Abstract
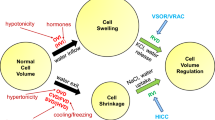
Volume regulation is an essential property of any living cell and needs to be tightly controlled. While different types of K+ channels have been found to participate in the regulation of cell volume, the newly identified volume-regulated anion channel (VRAC) LRRC8 has been claimed to be essential for volume regulation. In unbiased genome-wide small interfering RNA (siRNA) screens, two independent studies identified LRRC8A/Swell1 as an essential component of VRAC, thus being indispensable for cellular volume regulation. We reanalyzed the role of LRRC8A for VRAC and regulatory volume decrease (RVD) in several cell types and under various conditions. While the role of LRRC8A for VRAC and its contribution to RVD is confirmed, we find that it is not essential for swelling-activated anion currents or cellular volume regulation, or apoptotic cell shrinkage. The contribution of LRRC8A is variable and largely depending on the cell type.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cells regulate their volume when imbalances exist between intra- and extracellular osmolarity. Cell swelling is counteracted by the activation of K+ and Cl− channels or KCl (KCC) cotransporters, and subsequent release of KCl to the extracellular space, which is followed by exit of water [5, 13, 15]. A particular type of Cl− channel, the so called volume-regulated anion channel (VRAC) is thought to play a central role during volume regulation, particularly during regulatory volume decrease (RVD). Recently, LRRC8A has been identified as a ubiquitous and essential component of VRAC, and LRRC8A was shown to be indispensable for cellular volume regulation [20, 28].
Over the past 20 years, many Cl− channels were suggested to contribute to volume regulation, such as ClC-3, VDAC, p64, CLIC1, CFTR, TMEM16, and bestrophin [9, 18, 16]. However, while the contributions of some channels to volume regulation (e.g., Bestrophin 1, CFTR and TMEM16) have been examined also in naïve cells and original tissues [2, 4, 25], almost all other studies examined VRAC/RVD in cultured cells [5, 18]. Also for LRRC8A, the general physiological relevance is currently unclear. We therefore reexamined in a number of cell types, including those used for the identification of LRRC8A, whether LRRC8A is essential for VRAC and cellular volume regulation. While LRRC8A clearly determines the magnitude of VRAC, we could still activate VRAC in the absence of LRRC8A. The impact of LRRC8A on volume regulation was even less obvious. Thus swelling-activated Cl− currents, RVD and apoptotic cell shrinkage were still detected in the absence of LRRC8A.
Experimental procedures
Cells, cDNA, RT-PCR
HEK293 were grown in DMEM-F12 (GIBCO, Karlsruhe, Germany) supplemented with 10 % fetal bovine serum at 37 °C in the absence of antibiotics in a humidified atmosphere with 5 % CO2. HeLa and HCT116 [20, 28] cells were grown as described earlier [20, 28]. HCT-wt (LRRC8A+/+) and HCT-LRRC8A−/− cells were kindly provided by Dr. F. Voss/Prof. Dr. T. Jentsch (FMP, Berlin). BHY cells were grown in Opti-MEM (Gibco) supplemented with 10 % (v/v) heat-inactivated fetal bovine serum (FBS, Gibco) at 5 % CO2 and 37 °C. Human LRRC8A (IRAUp969D03104D, Source BioScience GmbH, Berlin, Germany) was subcloned into pcDNA31 by PCR using KAPA HiFi HotStart DNA Polymerase (KAPA Biosystems, Boston, MA, USA). The construct LRRC8AΔLRR was generated by PCR (KAPA HiFi HotStart DNA Polymerase) inducing a stop codon at position D367. pIRES2 LRRC8A-T44C was a generous gift from Zhaozhu Qiu [20]. CD8 MicroBeads were used to identify overexpressing cells. For semiquantitative RT-PCR total RNA (1 μg) was isolated from HeLa and HEK293B cells, reverse-transcribed using a random primer and M-MLV reverse transcriptase (Promega, Mannheim, Germany). The RT-PCR reaction contained sense and antisense primers for LRRC8A, KCC, cation chloride cotransporter (CCC), and GAPDH (0.5 μM; Table 1), 0.5 μl cDNA, and GoTaq polymerase (Promega). After 2 min at 95 °C, cDNA was amplified in 30 cycles for 30 s at 95 °C, 30 s at 56 °C, and 1 min at 72 °C and visualized by loading on ethidium bromide-containing agarose gels.
