekhov AN, Bobryshev YV, Chistiakov DA (2014) The complexity of cell composition of the intima of large arteries: focus on pericyte-like cells. Cardiovasc Res 103:438–451
Orekhov AN, Sobenin IA, Gavrilin MA, Gratchev A, Kotyashova SY, Nikiforov NG, Kzhyshkowska J (2015) Macrophages in immunopathology of atherosclerosis: a target for diagnostics and therapy. Curr Pharm Des 21:1172–1179
Pober JS, Sessa WC (2007) Evolving functions of endothelial cells in inflammation. Nat Rev Immunol 7:803–815
Rader DJ (2006) Molecular regulation of hdl metabolism and function: implications for novel therapies. J Clin Invest 116:3090–3100
Randolph GJ (2014) Mechanisms that regulate macrophage burden in atherosclerosis. Circ Res 114:1757–1771
Robbins CS, Hilgendorf I, Weber GF, Theurl I, Iwamoto Y, Figueiredo JL, Gorbatov R, Sukhova GK, Gerhardt LM, Smyth D, Zavitz CC, Shikatani EA, Parsons M, van Rooijen N, Lin HY, Husain M, Libby P, Nahrendorf M, Weissleder R, Swirski FK (2013) Local proliferation dominates lesional macrophage accumulation in atherosclerosis. Nat Med 19:1166–1172
Ross R (1999) Atherosclerosis—an inflammatory disease. N Engl J Med 340:115–126
Sanchis-Gomar F, Perez-Quilis C, Leischik R, Lucia A (2016) Epidemiology of coronary heart disease and acute coronary syndrome. Ann Transl Med 4:256
Sanson M, Distel E, Fisher EA (2013) Hdl induces the expression of the m2 macrophage markers arginase 1 and fizz-1 in a stat6-dependent process. PLoS One 8:e74676
Schopfer FJ, Cole MP, Groeger AL, Chen CS, Khoo NK, Woodcock SR, Golin-Bisello F, Motanya UN, Li Y, Zhang J et al (2010) Covalent peroxisome proliferator-activated receptor gamma adduction by nitro-fatty acids: selective ligand activity and anti-diabetic signaling actions. J Biol Chem 285:12321–12333
Seimon T, Tabas I (2009) Mechanisms and consequences of macrophage apoptosis in atherosclerosis. J Lipid Res 50(Suppl):S382–S387
Smith JD, Trogan E, Ginsberg M, Grigaux C, Tian J, Miyata M (1995) Decreased atherosclerosis in mice deficient in both macrophage colony-stimulating factor (op) and apolipoprotein E. Proc Natl Acad Sci U S A 92:8264–8268
Sobenin IA, Salonen JT, Zhelankin AV, Melnichenko AA, Kaikkonen J, Bobryshev YV, Orekhov AN (2014) Low density lipoprotein-containing circulating immune complexes: role in atherosclerosis and diagnostic value. Biomed Res Int 2014:205697
Stoger JL, Gijbels MJ, van der Velden S, Manca M, van der Loos CM, Biessen EA, Daemen MJ, Lutgens E, de Winther MP (2012) Distribution of macrophage polarization markers in human atherosclerosis. Atherosclerosis 225:461–468
Swirski FK, Libby P, Aikawa E, Alcaide P, Luscinskas FW, Weissleder R, Pittet MJ (2007) Ly-6chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. J Clin Invest 117:195–205
Swirski FK, Weissleder R, Pittet MJ (2009) Heterogeneous in vivo behavior of monocyte subsets in atherosclerosis. Arterioscler Thromb Vasc Biol 29:1424–1432
Tall AR, Yvan-Charvet L (2015) Cholesterol, inflammation and innate immunity. Nat Rev Immunol 15:104–116
Ter-Grigoryan V, Panosyan V, Panossian A, Wikman G (2003) Comparative evaluation of the efficacy and safety of multiple doses of Cardiohealth in comparison with Renitec in patients with mild to moderate hypertension. In 3rd International symposium on natural drugs, pp 223–226
