Abstract
Background
Gastric cancer is a major public health problem around the globe. With the standardization of tumor treatment, surgery continues to be the most important treatment method for gastric cancer. However, changes in body composition and nutrition index parameters in patients with Billroth II and Roux-en-Y anastomosis following totally laparoscopic distal gastrectomy (TLDG) remain unclear.
Methods
This was a single-center retrospective study. A total of 369 patients who underwent TLDG at the First Affiliated Hospital of Soochow University (Suzhou, China) between January 2016 and February 2019 were included and assigned to the Billroth II group or Roux-en-Y group according to the anastomosis method. After propensity score matching, body composition and relevant clinical data were compared between the two groups.
Results
The operation time for the Billroth II group was significantly shorter than for the Roux-en-Y group (174.12 ± 39.33 min vs. 229.19 ± 28.12 min, P < 0.001). In addition, the Billroth II group showed lower skeletal muscle loss. Specifically, the Billroth II group showed a − 4.77 ± 4.88% change in the skeletal muscle index (SMI), whereas the Roux-en-Y group showed a − 11.89 ± 8.68% change (P = 0.001). The Billroth II group also showed a smaller decrease in BMI than the Roux-en-Y group (− 6.67 ± 7.76% vs. − 13.12 ± 10.79%, P = 0.018).
Conclusions
These results suggest that Billroth II anastomosis after TLDG has advantages over Roux-en-Y for maintaining patient body composition, especially in terms of SMI, and may serve as a useful reference when choosing an anastomosis method.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastric cancer is a major health problem around the world [1]. China accounts for more than 40% of the total global gastric cancer deaths each year. In China, the incidence rate and mortality from gastric cancer rank second among all malignant tumor types, and gastric cancer is considered a serious threat to public health. With the popularization of standardized tumor treatment, surgical treatment continues to be the most important treatment method for gastric cancer. As gastric cancer cases in China most commonly occur in the lower third of the stomach, along with the continuous development of intracorporeal anastomosis techniques, totally laparoscopic distal gastrectomy (TLDG) has gradually become standard treatment, with Billroth II and Roux-en-Y anastomosis being the two most commonly used anastomosis methods [2,3,4]. Totally laparoscopic digestive tract reconstruction is different from laparoscopic assisted or open digestive tract reconstruction: In totally laparoscopic surgery, we use a linear stapler to complete digestive tract reconstruction, while in laparoscopic assisted or open surgery we prefer to use circular stapler to complete digestive tract reconstruction. As is commonly known, anastomoses of linear anastomosis are larger than those of circular anastomosis, which may affect patients’ postoperative food intake [5].
Radical surgery for gastric cancer is usually associated with weight loss and malnutrition. These effects are related to many factors including the characteristics of the malignant tumor itself, reduced food intake, problems due to postoperative complications, and gastrointestinal reactions to postoperative adjuvant chemotherapy, all of which result in a reduction in nutrient intake [6,7,8]. Many studies have shown that changes in body composition are common following gastrectomy for gastric cancer [9]. The reconstruction of the digestive tract after gastrectomy usually makes it different from the normal physiological process. Changes in anatomical structure, digestive fluid, and intestinal flora may lead to changes in the patient’s body composition [10].
Retrospective studies and meta-analysis suggest that there is a relationship between body composition and prognosis for gastric cancer patients [9, 11, 12]. Body mass index (BMI), adipose tissue distribution, muscle mass, and other nutrition-related indicators are standard parameters for evaluating a patient’s body composition and nutritional status. Better understanding of such parameters can help us to improve a patient’s prognosis after gastrectomy. In recent years, computer software has been developed to determine the distribution and proportion of body fat and muscle through analysis of computed tomography (CT) images. Such software allows us to conveniently and accurately obtain information on the composition of a patient’s body and makes early intervention possible [13].
