Abstract
The heat-sensitive transient receptor potential vanilloid 1 (TRPV1) channels are expressed in the peripheral and central nervous systems. However, there is no report on how the activation of TRPV1 causes the modulation of neuronal activity in the medullary respiratory center. We examined effects of capsaicin, a specific agonist of TRPV1 channels, on respiratory rhythm generation in brainstem-spinal cord preparation from newborn rats. Capsaicin induced a biphasic response in the respiratory rhythm (a transient decrease followed by an increase in the C4 rate). The second-phase excitatory effect (but not the initial inhibitory effect) in the biphasic response was partly blocked by capsazepine or AMG9810 (TRPV1 antagonists). Capsaicin caused strong desensitization. After its washout, the strength of C4 burst inspiratory activity was augmented once per four to five respiratory cycles. The preinspiratory and inspiratory neurons showed tonic firings due to membrane depolarization during the initial inhibitory phase. In the presence of TTX, capsaicin increased the fluctuation of the membrane potential of the CO2-sensitive preinspiratory neurons in the parafacial respiratory group (pFRG), accompanied by slight depolarization. The C4 inspiratory activity did not stop, even 60–90 min after the application of 50/100 μM capsaicin. Voltage-sensitive dye imaging demonstrated that the spatiotemporal pattern of the respiratory rhythm generating networks after application of capsaicin (50 μM, 70–90 min) was highly similar to the control. A histochemical analysis using TRPV1 channel protein antibodies and mRNA demonstrated that the TRPV1 channel-positive cells were widely distributed in the reticular formation of the medulla, including the pFRG. Our results showed that the application of capsaicin in the medulla has various influences on the respiratory center: transient inhibitory and subsequent excitatory effects on the respiratory rhythm and periodical augmentation of the inspiratory burst pattern. The effects of capsaicin were partially blocked by TRPV1 antagonists but could be also induced at least partially via the non-specific action. Our results also suggested a minor contribution of the TRPV1 channels to central chemoreception.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Capsaicin is known as a specific agonist of the heat-sensitive transient receptor potential vanilloid 1 (TRPV1) channels. It stimulates the sensation of pain via the nerves to cause an intense burning feeling. The TRPV1 channels are expressed in various peripheral nervous systems, including (but not limited to) the dorsal root ganglion, the trigeminal ganglion, the enteric ganglia, and the nerve plexus [35]. In the central nervous system, the TRPV1 channels are known to also be expressed in various brain regions, suggesting their involvement in various brain functions through the modulation of brain activity [5, 12, 27, 38]. Regarding the respiratory center in the lower brainstem, capsaicin is reported to evoke the dose-dependent release of glutamate and depletion of substance P (SP) in the fibers within the preBötzinger complex (preBötC), an inspiratory (Insp) rhythm generator in the ventral medulla. The depletion of SP and/or glutamate by capsaicin then causes the cessation of respiratory rhythm in neonatal rat slices [17]. Hirata and Oku [9] reported that the cells in glia-rich medullary cell cultures expressed the mRNA of TRPV1 as well as TRPA1 and TRPM8 and suggested that the TRP channels played a role in central chemoreception. We reported that a TRPM8 agonist caused depressive effects on the respiratory rhythm-generating neurons in the medulla [32] and that a TRPA1 agonist induced the long-lasting facilitation of respiratory rhythm [33]. These effects were thought to be primarily produced by the action of TRP agonists on preinspiratory (Pre-I) neurons in the parafacial respiratory group (pFRG) via their interaction with the preBötC Insp neurons. However, there are no reports on how the activation of TRPV1 causes the modulation of neuronal activity in the medullary respiratory center (including the pFRG and preBötC). In the present study, we examined the effects of capsaicin on respiratory rhythm generation in a brainstem-spinal cord preparation from newborn rats. Some of the results have previously been presented in abstract form [34].
Materials and methods
The experimental protocols were approved by the Animal Research Committee of Showa University, which operates in accordance with Law No. 105 for the care and use of laboratory animals of the Japanese Government.
Preparations
Experiments were performed with brainstem-spinal cord preparations from newborn (0 to 2 days old) Wistar rats. The newborn rats were deeply anesthetized with isoflurane, and the brainstem and spinal cord were isolated as reported previously [19, 20, 30]. In most experiments, the preparations were cut transversely at a level just rostral to the anterior inferior cerebellar artery (standard preparation), corresponding to the level between the roots of the sixth cranial nerve and the lower border of the trapezoid bod. In some experiments, the preparations were cut at a level of the more caudal medulla corresponding to the caudal end of the facial nucleus in which approximately 80% of the pFRG was removed (caudal preparation) [33]. The preparations were continuously superfused with artificial cerebrospinal fluid (ACSF [30]) (composition [in mM]: 124 NaCl, 5 KCl, 1.2 KH2PO4, 2.4 CaCl2, 1.3 MgCl2, 26 NaHCO3, 30 glucose, equilibrated with 95% O2 and 5% CO2; pH 7.4) at a rate of 2.5–3 ml/min in a 2-ml chamber and were maintained at a temperature of 25–26 °C. The inspiratory activity corresponding to phrenic nerve activity was monitored from the fourth cervical ventral root (C4).