Tertov VV, Kaplun VV, Sobenin IA, Orekhov AN (1998) Low-density lipoprotein modification occurring in human plasma possible mechanism of in vivo lipoprotein desialylation as a primary step of atherogenic modification. Atherosclerosis 138:183–195
Torzewski M, Lackner KJ (2006) Initiation and progression of atherosclerosis—enzymatic or oxidative modification of low-density lipoprotein? Clin Chem Lab Med 44:1389–1394
Torzewski M, Suriyaphol P, Paprotka K, Ochsenhirt V, Schmitt A, Han SR, Husmann M, Gerl VB, Bhakdi S, Lackner KJ (2004) Enzymatic modification of low-density lipoprotein in the arterial wall: a new role for plasmin and matrix metalloproteinases in atherogenesis. Arterioscler Thromb Vasc Biol 24:2130–2136
van Furth R, Cohn ZA (1968) The origin and kinetics of mononuclear phagocytes. J Exp Med 128:415–435
van Tits LJ, Stienstra R, van Lent PL, Netea MG, Joosten LA, Stalenhoef AF (2011) Oxidized ldl enhances pro-inflammatory responses of alternatively activated m2 macrophages: a crucial role for kruppel-like factor 2. Atherosclerosis 214:345–349
Younis N, Sharma R, Soran H, Charlton-Menys V, Elseweidy M, Durrington PN (2008) Glycation as an atherogenuc modification of LDL. Curr Opin Lipidol 19:552
Yvan-Charvet L, Pagler T, Gautier EL, Avagyan S, Siry RL, Han S, Welch CL, Wang N, Randolph GJ, Snoeck HW, Tall AR (2010) Atp-binding cassette transporters and hdl suppress hematopoietic stem cell proliferation. Science 328:1689–1693
Ziegler-Heitbrock L (2007) The cd14+ cd16+ blood monocytes: Their role in infection and inflammation. J Leukoc Biol 81:584–592
Ziegler-Heitbrock L, Ancuta P, Crowe S, Dalod M, Grau V, Hart DN, Leenen PJ, Liu YJ, MacPherson G, Randolph GJ, Scherberich J, Schmitz J, Shortman K, Sozzani S, Strobl H, Zembala M, Austyn JM, Lutz MB (2010) Nomenclature of monocytes and dendritic cells in blood. Blood 116:e74–e80
Zizzo G, Hilliard BA, Monestier M, Cohen PL (2012) Efficient clearance of early apoptotic cells by human macrophages requires m2c polarization and mertk induction. J Immunol 189:3508–3520
Acknowledgements
This work was supported by the Russian Science Foundation (Grant # 15-15-10022), Russian Federation. The authors wish to thank Dr. Ekaterina A. Ivanova, Katholieke Universiteit Leuven, Belgium, for the help with the preparation of the manuscript.
Conflict of Interest Disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Appendix
Appendix
1.1 Information about Allikor, SkQ1, Vezugen, Cellex and CardioHealth
1.1.1 Allicor
Manufacturer: Inat-Pharma (Russia
Composition: 1 tablet contains 300 mg of garlic powder.
Pharmacological effects: hypocholesterolaemic, antiagregatine, fibrinolytic, hypotensive. Reduces cholesterol and triglycerides in the plasma for hyperlipidaemia, slows the development of atherosclerosis, promotes the resorption of existing plaques, reduces blood sugar and blood pressure, inhibits platelet aggregation, normalises the increased blood clotting and promotes lysis of fresh thrombus.
Indications: atherosclerosis, hypertension, myocardial period, diabetes, migraine, impotence, decreased immunity, pregnancy; prevention of myocardial infarction and stroke; postoperative complications in patients with vascular disease, flu and colds.
Contraindications: Hypersensitivity to the drug.
Side effects: None known.