Previous studies of Billroth II and Roux-en-Y anastomosis have mostly focused on postoperative complications and postoperative quality of life. By contrast, there are few studies on changes in body composition and nutritional status of patients after two different anastomosis methods, especially in TLDG. Thus, we used a propensity score-matching method to compare changes in body composition and nutrition index parameters in patients with Billroth II and Roux-en-Y anastomosis after TLDG, to evaluate the prognosis of the two groups of patients, and to discuss possible causes of any differences.
Methods
Patients
The present study evaluated data from patients who underwent TLDG at the First Affiliated Hospital of Soochow University between January 2016 and February 2019. The inclusion criteria were (1) pathological confirmed gastric cancer, (2) stages I–III, (3) preoperative CT imaging data, and (4) CT imaging data and laboratory data 8–12 months after surgery. The exclusion criteria were (1) preoperative chemotherapy or radiotherapy, (2) distant metastasis confirmed during surgery, (3) combined multiple organ resection, (4) lack of any test parameter data, and (5) any other malignant tumors, endocrine disease, or neurodegenerative disorders. Two well-trained surgical clinical reviewers participated in collecting and recording defined demographic and clinical characteristics separately. The following data were recorded for all eligible patients: type of anastomosis, gender, age, BMI, pathological data (tumor node metastasis, TNM), CT data (skeletal muscle, visceral fat, and subcutaneous fat areas), laboratory data (hemoglobin, lymphocyte count, albumin, globulin), adjuvant chemotherapy, and postoperative complications.
Surgical procedure
Billroth II
After distal gastrectomy and duodenal stump closure with line stapler, lift the jejunum 15–20 cm away from the Treitz ligament, and an enterotomy and a gastrostomy were created for a 60-mm linear stapler to complete gastrojejunostomy. The common opening is closed using a line stapler or by hand stitching. Technical points include the following: (1) The input should not be too long and the mesentery should not be twisted. (2) The anastomosis can be placed on the side of the greater curvature or on the posterior. When placed on the posterior wall, attention should be paid to the presence of ischemia between the two cutting lines of the posterior gastric. (3) The anastomosis should be routinely checked for active bleeding and other conditions before closing the common opening.
Roux-en-Y
The jejunum was divided at 15–20 cm from the Treitz ligament and trim the jejunal mesentery. One incision is made at the greater curvature of remnant stomach, another one is made at 6 cm from the mesenteric part of the distal jejunal stump, the distal jejunum is lifted, and gastrojejunostomy is completed using a 60-mm linear stapler on the posterior greater gastric curvature. Antiperistaltic side-to-side anastomosis between the jejunum is approximately 30 cm from gastrojejunostomy. The common opening is closed and intestinal mesenteric fissure and Peterson’s fissure were sutured.
Body composition measurements
The skeletal muscle area was measured at the level of the L3 lumbar vertebra, which has been accepted by international consensus on the definition of cancer cachexia since 2011, such that the psoas, paraspinal, and abdominal wall muscles were all visible [14]. The cross-sectional composition was determined by manually delineating the required muscle or fat areas on a dedicated software platform. Next, Hounsfield units (HU) were used to further subdivide muscle (HU − 30 to 150) and fat (HU − 150 to − 30) areas. By comparing the CT parameters before and after surgery, the relative change was determined. The skeletal muscle index (SMI) was calculated as the skeletal muscle area divided by height squared. The visceral fat index (VFI) and subcutaneous fat index (SFI) were calculated in the same way. The prognostic nutritional index (PNI) was calculated as serum albumin (g/L) + 5 × total peripheral blood lymphocytes (× 109/L). Laboratory data were measured at the same clinical center. The tumor stage was determined by the 8th Edition of The AJCC Cancer Staging Manual for Gastric Cancer [15].
Propensity score matching
Patients were categorized into two groups based on Billroth II or Roux-en-Y anastomosis. Patients in the Billroth IIand Roux-en-Y groups were matched using the propensity score method. The 1:1 propensity score for an individual was calculated using age, gender, hypertension, diabetes, history of abdominal surgery, and tumor size.