siRNA, solutions, materials, and statistical analysis
Small interfering RNA (siRNA) sequence was 5′-CCAAGCUCAUCGUCCUCAA-3′ (LRRC8A), (Silencer Select, Life Technologies, Darmstadt, Germany), respectively. Experiments were performed 48 h after transfection of 2 × 105 cells using Lipofectamin 3000. For most experiments, cells were kept initially in Ringer solution (mM): NaCl 145, KH2PO4 0.4, K2HPO4 1.6, d-glucose 5, MgCl2 1, and calcium gluconate 1.3, pH 7.4. Ringer solution was then replaced by an isotonic solution (Iso) containing (mmol/l) NaCl 95.5, KH2PO4 0.4, K2HPO4 1.6, d-glucose 5, MgCl21, Ca-gluconate 1.3, and mannitol 100, pH 7.4. To induce cell swelling, a hypotonic solution (200 mosmol/l; 33 % Hypo) was produced by the removal of mannitol. Alternatively, Ringer solution was directly replaced by hypotonic solution. NS3728 was from Neurosearch (Hellerup, Denmak); RDIOA, CaCCinhAO1, staurosporine, ionomycin, BAPTA, Ba2+, TEA+, and Cs+ were from SIGMA. Osmolarity was measured using an osmometer. Data are presented as mean ± SEM. For statistical analysis, paired or unpaired t test or ANOVA were used where appropriate. A p value of <0.05 was accepted as significant.
Western blotting
Cells were collected and lysed in 0.5 % NonidetP40 lysis buffer. Proteins (50 μg) were separated by 8.5 % SDS-PAGE and transferred into PVDF membrane. Membrane was blocked with 5 % nonfat milk (NFM)/Tris-buffered saline and Tween 20 (TBS-T) or 5 % NFM/phosphate-buffered saline and Tween 20 (PBS-T) for 1 h at room temperature and incubated overnight at 4 °C with rabbit anti-LRRC8A (diluted 1:1000 in 1 % BSA/TBS-T; Sigma, Taufkirchen, Germany). Mouse anti β-actin (sc-47778) and anti-GAPDH were from Santa Cruz Biotech (Heidelberg, Germany). The membrane was incubated with horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG (diluted 1:10,000 in 1 % NFM/TBS-T) for 2 h at room temperature. Subsequently, the immunoreactive signals were detected using a SuperSignal West Pico chemiluminescence substrate (Pierce, Waltham, USA).
Stauro Caspase assay in HeLa cells
A total of 8 × 104 HeLa cells were seeded on fibronectin-coated glass coverslips. Cells were treated with 1 μM Staurosporine and non-fluorescent caspase-3 substrate DEVD-NucView488 (Biotium) for 6 h at 37 °C. Fluorescence intensity from enzymatic cleavage was detected by fluorescence microscopy at 488-nm excitation and 520-nm emission.
Flow cytometry
Cells were washed and re-dissolved in 10 ml Ringer, isotonic or hypotonic solution as described above. Cells were analyzed at 37 °C/pH 7.4 using a CASY flow cytometer (Roche Diagnostics, Mannheim, Germany). Cells were analyzed at a density of 106 cells/ml. Cell swelling and RVD were observed for up to 9 min after applying hypotonic bath solution. Data were fitted by an exponential fit (y = y 0 + A (R0x)) and time required for 50 % reduction of cell volume during RVD was determined.
Patch clamping
Fast whole-cell patch-clamp recordings were performed on cells grown on fibronectin-coated glass coverslips. If not indicated otherwise, patch pipettes were filled with a cytosolic-like solution containing KCl 30, K -gluconate 95, NaH2PO4 1.2, Na2HPO4 4.8, EGTA 1, Ca-gluconate 0.758, MgCl2 1.03, d-glucose 5, ATP 3, pH 7.2. The Ca2+ activity was 0.1 μM. We choose this solution because it enabled swelling/shrinkage behavior under physiological ion concentrations and allowed for direct comparison of the results from patch clamping and volume measurements. Additional pipette/bath solutions were used in which K+ was replaced by NMDG+. Hypotonic cell swelling was induced by (1) changing extracellular Ringer solution to an isotonic solution in which NaCl was partially (33 %) replaced by mannitol and then (2) removing mannitol from the solution thereby generating a hypotonic solution. Alternative protocols were applied as indicated. Coverslips were mounted in a perfused bath chamber on the stage of an inverted microscope (IM35, Zeiss) and kept at 37 °C. The bath was perfused continuously with Ringer solution at a rate of 8 ml/min. Patch pipettes had an input resistance of 2–4 MΩ when filled with the cytosolic-like (physiological) solution. Currents were corrected for serial resistance. The access conductance was measured continuously and was 60–140 nS. Currents (voltage clamp) and voltages (current clamp) were recorded using a patch-clamp amplifier (EPC 7, List Medical Electronics, Darmstadt, Germany), the LIH1600 interface and PULSE software (HEKA, Lambrecht, Germany) as well as Chart software (AD Instruments, Spechbach, Germany). Data were stored continuously on a computer hard disc and analyzed using PULSE software. In regular intervals, membrane voltage (Vc) was clamped in steps of 20 mV from −100 to +100 mV from a holding voltage of −100 mV. Current density was calculated by dividing whole cell currents by cell capacitance.