Drugs
For selective TRPV1 antagonists, we used capsazepine and AMG9810, (E)-3-(4-t-butylphenyl)-N-(2,3-dihydrobenzo[b] [1, 4]dioxin-6-yl)acrylamide [6]. Capsaicin, capsazepine, and AMG9810 were purchased from Sigma-Aldrich (Tokyo, Japan). Calmodulin inhibitor N-(6-aminohexyl)-1-naphthalenesulfonamide hydrochloride (W-7) was purchased from Wako Pure Chemical (Tokyo, Japan). Capsaicin was stocked as a 10 or 100 mM solution in ethanol. Capsazepine and W-7 were stocked as 10 mM solutions in ethanol. AMG9810 was stocked as a 10 mM solution in dimethyl sulfoxide (Wako Pure Chemical). All of the drugs were dissolved with ACSF and applied as a bath. The maximum concentration of vehicles in the final solution was 0.1%. We confirmed that this concentration of DMSO or ethanol has no effect on neuronal activity in the brainstem-spinal cord preparation.
Electrophysiology
The membrane potentials of Pre-I or Insp neurons in the rostral ventrolateral medulla, corresponding to the caudal or rostral part of the pFRG [22], were recorded by a blind whole-cell patch-clamp method [19]. The rostral pFRG neurons were recorded by an approach from the rostral cut surface at a level of 0.5–0.7 mm rostral to the caudal end of the facial nucleus (0 mm), and the caudal pFRG neurons were recorded by an approach from the ventral surface at the level of within ±100 μm from the caudal end of the facial nucleus [1, 33]. The electrodes, which had an inner tip diameter of 1.2–2.0 μm and a resistance of 4–8 MΩ, were filled with the following pipette solution (mM): 130 K-gluconate, 10 ethylene glycol tetra-acetic acid, 10 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid, 2 Na2-ATP, 1 CaCl2, and 1MgCl2, with pH 7.2–7.3 adjusted with KOH. For the histological analysis of the recorded cells, the electrode tips were filled with 0.5% Lucifer yellow (lithium salt). The membrane potential and input resistance at the resting level were measured between bursts. In the most experiments for membrane potential recordings, effects of 10 μM capsaicin were examined, whereas 20 μM was used in some experiments in the presence of TTX to obtain clearer effects of capsaicin. In experiments for capsaicin effects in the presence of TTX, the membrane potential fluctuation was estimated from the standard deviation (SD) at 5 points × 5-s period in each recording. After the experiments, preparations were fixed, and a histological analysis was then performed (see below).
Optical recording
The detailed procedure for making optical recordings using voltage-sensitive dye has been described previously [20]. In brief, brainstem-spinal cord preparations were incubated in ACSF (described above) containing a fluorescent voltage-sensitive dye (50 μg/ml Di-2-ANEPEQ; Molecular Probes, Eugene, OR, USA) for 40–50 min. In the present study, the purpose of the optical recordings was to investigate the spatiotemporal pattern of the respiratory neuron activity in the ventral medulla after long-term treatment (more than 50 min) with a high concentration of capsaicin. In preliminary experiments, we found that the respiratory rhythm became abruptly unstable due to the putative synergistic and toxic effects of the dye and capsaicin when the total time from the start of dye staining to the start of optical recording (after 50 min-capsaicin treatment) was more than 2 h. Therefore, to minimize the time until the start of optical recordings, 50 μM capsaicin was added together with voltage-sensitive dye and incubated during staining in most of the experiments. The preparation was then transferred to the recording chamber (1 ml) and superfused with ACSF containing 50 μM capsaicin. For control images, different preparations were stained by the dye in the absence of capsaicin and then optical recording was performed. The preparation was placed with the ventral surface facing up in a recording chamber mounted on the stage of an upright fluorescence microscope (BX50WIF-2; Olympus, Tokyo, Japan). The neuronal activity in the preparation was detected as changes in the fluorescence of the voltage-sensitive dye, which was observed using an optical recording apparatus (MiCAM02; BrainVision, Tokyo, Japan). The optical path contained a tungsten halogen lamp (150 W), a 510–550-nm excitation filter, a dichroic mirror, and a 590-nm emission filter (U-MWIG2 mirror unit; Olympus). The CCD-based camera head has a 4.80 × 6.40 mm2 imaging area consisting of 124 × 184 pixels. The magnification of the microscope was ×4.0 (XL Fluor 4×/340, NA 0.28; Olympus Optical, Tokyo, Japan), and the final magnification was adjusted to ×2.0 so that an area of 2.40 × 3.20 mm2 was covered by the image sensor. The recordings were made with an acquisition time (i.e., sampling clock) of 20 ms. The optical imaging data were averaged using the C4 activity as the trigger signal. The fluorescence signals for the 10.24 s/trial, including 2.5 s before the initiation of the C4 burst, were averaged for 40 trials.
Immunofluorescence and in situ hybridization
The brainstem-spinal cord preparations were fixed for 2–3 h in fixation solution at 4 °C, immersed in 18% sucrose in PBS overnight, embedded in optimal cutting temperature compound (OCT; Sakura Finetek, Torrance, CA, USA), and then frozen on dry ice. For histological analysis of the Lucifer yellow-stained neurons after electrophysiological measurements, preparations were cut into 50-μm thick transverse sections, followed by further immunohistochemical analysis. To identify motor neuron nuclei in the medulla, the sections from some preparations were stained with NeuroTrace (435/455 blue fluorescence, Invitrogen) for Nissl stain. In another series of immunofluorescence analyses, preparations were cut into 16–30-μm thick transverse sections, and then the expression of TRPV1 channel protein and Phox2b protein in the respiratory-related region of the medulla (and spinal cord and dorsal root ganglion [DRG] for the positive control) were examined. The following primary antibodies were used for immunofluorescence staining: rabbit anti-TRPV1 (1:400 dilution; Trans Genic Inc., Kobe, Japan) and Guinea pig anti-Phox2b (1:1000 dilution) [22]. Alexa Fluor 488 anti-rabbit IgG or Alexa Fluor 546 anti-rabbit IgG (Molecular Probes/Invitrogen) was used as the secondary antibody (1:1000 dilution). Images of the immunofluorescent samples were obtained with ×20 or ×40 objectives on an Olympus fluorescence microscope (BX60; Olympus Optical).