1.1.2 SkQ1
Manufacturer: Lomonosov Moscow State University (Russia)
Composition: SkQ1 is dissolved in 50% aqueous propylene glycol. The three most important segments of the molecule SkQ1 are Plastohinol, a powerful natural antioxidant carrying electrons from the chloroplasts of plants; C10, transports SkQ1 molecule in the cell membrane; Triphenylphosphonium, positively charged group delivering the components in the mitochondria.
Pharmacological effects: SkQ1 blocks and reduces the amount of free radicals formed by cells and thus prevents apoptosis-induced mitochondrial reactive oxygen species.
Indications: SkQ1 part of the eyedrops Vizomitin (Antioxidant, keratoprotektornoe agent for the treatment of early age-related cataract and the syndrome of “dry eye”), as well as part of the MitoVitan serum
Contraindications: Hypersensitivity to the drug.
Side effects: Allergic reactions.
1.1.3 Vezugen
Producer: JSC “pharm” (Russia)
Composition: peptide complex AC-2 (lysine, glutamic acid, aspartic acid). Other ingredients: microcrystalline cellulose, sugar, beet sugar, lactose, starch, Tween-80.
Pharmacological effects: Peptide complex AC-2 has directed tissue-specific effects on the vascular wall. Vezugen promotes normalisation of the functional state of vessels, regulates metabolism in the cells of the vascular wall, improves the condition of the vessel walls and normalises lipid metabolism.
Indications: general and cerebral arteriosclerosis; hypertension; coronary heart disease; endarteritis; of varicose veins of the lower extremities; systemic and local microcirculation disorders; vascular encephalopathy; hypercholesterolaemia; vascular dystonia; psycho-emotional stress; effects of acute stroke; the impact of various factors on the extreme. Vezugen also used for the prevention of vascular disease in the elderly.
Contraindications: individual intolerance to the components of dietary supplements, pregnancy, breastfeeding.
Side effects: None known.
1.1.4 Cellex
Producer: JSC “Pharm-Sintez” (Russia)
Composition in 1 ml: active substance: polypeptides from hog brain of embryos based on 0.9–2.4 mg of total protein (nominal total protein content—1.65 mg per 1 ml of substance); excipients: 3.75 mg of glycine, 0.1 M disodium hydrogen phosphate solution, 5.85 mg of sodium chloride, 0.005 mg of Polysorbate 80, purified water.
Pharmacological effects: The presence of tissue-specific signalling proteins and polypeptides leads to neuroreparation. The drug activates the secondary neuroprotection by stimulating synaptogenesis processes of autophagy recovery signals. Tissue-specific and systemic restorative effect was found as well as the restoration of the regenerative and reparative potential of the brain cells reducing the number of damaged cells and the severity of perifocal oedema in the penumbra, the restoration of microcirculation and perfusion. Recovers and regulates stimulation of different compartments of central nervous system. The therapeutic effect usually develops within 3–5 days after the start of administration.
Indications: Cerebrovascular diseases.
Contraindications: Epilepsy; Manic psychosis; age of 18 years (due to the lack of clinical data).
Side effects: allergic reactions.
1.1.5 CardioHealth
CardioHealth is a plant complex from the leaves of the European Olive, standardised to oleuropein content (4 mg), Potentilla goose and Andrographis paniculata. CardioHealth has antihypertensive and moderate hypoglycaemic and hypolipidaemic effects.
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Bobryshev, Y.V., Nikiforov, N.G., Elizova, N.V., Orekhov, A.N. (2017). Macrophages and Their Contribution to the Development of Atherosclerosis. In: Kloc, M. (eds) Macrophages. Results and Problems in Cell Differentiation, vol 62. Springer, Cham. https://doi.org/10.1007/978-3-319-54090-0_11
Download citation
DOI: https://doi.org/10.1007/978-3-319-54090-0_11
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-54089-4
Online ISBN: 978-3-319-54090-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)