Hypotonicity-induced YFP quenching
Hypotonicity-induced anion (VRAC) conductance was assessed in HeLa cells stably expressing halide-sensitive YFP-H148Q/I152L (kindly provided by Prof. Dr. M. Amaral, University of Lisbon, Portugal) [3]. Cells were exposed to hypotonic bath solutions as described above. Twenty millimolar I− was added either simultaneously with hypotonic bath solution or was added after pre-stimulation with Hypo. Quenching was more pronounced when I− and Hypo were applied simultaneously, as VRAC rapidly inactivated due to RVD (Supplement 2B). All experiments were performed at the physiological temperature of 37 °C, where activation of VRAC was faster and more pronounced. VRAC was activated dose-dependently by exposing cells to variable hypotonicity (10–45 % hypotonicity). Both absolute fluorescence quenching (in arbitrary units) and rate of quenching (au/s) was determined.
Results and discussion
Contribution of LRRC8A to VRAC and RVD in HeLa cells
HeLa cells express LRRC8A along with a number of other Cl−-transporting proteins, known to regulate cell volume like KCl (KCC) and cation chloride cotransporter (CCC) (Fig. 1). This cell line has been used to identify LRRC8A as essential VRAC component [20, 28]. Indeed LRRC8A is expressed in HeLa cells and in every other cell line examined, probably at very different expression levels according to RT-PCR (Fig. 1a). Similar to previous studies [20, 28], we made use of yellow fluorescence protein (YFP) quenching to examine the role of LRRC8A for VRAC. HeLa cells stably expressing YFP were exposed to 20 mmol/l iodide, which induced fluorescence quenching by iodide influx upon exposure to hypotonic bath solution (Hypo). Importantly, we performed all experiments at the physiological temperature of 37 °C. In fact, we found a much larger VRAC at 37 °C when compared to 20 °C (Figs. 2a, 7i, j) [22]. Hypo-induced I− quenching was significantly reduced by the siRNA-knockdown of LRRC8A (Figs. 2b and 3d). Quenching was also inhibited by high extracellular K+ concentrations (Fig. 2c, d), but was otherwise little affected by the inhibition of K+ channels with Ba2+/TEA+ (Fig. 2e), suggesting a significant contribution of I− transport by KCC and possibly CCC.
Expression of LRRC8A, KCC, and CCC in various human cell lines. a RT-PCR analysis of the LRRC8A expression in retinal pigment epithelial cells (RPE), HeLa cells, airway epithelial cells (H441, CFBE, A549), cancer cell lines (Cal27, Cal33, HT29, T84, HEK293, CFPAC), B- and T-lymphocytes, THP1-macrophages, Cos-7, IHKE-1 renal cells, and human osteoblasts (Ob). b, c RT-PCR analysis of the expression of KCl cotransporter (KCC) and cation chloride cotransporter (CCC) in HeLa and HEK293 cells. RT reverse transcriptase
Role of LRRC8A for VRAC in HeLa cells. a Hypotonicity-dependent activation of VRAC measured by I− quenching. Quenching, i.e., the activation of VRAC was more pronounced at 37 °C when compared to 20 °C. b Inhibition of VRAC activation (iodide quenching) by siRNA-knockdown of LRRC8A. c, d Inhibition of iodide quenching by increasing concentrations of extracellular K+, suggesting a K+-dependent Cl− transport by KCC or CCC transporter. e Inhibition of K+ channels by Ba2+ and TEA+ only marginally inhibits quenching. Mean ± SEM, (number of experiments). #Significant difference when compared to 20 °C (a), scrambled (b; c), and 1 mM K+ (d)
LRRC8A has little impact on RVD in HeLa cells. a YFP fluorescence quenching by application of 20 mM I− in hypotonic buffer (Hypo; 200 mosmol/l). b Quenching in isotonic and hypotonic solution in mock and LRRC8A-overexpressing cells. c Volume regulation (RVD) upon application of hypotonic buffer (Hypo) in mock and LRRC8A-overexpressing cells, detected by flow cytometry. d Knockdown of LRRC8A-expression by siRNA. e, f Volume regulation assessed by two different methods. Curve fitting by exponential equation (c.f. Methods) and assessment of time for half maximal recovery (T 50 %) from hypotonic swelling. g Effect of dihydroindenyl)oxy]alkanoic acid (DIOA; 50 μM), and NS3728 (10 μM) on volume regulation. h Summary of cell volume (under isotonic conditions), T 50 %, and -R 0 values obtained from volume regulation under hypotonic solution. Effect siRNAs, as well as the VRAC inhibitor NS3728 (10 μM), the KCC blocker RDIOA (50 μM), the TRPM7-inhibitor NS8593 (10 μM), the TRPC inhibitor SK&F96365 (10 μM), and the VRAC and anoctamin inhibitors tamoxifen (10 μM) and CaCCinhAO1 (10 μM). Mean ± SEM; #significant difference when compared to mock or scrambled RNA (scrbld). §Significant difference when compared Iso (ANOVA). (number of cells or flow cytometry assays)
Overexpression of LRRC8A enhanced basal I− quenching, while Hypo-induced quenching was not affected. RVD was even slightly inhibited by overexpression of LRRC8A (Fig. 3a-c). Inhibition of LRRC8A-expression by siRNA only slightly attenuated RVD (Fig. 3d-h). As knockdown or overexpression of LRRC8A affected baseline conductances (Fig. 3b), the assessment of RVD might be compromised when using the standard protocol (Ringer → Iso → Hypo; c.f. Methods) (Fig. 3e). We therefore also used a modified protocol and exchanged Ringer solution directly by hypotonic solution, which, however, showed similar results (Figs. 3f, g and 4). Values for half maximal RVD (T 50 %) were determined and an exponential function was fitted (R 0) to describe RVD more accurately (Fig. 3f, h). The data confirm earlier reports in that knockdown of LRRC8A attenuates RVD [20, 28]. Nevertheless, RVD is still clearly detectable in the absence of LRRC8A (Fig. 3f). Moreover, the VRAC inhibitor NS3728 only slightly reduced RVD, in contrast to the inhibition of KCl cotransport by RDIOA which strongly delayed RVD (Fig. 3g). Simultaneous inhibition of VRAC and KCl cotransport practically abolished RVD. T 50 % and R 0 values illustrate the marginal contribution of LRRC8A to volume regulation in HeLa cells (Fig. 3h).