In situ hybridization was performed on brainstems (and DRG for the positive control) that were isolated from three independent Wistar rat neonates. The brainstem was isolated and fixed in fixation solution at 4 °C for 1–2 h. The samples were then immersed in 18% sucrose in PBS, embedded in OCT compound, frozen on dry ice, and cut into 20-μm thick cryosections. Partial cDNAs of rat Trpv1 (nucleotide number 2451–3378 of NM_207608) were obtained via an RT-PCR using rat brain total RNA, subcloned into pGEM-T Easy Vector (Promega, Madison, WI, USA), confirmed by sequencing, and were then used as a riboprobe template. In situ hybridization was performed as described previously with a digoxigenin-UTP (Roche Diagnostics, Basel, Switzerland)-labeled riboprobe [10]. Proteinase K (1 μg/ml) was applied for 3–4 min at 26 °C, and the hybridization temperature was 50 °C. Signals were detected using an antidigoxigenin antibody conjugated with alkaline phosphatase (Roche) and NBT/BCIP (Roche) for chromogen.
Data analyses
All data analyses were performed using the LabChart 7 Pro software program (ADInstruments, Castle Hill, Australia). To assess the effects of capsaicin on C4 activity, the burst rate (bursts/min) was calculated from the mean rate for 3–5 min. The data are presented as the mean and SD for all preparations. The significance of the values was analyzed by a paired t test or by a one-way ANOVA followed by a Tukey-Kramer multiple comparisons test (GraphPad InStat; GraphPad Software Inc., La Jolla, CA, USA). P values of <0.05 were considered to indicate statistical significance.
Results
Effects of capsaicin on C4 activity
Bath-applied capsaicin (1–100 μM) induced a sudden decrease in the C4 burst rate at 2–3 min after the application of capsaicin and a subsequent gradual increase at 10–15 min after the application (Figs. 1 and 2). The initial inhibitory effect, which was dose dependent, appeared at 1–100 μM, whereas the excitatory effect at 10–15 min of application reached the maximum level at 10 μM (Fig. 2). After washout, the C4 rate gradually decreased to the same rate as the control (Fig. 2). The effects of 10 μM capsaicin were partially reversed by TRPV1 antagonists, 10 μM capsazepine or 1 μM AMG9810. As shown in Fig. 2c, d, capsazepine (as well as AMG9810) typically blocked the second phase of the capsaicin effects, i.e., the excitation following the initial decrease of the C4 burst rate.
The effects of capsaicin on C4 activity. Upper traces, raw data; lower traces, integrated C4 activity. a A typical example of the effects of 10 μM capsaicin on C4 activity. Note that the 15-min application of 10 μM capsaicin induced strong tonic discharges as well as transient inhibition followed by the facilitation of respiratory rhythm. b The effects of the second application of 10 μM capsaicin, 15 min after the first application. Note that the second application induced no significant effects on C4 activity and that tonic activity was not induced
The dose-dependent effects of capsaicin on C4 activity. a, b Typical examples of the time course of the changes in the C4 burst rate in response to the application of 1 or 10 μM capsaicin. c The effects of 10 μM capsazepine on the C4 burst rate induced by 10 μM capsaicin. Note that the increase in the burst rate after transient inhibition by capsaicin is not clear in the presence of capsazepine. d A summary of the dose-dependent effects of capsaicin and effects of TRPV1 antagonists, capsazepine, and AMG9810 on the C4 burst rate. Solid bars, control; gray bars, at 2–3 min application; white bars, at 15 min application. The “10 μM + capsazepine” and “10 μM + AMG9810” columns denote the effects of 10 μM capsaicin in the presence of 10 μM capsazepine or 1 μM AMG9810, respectively. The number in each bar denotes the sample number. The bars show the mean values and standard deviations. The significance (in comparison to control) was *P < 0.05; **P < 0.01; ***P < 0.01. The significance of 15-min application in comparison to 3-min application was $$ P < 0.01; $$$ P < 0.001
It is well known that capsaicin causes strong desensitization [36]. We investigated whether the effects of capsaicin on respiratory rhythm generation are desensitized. As shown in Figs. 1 and 3, a second application of 10 μM capsaicin at 15 or 30 min after washout of the first application induced no significant effects on the C4 activity and did not induce tonic activity. Because the desensitization is thought to be induced by the binding of Ca2+ to calmodulin [18], we examined the effects of a calmodulin inhibitor on the capsaicin-induced desensitization. The coapplication of W-7 (50 μM), a calmodulin antagonist, with capsaicin (10 μM) induced biphasic responses on C4 activity that were similar to the sole application of capsaicin. However, after 15 min of washout, the second application of capsaicin induced no significant change in the C4 activity (n = 3, Fig, 3c), indicating that W-7 could not prevent TRPV1 desensitization.