Statistical analysis
All data were analyzed using the SPSS 26.0 (SPSS Inc., Chicago, IL, USA), GraphPad Prism 8 (San Diego, CA), and R software. The Chi-squared test or Fisher’s exact test was used for categorical variables, which are presented as a number. The Student’s t test or Mann–Whitney U test was used for continuous variables, which are expressed as means ± SD. Principal co-ordinate analysis (PCoA), which is a non-constrained ranking analysis that reflects the similarity and difference in structure between samples, was performed using the “vegan” package (version 2.5–6, https://cran.r-project.org/web/packages/vegan/index.html) and “ape” package (version 5.3, https://cran.r-project.org/web/packages/ape/index.html), and Kaplan–Meier survival curves were analyzed using GraphPad Prism 8. The log-rank test was used for statistical comparison. The level of statistical significance was set at P < 0.05.
Results
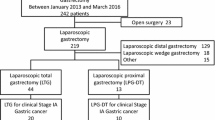
The flow of patients through this retrospective study is summarized in Fig. 1. Ultimately, 369 patients who underwent TLDG with Billroth II (n = 217) or Roux-en-Y (n = 152) anastomosis for distal gastric cancer were enrolled. After propensity score matching, finally 122 patients were matched. In a preliminary study, 46 patients were enrolled and PCoA cluster analysis was performed based on the CT parameters of body composition (SMI, SFI, VFI), BMI, PNI, albumin, hemoglobin, and lymphocytes; P = 0.008, Fig. 2. It could be clearly distinguished on the PC1 axis (P = 0.013). Thus, there may be significant differences in the above indicators for gastric cancer patients with Roux-en-Y and Billroth II digestive tract reconstruction after digital gastrectomy.
The clinical and pathological characteristics of the 122 propensity score-matched patients are shown in Table 1. As determined by the study design, age, gender, hypertension, diabetes, history of abdominal surgery, and tumor size in the Billroth IIand Roux-en-Y groups were comparable. There were no significant group differences in age, gender, hypertension, diabetes, history of abdominal surgery, and tumor size.
The surgical and pathological outcomes both before and after PSM are shown in Table 2. After propensity score matching, there was no significant between-group difference in the depth of tumor invasion, lymph node metastasis, degree of differentiation, vascular invasion, neural invasion, TNM staging, and estimated blood loss, but the operation time in the Billroth II group was significantly shorter than in the Roux-en-Y group (174.12 ± 39.33 min vs. 229.19 ± 28.12 min, P < 0.001, Table 2). In this study, early postoperative complication is defined as the complication of Clavien-Dindo (CD) grade III and above, mainly including complications related to anastomosis, serious cardiopulmonary complications, intestinal obstruction, pancreatic fistula, abdominal abscess, and incisional surgical site infection. The incidence of complications in Billroth II is lower than that in Roux-en-Y both before and after PSM. At the same time, the rate of postoperative blood transfusion in the Billroth II group was lower (P = 0.039, Table 2).
In addition, the differences in CT parameters relevant to body composition (SMI, SFI, VFI), albumin, hemoglobin, lymphocyte count, PNI, and BMI before surgery did not differ significantly between the two groups (P > 0.05, Table 3) both before and after matching. Preoperative and postoperative nutritional changes in the two patient groups are shown in Table 3. The Billroth II group showed lower skeletal muscle loss than the Roux-en-Y group. Specifically, SMI change was − 4.77 ± 4.88% in the Billroth II group and − 11.89 ± 8.68% in the Roux-en-Y group (P = 0.001, Table 3). At the same time, the Billroth II group showed less BMI loss than the Roux-en-Y group (− 6.67 ± 7.76% vs. − 13.12 ± 10.79%, P = 0.018, Table 3). Changes in other nutritional indicators, including hemoglobin, albumin, lymphocyte count, SFI, and VFI, were not significantly different between the two groups (P > 0.05, Table 3).