Effects of hypotonic bath solution at variable baseline Cl− conductances. a If cellular baseline Cl− conductance is low, replacement of Ringer solution by isotonic solution (partial replacement of Cl− (and Na+) by mannitol (M) will not affect cell volume, subsequent swelling by hypotonic bath solution and regulatory volume decrease (RVD). If cellular baseline conductance is high, a change from Ringer solution to isotonic solution will shrink the cells and this will affect subsequent swelling by hypotonic bath solution and RVD. b Direct change from Ringer to hypotonic bath solution will lead to immediate water influx, swelling, and subsequent uncompromised RVD, independent of baseline conductances
Under the present experimental conditions (37 °C), current activation was very fast and volume regulation (RVD) was maximal already 3 min after exposure to Hypo (Fig. 5a). Both VRAC currents and volume regulation were attenuated at 20 °C (Fig. 2a). VRAC (measured by whole-cell patch clamping) did not inactivate, as the cytosolic compartment stays hypertonic relative to the bath solution throughout the time during Hypo exposure, thus confirming earlier findings by the Nilius team [14] (Fig. 5a). In contrast, when VRAC time dependence was assessed by YFP quenching (by applying I− at variable time points during Hypo), the inactivation of VRAC is clearly detectable (Fig. 5b). Notably, the effect of LRRC8A-knockdown on I− quenching could not be detected when I− was applied only 12 min after the onset of Hypo, confirming transient activation of VRAC/LRRC8A (Fig. 5c) [14]. Moreover, quenching was almost abolished by the removal of extracellular Ca2+, confirming Ca2+ dependence of VRAC activation as described earlier (Fig. 5d) [1, 13, 22]. Notably, a transient and small Ca2+-induced shrinkage due to activation of purinergic receptors with ATP was also attenuated in the absence of LRRC8A, suggesting a role of LRRC8A for receptor-mediated cell shrinkage (Fig. 5e).
Fast activation of VRAC and RVD in HeLa cells. a Time course for swelling (33 % hypotonicity; Hypo) activated whole-cell currents (patch clamp) and regulatory volume decrease (RVD) measured by flow cytometry. Note that maximal VRAC current was reached 2.5 min after applying Hypo and RVD was essentially completed 3.5 min after Hypo. Whole-cell currents did not inactivate as cells under whole-cell patch-clamp conditions demonstrate continues osmotic water influx and activation of VRAC [13]. b In contrast, VRAC, when measured by I− quenching, inactivated under continuous exposure to Hypo and with ongoing cellular RVD. c VRAC (measured by I− quenching) 3 min and 12 min after onset of Hypo. Inhibition of VRAC by siRNA knockdown of LRRC8A was detectable 3 min but not 12 min after Hypo, indicating inactivation of VRAC. d Activation of VRAC (I− quenching) in the presence and absence of extracellular Ca2+. e Transient cell shrinkage (rel. Volume in arbitrary units) upon stimulation of purinergic receptors by 100 μM ATP assessed by flow cytometry in the presence or absence of LRRC8A. Mean ± SEM, (number of experiments). #significant difference when compared to 12 min and presence of Ca2+, respectively (unpaired t test)
As reported initially [28], we also found that VRAC was reduced by overexpression of LRRC8A. Moreover, overexpression of a LRRC8A mutant, lacking the leucine-rich repeat (LRRC8AΔLRR) also attenuated VRAC (Fig. 6a). In contrast to LRRC8A-wt, LRRC8AΔLRR was not expressed in the cell membrane (Fig. 6b). In HeLa cells, a substantial portion of the swelling-induced whole cell current is carried by K+, as demonstrated by the K+ channel inhibitors Ba2+/TEA+ (Fig. 6c). Even in the presence of Ba2+/TEA+, siRNA-knockdown of LRRC8A was unable to abolish completely swelling-activated Cl− currents, suggesting that LRRC8A determines the magnitude of VRAC but is not essential for its activation in HeLa cells (Fig. 6c).