The desensitization of the C4 burst rate change in response to the application of capsaicin. The burst rates of the control and 2–3 and 15 min after the application of 10 μM capsaicin are plotted. a The second application of capsaicin was performed after 15 min of washout. b The second application of capsaicin was performed after 30 min of washout. c 50 μM W-7 was coapplied with capsaicin. Note that the desensitization to the second application was preserved in the presence of W-7
Effects of capsaicin on Pre-I and Insp neurons
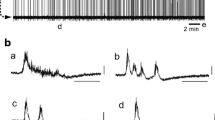
We examined the effects of 10 μM capsaicin on the membrane potentials and burst activity of Pre-I (n = 5) and Insp (n = 7) neurons in the rostral ventrolateral medulla. Capsaicin induced typically biphasic responses in the membrane potentials: initial depolarization in association with tonic firing and a subsequent recovery phase (Figs. 4 and 5), whereas initial transient hyperpolarization, in association with the inhibition of the firing, was induced in two Pre-I and one Insp neuron (data not shown). The group data are shown in Table 1. During and after the application of 10 μM capsaicin, an augmented inspiratory burst was more frequently and clearly observed in 75% of the preparations at a rate of approximately once per four to five respiratory cycles. Typical examples are shown in Fig. 5 (B-3 and C). The inspiratory burst activity was smallest just after the augmented inspiratory burst; the burst amplitude then tended to increase until the next augmented burst (Fig. 5 (C)).
The effects of capsaicin on a preinspiratory neuron in the medulla. Upper traces, membrane potentials; lower traces, C4 activity. A, A slower sweep representation of the membrane potential response to the application of 10 μM capsaicin. B-1–B-4, Faster sweep representations of the membrane potential responses. The application of capsaicin depolarized this neuron, and the burst discharge turned into a tonic firing pattern after 5 min of application (B-2); this was accompanied by a decrease in the C4 rate and tonic discharges. After 15 min of application, the preinspiratory neuron and C4 rhythm increased (B-3)
The effects of capsaicin on an inspiratory neuron in the medulla. Upper traces, membrane potentials; lower traces, C4 activity. A, A slower sweep representation of the membrane potential response to the application of 10 μM capsaicin. B-1–B-3, Faster sweep representations of the membrane potential responses. The application of capsaicin depolarized this neuron, and the burst discharge turned into a tonic firing pattern after 5 min of application (B-2, in which action potentials are truncated); this was accompanied by a decrease in the C4 rate and tonic discharges. Note that an augmented inspiratory burst was clearly identified after 15 min of washout (B-3, the first and fifth burst). C, The augmented inspiratory burst appeared at every four respiratory cycles (arrows). Note that the burst activity was smallest just after the augmented inspiratory burst and then tended to increase the burst amplitude until the next augmented burst (see the lowest trace, which indicates integrated C4 burst activity)
In Pre-I neurons (n = 4) in the rostral pFRG, the effects of 20 μM capsaicin and hypercapnic stimulation were examined in the presence of TTX (0.5 μM). A typical example was shown in Fig. 6 (A). After the application of capsaicin, the Pre-I neurons tended to show slight depolarization (−45.5 ± 3.8 mV in control, −43.0 ± 3.6 mV in capsaicin; P < 0.01, n = 4 by paired t test, summarized in Fig. 6 (B-3)) with a minor change in their input resistance (625 ± 263 MΩ in control, 675 ± 366 MΩ in capsaicin, not significant) (Fig. 6 (A)). The fluctuation of the membrane potential increased in response to capsaicin application in the presence of 0.5 μM TTX (Fig. 6 (B-1, B-2); SD value of 153 ± 6.3% of control). Hypercapnic stimulation (2 → 8%) in the above four cells induced 9.0 ± 3.6 mV depolarization (P < 0.05, n = 4) with an increase in the input resistance (625 ± 299 → 875 ± 457 MΩ, P = 0.08, n = 4), consistent with results of our previous studies [22, 23] (Fig. 6 (A)). Furthermore, we examined effects of 20 μM capsaicin in the presence of 0.5 μM TTX, 0.1 mM Cd2+, and 1 μM CNQX on CO2-sensitive Pre-I neurons in the rostral pFRG (n = 4, data not shown). The membrane fluctuation was at least partially blocked (SD value of 121 ± 3.1% of control). All of the tested neurons were identified as being Phox2b positive (Fig. 6 (C, D)). As shown in an example in Fig. 6 (A), hypercapnic stimulation immediately after 20 μM capsaicin application still induced membrane depolarization. Because capsaicin induced strong desensitization (see above), this result suggested that the CO2 response of pFRG-Pre-I neurons was less affected by the desensitization of the capsaicin response. This was also confirmed in the absence of TTX; an increase in the C4 burst rate by hypercapnic stimulation (2 → 8% CO2) was observed in the presence of 10 μM capsaicin (at more than 30 min) (197 ± 52% without capsaicin and 222 ± 144% with capsaicin, n = 5, not significant). We also tested the membrane potential response to 10 μM capsaicin application in the presence of TTX for Pre-I neurons in the caudal pFRG that were Phox2b negative and CO2 insensitive [22]. Application of capsaicin induced slight membrane depolarization (−42.3 ± 2.1 mV, 383.3 ± 104.1 MΩ in control; −40.3 ± 1.5 mV, 363.3 ± 80.8 MΩ in capsaicin; n = 3, not significant).