VRAC can be activated in HeLa cells lacking expression of LRRC8A. a Current/voltage (I/V) relationships and original recordings of whole-cell currents activated by Hypo in mock transfected, LRRC8A, and LRRC8AΔLRR expressing cells. b Membrane localization of overexpressed GFP-LRRC8A but not GFP- LRRC8AΔLRR. c Activation of VRAC in the absence or presence of K+ channel inhibitors Ba2+/TEA+ (5 mM/10 mM). siRNA-knockdown of LRRC8A reduced but not abolished swelling activated Cl− currents. Mean ± SEM. *Significant increase of whole cell currents by Hypo (paired t test). #Significant inhibition by Ba/TEA (unpaired t test). §Significant difference when compared to scrambled (unpaired t test). (number of cells)
Contribution of LRRC8A to VRAC and RVD in HCT116 cells
Stable knockout of LRRC8A in HCT116 cells has been reported by Voss et al. [28]. Voss and collaborators (FMP, Berlin) generously supplied us with HCT116 parental (LRRC8A+/+) and knockout (LRRC8A−/−) cell lines. We measured slightly enhanced basal currents in HCT-LRRC8A−/− cells, while swelling-activated whole-cell currents were reduced (Fig. 7a, c). In the presence of the K+ channel inhibitors Ba2+ and TEA+, VRAC was clearly reduced in HCT-LRRC8A−/− compared to HCT-LRRC8A+/+ cells, albeit a residual VRAC was still detected in KO cells (Fig. 7b, d). The enhanced basal currents in both LRRC8A+/+ and LRRC8A−/− cells were due to enhanced Cl− permeability, as demonstrated by extracellular Cl− replacement (Fig. 7e, f).
RVD and VRAC are present in HCT116 cells lacking expression of LRRC8A. a, b Whole-cell currents activated by hypotonic bath solution (Hypo, 200 mosmol/l) in HCT-LRRC8A+/+ and HCT-LRRC8A−/− cells, in the absence or presence of Ba2+ (5 mM) and TEA+ (10 mM). Note that Hypo-activated whole-cell currents in HCT-LRRC8A−/− cells, even in the presence of the K+ channel inhibitors Ba/TEA, which completely inhibited K+ currents (not shown). c, d Corresponding I/V curves. e, f Baseline Cl− currents in HCT-LRRC8A+/+ and HCT-LRRC8A−/− cells, as demonstrated by removal of extracellular Cl− (5Cl−). g Hypo-induced current densities and effect of removal of extracellular Cl− in HCT-LRRC8A+/+ and HCT-LRRC8A−/− cells (NMDG+Cl− in patch pipette and bath). Data indicate activation of an attenuated Cl− current in HCT-LRRC8A−/− cells. h Inhibition of VRAC in HCT-LRRC8A+/+ and HCT-LRRC8A−/− cells by NS3728 (10 μM) and CaCCinhAO1 (10 μM). i Hypo-induced VRAC in HCT-LRRC8A+/+ cells is attenuated and shows time-dependent inactivation at lower temperature (20 °C). j Hypo-induced VRAC in HCT-LRRC8A+/+ cells is attenuated and shows time dependent inactivation in the presence of the PLA2 inhibitor ACA (20 μM). Mean ± SEM; *significant activation by Hypo and inhibition by 5Cl− (paired t-test). #significantly different from HCT-LRRC8A+/+ cells (ANOVA)
Further characterization of VRAC in LRRC8A+/+ and LRRC8A−/− cells using NMDG+Cl− in bath and patch pipette indicated that (i) extracellular Cl− removal inhibited VRAC in both cell lines (Fig. 7g), (ii) the anion selectivity was I− > Cl− for VRAC in both cell lines, (iii) VRAC in both cell lines was inhibited by the VRAC and anoctamin inhibitors NS3728 and CaCCinhAO1 (Fig. 7h), (iv) the VRAC whole-cell currents were outwardly rectifying in both cell lines. (v) Time-dependent inactivation for VRAC was typically not observed (at 37 °C). However, when measured at 20 °C, VRAC in LRRC8A+/+ cells was significantly reduced and demonstrated the “typical” time-dependent inactivation (Fig. 7i). vi) Moreover, the inhibition of phospholipase A2 (PLA2) by ACA also largely inhibited VRAC and induced time-dependent inactivation, as observed recently in other cell types [22]. These results suggest that VRAC is largely reduced in LRRC8A−/− cells, but has otherwise similar properties.
Putative pore formation by LRRC8A
A change in the halide permeability sequence was reported for the putative pore mutant LRRC8A-T44C (kindly provided by Dr. Zhaozhu Qiu, The Scripps Research Institute, La Jolla, USA) [20]. When overexpressed in LRRC8A−/− cells, we also observed a shift in the Cl−/I− permeability for LRRC8A-T44C, albeit the shift was marginal (Fig. 8b, d). Importantly, VRAC currents generated by LRRC8A-T44C were largely reduced (Fig. 8a, c). While these results confirm a possible pore formation by LRRC8A [20], the data need to be interpreted with caution as LRRC8A-independent anion background currents may be relatively enhanced in LRRC8A−/− cells. Finally, we analyzed regulatory volume decrease after hypotonic cell swelling by flow cytometry and found that although attenuated in LRRC8A−/− cells, RVD was still clearly detectable in cells lacking LRRC8A expression (Fig. 8e, f). The data clearly confirm the role of LRRC8A for VRAC and hypotonic volume regulation, but do not support the concept of LRRC8A being an indispensable subunit.