The effects of capsaicin on preinspiratory neurons in the presence of 0.5 μM TTX. A, The membrane potential response to hypercapnia (2→8% CO2, blue lines) and 20 μM capsaicin (red lines). A square current pulse (500 ms, 0.1 Hz, 20 pA) was applied to monitor change of input resistance. Negative deflections of the baseline membrane potential are proportional to the input resistance. A’, The burst pattern of this preinspiratory neuron in a control (upper trace, membrane potential; lower trace, C4 activity). Note that hypercapnic stimulation induced membrane depolarization, which was accompanied by an increase in the input resistance. The application of 20 μM capsaicin induced slight depolarization and an increase of the membrane potential fluctuation. Second application of 8% CO2 immediately after 20 μM capsaicin also induced membrane depolarization. B-1, B-2, Fast sweep representations of B-1 and B-2, respectively, in A. Note that the membrane potential fluctuation increased during the application of capsaicin (B-2). B-3, Summary of membrane potential changes in four preinspiratory neurons in response to the application of 20 μM capsaicin in TTX; Pre, in control; Post, after 15 min in capsaicin. C, The location of the recorded neuron (Lucifer yellow [cyan] staining). This neuron is Phox2b-positive (red). D-1, D-2, Higher magnification views of the highlighted square in the left image. FN facial nucleus
Effects of high capsaicin concentration
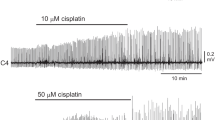
A previous paper reported that high concentrations (e.g., 50 μM, 55–60 min) of capsaicin stopped inspiratory burst activity in medullary slice preparations, including the preBötC [17]. We also tested this effect in en bloc preparations. C4 inspiratory activity did not stop, even after 60–90 min application of 50 or 100 μM capsaicin. The C4 burst rate was 7.0 ± 0.6 bursts/min (n = 5) and 11 ± 1.0 bursts/min (n = 3) after the 60-min application of 50 and 100 μM capsaicin, respectively. To clarify the network mechanisms that produce respiratory activity in the presence of 50 μM capsaicin, we performed optical recordings using a voltage-sensitive dye. Figure 7 shows that an example of optical recording in the presence of 50 μM capsaicin (60–80 min) was basically the same as that under control conditions (Fig. 7 (B)) [20]; the preinspiratory activity initially appeared in the rostral ventrolateral medulla (corresponding to the pFRG), and then the activity propagated to the more caudal medulla (probably corresponding to the preBötC). The duration of the preinspiratory phase tended to increase in the presence of capsaicin, but the change was not statistically significant (356 ± 220 ms in control [n = 10] and 520 ± 166 ms in capsaicin [n = 7]).
Optical imaging of the respiratory neuron activity in the ventral medulla. A, B Images in a preparation under the control condition; C, D images in another preparation under the presence of 50 μM capsaicin (70–80 min). The results represent the average from 40 consecutive respiratory cycles with a C4 burst used as the trigger. A, C, Optical images of respiratory neuron activity at the onset of the C4 burst (blue dotted vertical bar in A’, C’) superimposed on the ventral surface of the medulla. A’ , C’, Fluorescence changes at the two spots indicated in the left panel (A, C): red, in the rostral ventrolateral medulla at a level of the facial nucleus corresponding to the parafacial respiratory group; blue, in the more caudal ventrolateral medulla at the level of the rostral roots of the 12th cranial nerve corresponding to the preBötzinger complex. The fluorescence decrease, i.e., depolarization, was upward. Light blue bars under the C4 trace denote the inspiratory phase. B, D, The spatiotemporal pattern of optical respiratory neuron activity. Panels a–c correspond to a–c in A’ and C’, respectively. Note that the optical signals reflecting the respiratory neuron activity appear in the rostral ventrolateral medulla during the preinspiratory phase (similar to the pattern in the control of previous reports) [20]
To further investigate the role of the pFRG on respiratory rhythm generation after treatment with a high concentration of capsaicin, we examined effects of 50 μM capsaicin in the caudal preparations in which approximately 80% of the rostral part of the pFRG was removed. The C4 burst rate of the caudal preparations was lower than that of the standard preparation (2.2 ± 0.8 bursts/min [n = 6], as reported previously [33]). As shown in Fig. 8, C4 inspiratory rhythm stopped within 20 min (16.8 ± 2.7 min, n = 6) after capsaicin application.
Effects of a high concentration of capsaicin on C4 inspiratory activity in the caudal preparation. a C4 activity in the caudal preparation. C4 inspiratory activity was stopped at 16 min after 50 μM capsaicin was applied. b Representation in a sagittal slice with fluorescent Nissl stain of the transection level of this caudal preparation in which approximately 80% of the rostral part of the pFRG was removed. C4 inspiratory activity did not recover even after washout of capsaicin (50-min application and 50-min washout). FN facial nucleus
Immunohistochemistry
The histochemical analysis demonstrated a wide distribution of TRPV1 channel-positive cells in the reticular formation of the medulla, including the pFRG and preBötC, with stronger expression in the motor neuron pools in the medulla as well as the spinal cord and dorsal root ganglion (Fig. 9a, d). A similar distribution was also confirmed by the in situ hybridization of mRNA for TRPV1 channel protein (Fig. 9e). We confirmed that the Phox2b-positive cells in the pFRG region expressed TRPV1 channel protein (Fig. 9b, c).
TRPV1 immunostaining of the ventral medulla of the neonatal rat (postnatal day 1). a TRPV1 expression at the level of the rostral pFRG (0.6 mm rostral to the caudal end of the facial nucleus). b Phox2b expression. c Merged TRPV1 (green) and Phox2b (red) expression. The region of the rostral pFRG is indicated by the white dotted line. d A dorsal root ganglion (left) and spinal cord (right) as positive controls. e Expression of the genes encoding TRPV1 in the region of the pFRG of a neonatal rat on postnatal day 1. In situ hybridization using a riboprobe for rat Trpv1. The arrowheads indicate trpv1-positive cells in the pFRG region. f A dorsal root ganglion as a positive control. FN facial nucleus
Discussion
Capsaicin induced a biphasic response in the respiratory rhythm (i.e., a transient decrease followed by an increase in the C4 rate) in brainstem-spinal cord preparations from newborn rats. The second-phase excitatory effect in the biphasic response was partially blocked by TRPV1 antagonists, capsazepine, or AMG9810, whereas the initial inhibitory effect was not suppressed by these antagonists. Our histochemical analysis using antibodies and mRNA for TRPV1 channel protein demonstrated that TRPV1 channel-positive cells were widely distributed in the reticular formation of the medulla. Although the partial blockade by antagonists on capsaicin effects might be due to the potency of antagonists used [15, 39], the effects of capsaicin might be due to the partially TRPV1-independent or non-specific action of capsaicin [7, 25]. The Pre-I and Insp neurons showed tonic firings due to membrane depolarization during this inhibitory phase. In the brainstem-spinal cord preparation, it has been hypothesized that Pre-I neurons in the pFRG periodically trigger the Insp burst generation in the preBötC [11, 16, 20, 28]. Thus, the initial inhibitory phase may be explained by the disorganization of the respiratory rhythm-generating neuron networks.
One of the characteristic effects of capsaicin is that it causes strong desensitization [36]. We confirmed that this property also appeared in capsaicin’s effects on the generation of respiratory rhythm. It has been suggested that the Ca2+-dependent phosphorylation/dephosphorylation process is involved in this desensitization [36]. As one of the mechanisms, calmodulin is involved in Ca2+-dependent desensitization of TRPV1 [18, 36]. In the present study, the capsaicin-induced desensitization was not blocked by the coapplication of W-7 (a calmodulin antagonist) with capsaicin. Nevertheless, as discussed previously, this negative result does not necessarily suggest that a calmodulin-independent mechanism is involved in the desensitization [18].
With regard to the other characteristics of the effects of capsaicin, we note that the augmented C4 inspiratory activity appeared at a rate of once per four to five respiratory cycles after washout. The inspiratory burst activity was typically the smallest just after the augmented inspiratory burst, and then the burst amplitude tended to increase until the next augmented burst. This gradual recovery during four to five respiratory cycles might reflect a gradual increase in the number of active inspiratory neurons (i.e., the recovery process of excitability of the inspiratory neuron network after the generation of the preceding burst). This augmented inspiratory activity might correspond to sigh activity [14, 21, 26]. A recent paper reported that specific peptidergic neurons in the parafacial region were responsible for the generation of sigh activity in the preBötC [13]. Whether a similar mechanism is involved in the generation of the augmented activity that was observed in the present study remains a topic for future research.
Cells in the pFRG that have been reported to be important in central chemoreception [8, 22, 29] expressed TRPV1 channels. Although it is known that TRPV1 is sensitive to extracellular acidification [36, 37], recent studies suggested that extracellular acidosis may not significantly modulate TRPV1 activity in physiological conditions due to the attenuation of TRPV1 channel activity by intracellular acidification [3, 4]. However, TRP channel broad-spectrum blockers and TRPV1- and TRPM8-specific blockers attenuated the hypercapnic acidosis-induced Ca2+ response in glia-rich cell cultures from the medulla in which the mRNA of TRPV1, TRPA1, and TRPM8 were expressed [9]. Thus, the members of the TRP channel family partially mediate the fast hypercapnic acidosis-induced Ca2+ response and are candidate central chemosensing proteins [9]. In the pFRG of the brainstem-spinal cord preparation, capsaicin slightly depolarized CO2-sensitive Pre-I neurons in the presence of TTX, whereas the response was insufficient to engage membrane depolarization by hypercapnic stimulation (2 → 8% CO2). We found that capsaicin increased the fluctuation of the membrane potential in the presence of TTX with a minor change in the input resistance and that the fluctuation was partially blocked in solution containing TTX, Cd2+, and CNQX. Similar responses were also observed in CO2-insensitive Pre-I neurons in the caudal pFRG. These results suggest that capsaicin mainly acts on the presynaptic sites (and/or intracellular targets such as the endoplasmic reticulum) [24, 40] of the pFRG-Pre-I neurons and that the contribution of TRPV1 to the hypercapnic response of Pre-I neurons may be limited. Our results that capsaicin-induced desensitization did not affect the hypercapnic response in rostral pFRG Pre-I neurons (in TTX) and the C4 burst rate (without TTX) also supported the minor contribution of TRPV1 channels to central chemoreception in the newborn rat en bloc preparation. In our previous paper, we investigated the effects of the combined application of capsaicin with QX-314 (a quaternary lidocaine derivative) on respiratory rhythm generation [31]. Although it was reported that QX-314 passed through activated TRPV1 channels of the dorsal root ganglion neurons [2], the combined application of capsaicin with QX-314 did not cause additional effects on respiratory activity in the en bloc preparation compared to the sole application of QX-314. These and the present results are consistent with the presynaptic (and/or non-specific) action of capsaicin on pFRG-Pre-I neurons.