Possible pore formation by LRRC8A and role in volume regulation. a–d Shifts in the reversal potentials by replacement of extracellular Cl− by I−. Note the larger shift for LRRC8A-T44C, when compared to LRRC8A-wt that becomes only evident after spreading of the x-axis. e Volume regulation (flow cytometry) in HCT-LRRC8A+/+ and HCT-LRRC8A−/− cells after exposure to hypotonic bath solution (Hypo, 200 mosmol/l). f Expression of LRRC8A in HCT-LRRC8A+/+ and HCT-LRRC8A−/− cells
We also analyzed the role of LRRC8A for VRAC and volume regulation in the head and neck cancer cell line BHY. The expression of LRRC8A was strongly suppressed by siRNA (Fig. 9a). Hypo-induced VRAC was almost abolished in BHY cells lacking the expression of LRRC8A (Fig. 9b, c). Moreover, regulatory volume decrease, particularly the fast initial component of RVD, was clearly attenuated in the BHY cells lacking the expression of LRRC8A (Fig. 9d, e). The data confirm the variable, cell type-dependent input of LRRC8A on VRAC and volume regulation and support our earlier finding that VRAC and RVD are possible in the virtual absence of LRRC8A [12].
VRAC but not RVD is absent in BHY cells lacking expression of LRRC8A. a Knockdown of LRRC8A by siRNA as shown by Western blotting. b Whole-cell currents activated by hypotonic bath solution (Hypo, 200 mosmol/l) in cells treated with scrambled RNA or siRNA for LRRC8A. c Corresponding i/v curves. d, e Volume regulation (flow cytometry) after exposure to hypotonic bath solution (Hypo, 200 mosmol/l) in cells treated with scrambled RNA, or after siRNA-knockdown of LRRC8A. Mean ± SEM; #significant difference when compared to scrambled (unpaired t test). (number of cells or flow cytometry assays)
LRRC8A is not essential for apoptosis of HeLa cells
VRAC (VSOR, VSOAC) has been claimed to be in charge for apoptotic cell death [11]. Recent reports point out the role of LRRC8 proteins in cancer drug resistance and apoptosis as well as cisplatin and taurine transport [8, 19]. We therefore examined the impact of LRRC8A on HeLa cell growth and staurosporine-induced apoptotic cell death. siRNA-knockdown of LRRC8A did not affect cell proliferation, in contrast to knockdown of the papilloma virus product E6/E7 [6] (Fig. 10a). Incubation with staurosporine induced cell shrinkage within 6 h and increased caspase-3 activity (Fig. 10b-e). Cells were shrunken and hypotonic swelling/volume regulation was compromised after incubation with staurosporine (Fig. 10b, c). We found that apoptotic shrinkage was not inhibited by knockdown of LRRC8A or ANO10, and was not affected by the VRAC blocker NS37128. Also, caspase activity was not affected by these maneuvers (Fig. 10e). In contrast, knockdown of ANO6 and inhibition of KCl cotransporters by RDIOA blocked apoptotic shrinkage and caspase activity. Taken together, LRRC8A has a variable impact on VRAC and volume regulation and may be a necessary, but not an indispensable component of VRAC.
LRRC8A has no effect on apoptotic cell death in HeLa cells. a Proliferation of cells. Effect of siRNA-knockdown of LRRC8A, or the human papillomavirus E6/E7 oncogenes. b Volume regulation after exposure to hypotonic bath solution (Hypo, 200 mosmol/l) under control and after induction of apoptosis by staurosporine (1 μM/ 4 h). c Cell volumes before and after induction of apoptosis by staurosporine. Note the staurosporine induced cell shrinkage indicating induction of apoptosis. siRNA-knockdown of the Cl− channel ANO6 (9;16) and inhibition of KCl cotransport by DIOA (50 μM) blocked apoptotic cell shrinkage, while siRNA-knockdown of LRRC8A, NS3728 (10 μM) and siRNA-knockdown of ANO10 did not inhibit apoptotic cell shrinkage. d, e Staurosporine (ST) induced caspase activity (number of green cells) and effects of siRNA and inhibitors on the number of caspase positive cells. Mean ± SEM; #significant effect of staurosporine (unpaired t test). Significant inhibition of activation of caspase by siRNA and inhibitors (ANOVA). (number of cells or assays)
The major findings of the present study concern the variable and cell-dependent impact of LRRC8A on VRAC and volume regulation. Despite the strong attenuation of VRAC and impairment of RVD in some cell types lacking LRRC8A expression, both VRAC and RVD are still detectable in these cells. Particularly, volume regulation is only partially compromised by knockdown of LRRC8A. The present data correspond to our recent report indicating that LRRC8A has little effects on VRAC in mouse sperm and human retinal pigment epithelium. Bestrophin1 was detected as the volume-regulated anion channel in these cells [12]. Disruption of mouse Best1−/− leads to a severe subfertility phenotype due to reduced motility and abnormal sperm morphology, which might be explained by a compromised RVD [12]. Mouse retinal pigment epithelial cells (RPE) does not express Best1, and human RPE cells rely on BEST1 for volume regulation.