A previous paper reported that treatment with a high concentration (55–60 min treatment with 50 μM) of capsaicin stopped inspiratory burst activity in medullary slice preparations, including the preBötC [17]. This was due to the depletion of SP in the nerve terminal of the preBötC. In the present study, we found that C4 inspiratory activity did not stop, even after 60–90 min of application of 50 or 100 μM capsaicin. Voltage-sensitive dye imaging demonstrated that the spatiotemporal pattern of respiratory rhythm-generating networks after the application of capsaicin (50 μM, 70–90 min) were basically the same as that of the control [1, 16, 20]. In contrast, application of 50 μM capsaicin stopped C4 Insp activity in preparations without most of the pFRG. The present results suggest that respiratory rhythm is driven by Pre-I neurons, regardless of the SP content in the preBötC and possibly in the pFRG regions in the en bloc preparation [1, 16, 20].
Conclusion
Application of capsaicin in the medulla had various influences on the respiratory center. These included potent (but transient) inhibitory and subsequent excitatory effects on the respiratory rhythm and the periodical augmentation of the inspiratory burst pattern. The effects of capsaicin were partially blocked by TRPV1 antagonists but could also be induced at least partially via the non-specific action. Further study is necessary to elucidate the cellular mechanisms underlying the desensitizing effects of capsaicin on respiratory activity. Our results also suggested that the contribution of the TRPV1 channels to central chemoreception was limited, although capsaicin could modulate the activity of CO2-sensitive Phox2b-expressing Pre-I neurons.
References
Ballanyi K, Ruangkittisakul A, Onimaru H (2009) Opioids prolong and anoxia shortens delay between onset of preinspiratory (pFRG) and inspiratory (preBötC) network bursting in newborn rat brainstems. Pflugers Arch 458:571–587
Binshtok AM, Bean BP, Woolf CJ (2007) Inhibition of nociceptors by TRPV1-mediated entry of impermeant sodium channel blockers. Nature 449:607–610
Chung S, Kim YH, Koh JY, Nam TS, Ahn DS (2011) Intracellular acidification evoked by moderate extracellular acidosis attenuates transient receptor potential V1 (TRPV1) channel activity in rat dorsal root ganglion neurons. Exp Physiol 96:1270–1281
Dhaka A, Uzzell V, Dubin AE, Mathur J, Petrus M, Bandell M, Patapoutian A (2009) TRPV1 is activated by both acidic and basic pH. J Neurosci 29:153–158
Edwards JG (2014) TRPV1 in the central nervous system: synaptic plasticity, function, and pharmacological implications. Prog Drug Res 68:77–104
Gavva NR, Tamir R, Qu Y, Klionsky L, Zhang TJ, Immke D, Wang J, Zhu D, Vanderah TW, Porreca F, Doherty EM, Norman MH, Wild KD, Bannon AW, Louis JC, Treanor JJ (2005) AMG 9810 [(E)-3-(4-t-butylphenyl)-N-(2,3-dihydrobenzo[b][1,4] dioxin-6-yl)acrylamide], a novel vanilloid receptor 1 (TRPV1) antagonist with antihyperalgesic properties. J Pharmacol Exp Ther 313:474–484
Gupta S, Lozano-Cuenca J, Villalon CM, de Vries R, Garrelds IM, Avezaat CJ, van Kats JP, Saxena PR, Maassen VanDenBrink A (2007) Pharmacological characterisation of capsaicin-induced relaxations in human and porcine isolated arteries. Naunyn Schmiedeberg’s Arch Pharmacol 375:29–38
Guyenet PG, Mulkey DK (2010) Retrotrapezoid nucleus and parafacial respiratory group. Respir Physiol Neurobiol 173:244–255
Hirata Y, Oku Y (2010) TRP channels are involved in mediating hypercapnic Ca2+ responses in rat glia-rich medullary cultures independent of extracellular pH. Cell Calcium 48:124–132
Ikeda K, Satake S, Onaka T, Sugimoto H, Takeda N, Imoto K, Kawakami K (2013) Enhanced inhibitory neurotransmission in the cerebellar cortex of Atp1a3-deficient heterozygous mice. J Physiol 591:3433–3449
Ikeda K, Kawakami K, Onimaru H, Okada Y, Yokota S, Koshiya N, Oku Y, Iizuka M, Koizumi H (2016) The respiratory control mechanisms in the brainstem and spinal cord: integrative views of the neuroanatomy and neurophysiology. J Physiol Sci
Kauer JA, Gibson HE (2009) Hot flash: TRPV channels in the brain. Trends Neurosci 32:215–224
Li P, Janczewski WA, Yackle K, Kam K, Pagliardini S, Krasnow MA, Feldman JL (2016) The peptidergic control circuit for sighing. Nature 530:293–297
Lieske SP, Thoby-Brisson M, Telgkamp P, Ramirez JM (2000) Reconfiguration of the neural network controlling multiple breathing patterns: eupnea, sighs and gasps. Nat Neurosci 3:600–607
McIntyre P, McLatchie LM, Chambers A, Phillips E, Clarke M, Savidge J, Toms C, Peacock M, Shah K, Winter J, Weerasakera N, Webb M, Rang HP, Bevan S, James IF (2001) Pharmacological differences between the human and rat vanilloid receptor 1 (VR1). Br J Pharmacol 132:1084–1094
Mellen NM, Janczewski WA, Bocchiaro CM, Feldman JL (2003) Opioid-induced quantal slowing reveals dual networks for respiratory rhythm generation. Neuron 37:821–826
Morgado-Valle C, Feldman JL (2004) Depletion of substance P and glutamate by capsaicin blocks respiratory rhythm in neonatal rat in vitro. J Physiol 555:783–792
Numazaki M, Tominaga T, Takeuchi K, Murayama N, Toyooka H, Tominaga M (2003) Structural determinant of TRPV1 desensitization interacts with calmodulin. Proc Natl Acad Sci U S A 100:8002–8006
Onimaru H, Homma I (1992) Whole cell recordings from respiratory neurons in the medulla of brainstem-spinal cord preparations isolated from newborn rats. Pflugers Arch 420:399–406
Onimaru H, Homma I (2003) A novel functional neuron group for respiratory rhythm generation in the ventral medulla. J Neurosci 23:1478–1486
Onimaru H, Ikeda K, Kawakami K (2007) Defective interaction between dual oscillators for respiratory rhythm generation in Na+, K+-ATPase {alpha}2 subunit-deficient mice. J Physiol 584:271–284
Onimaru H, Ikeda K, Kawakami K (2008) CO2-sensitive preinspiratory neurons of the parafacial respiratory group express Phox2b in the neonatal rat. J Neurosci 28:12845–12850
Onimaru H, Ikeda K, Kawakami K (2012) Postsynaptic mechanisms of CO2 responses in parafacial respiratory neurons of newborn rats. J Physiol 590(7):1615–1624
Peters JH, McDougall SJ, Fawley JA, Smith SM, Andresen MC (2010) Primary afferent activation of thermosensitive TRPV1 triggers asynchronous glutamate release at central neurons. Neuron 65:657–669
Reynolds PJ, Fan W, Andresen MC (2006) Capsaicin-resistant arterial baroreceptors. J Negat Results Biomed 5:6
Ruangkittisakul A, Schwarzacher SW, Secchia L, Ma Y, Bobocea N, Poon BY, Funk GD, Ballanyi K (2008) Generation of eupnea and sighs by a spatiochemically organized inspiratory network. J Neurosci 28:2447–2458
Saffarzadeh F, Eslamizade MJ, Mousavi SM, Abraki SB, Hadjighassem MR, Gorji A (2016) TRPV1 receptors augment basal synaptic transmission in CA1 and CA3 pyramidal neurons in epilepsy. Neuroscience 314:170–178
Smith JC, Ellenberger HH, Ballanyi K, Richter DW, Feldman JL (1991) Pre-Bötzinger complex: a brainstem region that may generate respiratory rhythm in mammals. Science 254:726–729
Stornetta RL, Moreira TS, Takakura AC, Kang BJ, Chang DA, West GH, Brunet JF, Mulkey DK, Bayliss DA, Guyenet PG (2006) Expression of Phox2b by brainstem neurons involved in chemosensory integration in the adult rat. J Neurosci 26:10305–10314
Suzue T (1984) Respiratory rhythm generation in the in vitro brain stem-spinal cord preparation of the neonatal rat. J Physiol 354:173–183
Takahashi K, Hayakawa C, Onimaru H (2016) Effects of a quaternary lidocaine derivative, QX-314, on the respiratory activity in brainstem-spinal cord preparation from newborn rats. Neurosci Lett 619:121–125
Tani M, Onimaru H, Ikeda K, Kawakami K, Homma I (2010) Menthol inhibits the respiratory rhythm in brainstem preparations of the newborn rats. Neuroreport 21:1095–1099
Tani M, Yazawa I, Tsuzawa K, Onimaru H (2013) Long-lasting facilitation of respiratory rhythm by treatment with TRPA1 agonist, cinnamaldehyde. J Physiol Sci 63:s221
Tani M, Lin S-T, Onimaru H (2014) Cellular mechanisms of capsaicin actions on the respiratory neurons in brainstem-spinal cord preparations from the newborn rat. J Physiol Sci 64:s280
Tominaga M, Caterina MJ (2004) Thermosensation and pain. J Neurobiol 61:3–12
Tominaga M, Tominaga T (2005) Structure and function of TRPV1. Pflugers Arch 451:143–150
Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, Raumann BE, Basbaum AI, Julius D (1998) The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron 21:531–543
Toth A, Boczan J, Kedei N, Lizanecz E, Bagi Z, Papp Z, Edes I, Csiba L, Blumberg PM (2005) Expression and distribution of vanilloid receptor 1 (TRPV1) in the adult rat brain. Brain Res Mol Brain Res 135:162–168
Walker KM, Urban L, Medhurst SJ, Patel S, Panesar M, Fox AJ, McIntyre P (2003) The VR1 antagonist capsazepine reverses mechanical hyperalgesia in models of inflammatory and neuropathic pain. J Pharmacol Exp Ther 304:56–62
Wong CO, Chen K, Lin YQ, Chao Y, Duraine L, Lu Z, Yoon WH, Sullivan JM, Broadhead GT, Sumner CJ, Lloyd TE, Macleod GT, Bellen HJ, Venkatachalam K (2014) A TRPV channel in drosophila motor neurons regulates presynaptic resting Ca2+ levels, synapse growth, and synaptic transmission. Neuron 84:764–777
Acknowledgments
This work was supported by Grants-in-Aid for Scientific Research (KAKENHI: 25430012).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tani, M., Kotani, S., Hayakawa, C. et al. Effects of a TRPV1 agonist capsaicin on respiratory rhythm generation in brainstem-spinal cord preparation from newborn rats. Pflugers Arch - Eur J Physiol 469, 327–338 (2017). https://doi.org/10.1007/s00424-016-1912-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-016-1912-8