Although a detailed analysis of the molecular counterparts of VRAC in naïve tissues is currently not provided, it is likely that highly differentiated tissues provide individual mechanisms for controlling their cell volume. While bestrophin is essential in human RPE cells, CFTR is required in the intestinal epithelium and respiratory tract. Knockout of CFTR has been shown to compromise volume regulation in intestinal crypt and tracheal epithelial cells [24–27]. Possibly, anoctamin 1 might be relevant for volume regulation in exocrine glands and the choroid plexus [7, 17, 23]. Individuals carrying LRRC8A mutations and LRRC8A knockout mice demonstrate defects in the lymphatic system, and we therefore may expect LRRC8A being important for VRAC and volume regulation in lymphocytes [10, 21].
The temperature dependence of VRAC and RVD detected here may provide an explanation for our findings. As reported recently, we found that Ca2+ is essential for proper activation and VRAC [22]. Time-dependent inactivation of VRAC currents was only detected at 20 °C but not at 37 °C, where currents are larger and activated more rapidly. Although other studies are typically performed at 20 °C, we believe that only at 37 °C, both currents and volume regulation are readily detected. Thus, VRAC and RVD are more pronounced in the present report when compared to earlier studies [20, 28]. Our data suggest a crucial role of temperature-sensitive PLA2 for both VRAC and RVD, which possibly may facilitate facilitates the access of Ca2+ to VRAC [22].
References
Akita T, Okada Y (2011) Regulation of bradykinin-induced activation of volume-sensitive outwardly rectifying anion channels by Ca2+ nanodomains in mouse astrocytes. J Physiol 589:3909–3927
Almaca J, Tian Y, AlDehni F, Ousingsawat J, Kongsuphol P, Rock JR, Harfe BD, Schreiber R, Kunzelmann K (2009) TMEM16 proteins produce volume regulated chloride currents that are reduced in mice lacking TMEM16A. J Biol Chem 284:28571–28578
Galietta LJ, Haggie PM, Verkman AS (2001) Green fluorescent protein-based halide indicators with improved chloride and iodide affinities. FEBS Lett 499:220–224
Gawenis LR, Franklin CL, Simpson JE, Palmer BA, Walker NM, Wiggins TM, Clarke LL (2003) cAMP inhibition of murine intestinal Na/H exchange requires CFTR-mediated cell shrinkage of villus epithelium. Gastroenterology 125:1148–1163
Hoffmann EK, Lambert IH, Pedersen SF (2009) Physiology of cell volume regulation in vertebrates. Physiol Rev 89:193–277
Honegger A, Leitz J, Bulkescher J, Hoppe-Seyler K, Hoppe-Seyler F (2013) Silencing of human papillomavirus (HPV) E6/E7 oncogene expression affects both the contents and the amounts of extracellular microvesicles released from HPV-positive cancer cells. Int J Cancer 133:1631–1642
Jang Y and Oh U (2014) Anoctamin 1 in secretory epithelia. Cell Calcium 55:355-361
Jentsch TJ, Lutter D, Planells-Cases R, Ullrich F, and Voss FK (2015) VRAC: molecular identification as LRRC8 heteromers with differential functions. Pflugers Arch (in press)
Juul CA, Grubb S, Poulsen KA, Kyed T, Hashem N, Lambert IH, Larsen EH, Hoffmann EK (2014) Anoctamin 6 differs from VRAC and VSOAC but is involved in apoptosis and supports volume regulation in the presence of Ca. Pflugers Arch 466:1899–1910
Kumar L, Chou J, Yee CS, Borzutzky A, Vollmann EH, von Andrian UH, Park SY, Hollander G, Manis JP, Poliani PL, Geha RS (2014) Leucine-rich repeat containing 8A (LRRC8A) is essential for T lymphocyte development and function. J Exp Med 211:929–942
Lang F, Hoffmann EK (2012) Role of ion transport in control of apoptotic cell death. Compr Physiol 2:2037–2061
Milenkovic A, Brandl C, Milenkovic VM, Jendrike T, Sirianant L, Wanitchakool P, Zimmermann S, Reif CM, Horling F, Schrewe H, Strünker T, Alvarez L, Schreiber R, Kunzelmann K, Wetzel CH, and Weber BHF (2015) Bestrophin1 is the volume-regulated anion channel in mouse sperm and human retinal pigment epithelium. Proc.Natl.Acad.Sci U.S A. 112: E2630-E2639
Nilius B, Eggermont J, Voets T, Droogmans G (1996) Volume-activated Cl- channels. Gen Pharmacol 27:1131–1140
Nilius B, Prenen J, Voets T, Eggermont J, Droogmans G (1998) Activation of volume-regulated chloride currents by reduction of intracellular ionic strength in bovine endothelial cells. J Physiol 506:353–361
Okada Y (2006) Cell volume-sensitive chloride channels: phenotypic properties and molecular identity. Contrib Nephrol 152:9–24
Ousingsawat J, Wanitchakool P, Kmit A, Romao AM, Jantarajit W, Schreiber S, Kunzelmann K (2015) Anoctamin 6 mediates effects essential for innate immunity downstream of P2X7-receptors in macrophages. Nat Commun 6:6245
Pedemonte N, Galietta LJ (2014) Structure and function of TMEM16 proteins (anoctamins). Physiol Rev 94:419–459
Pedersen SF, Klausen TK, and Nilius B (2015) The identification of VRAC (volume regulated anion channel): an amazing Odyssey. Acta Physiol (Oxf). 213:268-281
Planells-Cases R, Lutter D, Guyader C, Gerhards NM, Ullrich F, Elger DA, Kucukosmanoglu A, Xu G, Voss FK, Reincke SM, Stauber T, Blomen VA, Vis DJ, Wessels LF, Brummelkamp TR, Borst P, Rottenberg S, and Jentsch TJ (2015) Subunit composition of VRAC channels determines substrate specificity and cellular resistance to Pt-based anti-cancer drugs. EMBO J. 34:2993-3008
Qiu Z, Dubin AE, Mathur J, Tu B, Reddy K, Miraglia LJ, Reinhardt J, Orth AP, Patapoutian A (2014) SWELL1, a plasma membrane protein, is an essential component of volume-regulated anion channel. Cell 157:447–458
Sawada A, Takihara Y, Kim JY, Matsuda-Hashii Y, Tokimasa S, Fujisaki H, Kubota K, Endo H, Onodera T, Ohta H, Ozono K, Hara J (2003) A congenital mutation of the novel gene LRRC8 causes agammaglobulinemia in humans. J Clin Invest 112:1707–1713
Sirianant L, Ousingsawat J, Wanitchakool P, Schreiber R, and Kunzelmann K (2016) Cellular volume regulation by anoctamin 6: Ca2+, phospholipase A2, osmosensing. Pflügers Arch 468:335-349
Takayama Y, Shibasaki K, Suzuki Y, Yamanaka A, Tominaga M (2014) Modulation of water efflux through functional interaction between TRPV4 and TMEM16A/anoctamin 1. FASEB J 28:2238–2248
Valverde MA, O'Brien JA, Sepulveda FV, Ratcliff R, Evans MJ, Colledge WH (1993) Inactivation of the murine cftr gene abolishes cAMP-mediated but not Ca2+-mediated secretagogue-induced volume decrease in small-intestinal crypts. Pflugers Arch 425:434–438
Valverde MA, O'Briens JA, Sepulveda FV, Ratcliff RA, Evans MJ, Colledge WH (1995) Impaired cell volume regulation in intestinal crypt epithelia of cystic fibrosis. Proc Natl Acad Sci 92:9038–9041
Valverde MA, Vazquez E, Munoz FJ, Nobles M, Delaney SJ, Wainwright BJ, Colledge WH, Sheppard DN (2001) Murine CFTR channel and its role in regulatory volume decrease of small intestine crypts. Cell Physiol Biochem 10:321–328
Vazquez E, Nobles M, Valverde MA (2001) Defective regulatory volume decrease in human cystic fibrosis tracheal cells because of altered regulation of intermediate conductance Ca2+-dependent potassium channels. Proc Natl Acad Sci U S A 98:5329–5334
Voss FK, Ullrich F, Munch J, Lazarow K, Lutter D, Mah N, Andrade-Navarro MA, von Kries JP, Stauber T, Jentsch TJ (2014) Identification of LRRC8 heteromers as an essential component of the volume-regulated anion channel VRAC. Science 344:634–638
Acknowledgments
This study was supported by DFG SFB699-A7/A12, DFG KU756/12-1, and Volkswagenstiftung AZ 87 499. HeLa cells stably expressing halide-sensitive YFP-H148Q/I152L were generously provided by Prof. Dr. M. Amaral, University of Lisbon, Portugal. HCT-wt (LRRC8A+/+) and HCT-LRRC8A−/− cells were from Dr. Voss/Prof. Dr. Jentsch (FMP, Berlin). pIRES2 LRRC8A-T44C was a generous gift from Dr. Zhaozhu Qiu (The Scripps Research Institute, La Jolla, USA). The excellent technical assistance by Mss. B. Wild, P. Seeberger, and E. Tartler is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sirianant, L., Wanitchakool, P., Ousingsawat, J. et al. Non-essential contribution of LRRC8A to volume regulation. Pflugers Arch - Eur J Physiol 468, 805–816 (2016). https://doi.org/10.1007/s00424-016-1789-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-016-1789-6