All patients who participated in the survival analysis were followed up for 2 years. Kaplan–Meier survival analysis at 1 year and 2 years was conducted for 312 and 144 patients respectively. The remaining patients were excluded because of death, declined follow-up, or dissatisfaction with post-op time. There was no significant difference in survival between the two groups in the short term after surgery. Specifically, the 1-year survival rate was not significantly different between the two groups (P = 0.950), nor was the 2-year survival rate (P = 0.317).
Discussion
Advanced lower gastric cancer accounts for the majority of gastric cancer cases in China, and major distal gastric resection continues to be one of the main surgical treatment methods. The method of gastrointestinal reconstruction is an important factor affecting recovery and quality of life after distal gastrectomy. Billroth II and Roux-en-Y anastomosis are two commonly used anastomosis methods, and their safety and feasibility have been verified [16].
Billroth II and Roux-en-Y have their advantages and disadvantages in different ways, including the difficulty of the procedure and postoperative reflux, and research is still ongoing. The choice of anastomosis is still largely a matter of surgeon’s preference. In our usual practice, the choice of anastomosis in our institution was decided jointly by two surgeons with more than 15 years of surgical experience.
Both Billroth II and Roux-en-Y are frequently performed in different parts of the world. Roux-en-Y is more complicated than Billroth II because it is to prevent bile reflux, although the significance of bile reflux is not clear, and Roux-en-Y has some unique complications, like Roux stasis syndrome. Although Billroth II combined with a Braun anastomosis was developed, it is believed that it can reduce bile reflux, and the effect is not exact [17, 18]. It has been reported that although in Roux-en-Y, a reduction in reflux can be found through endoscopy, but there is no statistical difference in the incidence of reflux gastritis [19]. Simultaneously others also consider that the findings under endoscopy have no obvious relationship with clinical symptoms [20]. Some scholars have also found that Roux-en-Y can significantly reduce the occurrence of reflux gastritis, and reduce the risk of carcinogenesis in the gastric remnant [21, 22]. Shimoda et al. founded the high incidence of delayed gastric emptying in Roux-en-Y [18]. Roux-en-Y stasis syndrome is considered to be related to the length of the limb, and shorter than 40 cm is more appropriate, and is related to abnormal small bowel motility [19, 23, 24].
To the best of our knowledge, this is the first study to focus on body composition changes in patients undergoing different anastomosis methods in TLDG. Interestingly, previous studies have shown that changes in body composition parameters can predict the prognosis of patients with gastric cancer [9]. About 85% of gastric cancer patients suffer from malnutrition, and BMI, which reflects the condition of subcutaneous and visceral fat, is often used as an index to evaluate the nutritional status of patients [25,26,27]. Subsequent studies have used CT cross-sectional parameters at the L3 level to measure body composition and have shown that subcutaneous fat is a good prognostic factor, whereas sarcopenia and high visceral fat predict poor survival [28, 29]. In the present study, we first tried to normalize the data on the rate of postoperative nutrition-related changes in the two groups. In a preliminary study, we performed PCoA, so as to analyze whether the samples could be clearly distinguished between two groups. The PCoA result showed that the patients’ overall nutritional status after the two different anastomosis methods was significantly different, after which we analyzed the body composition and nutritional indicators.
Many past studies linked BMI as an indicator of nutritional status to the prognosis of patients, as BMI can assess levels of abdominal subcutaneous and visceral fat. Such studies proved that class 2 and 3 obesity (BMI > 35) often predict a poor survival rate [9, 12, 26, 30]. However, there is insufficient evidence that BMI is an independent predictive factor, and there is evidence that BMI is not statistically significant at predicting the prognosis of patients with stages II–III gastric cancer undergoing surgery [9, 30]. In our study, the decrease in BMI between the Billroth II and Roux-en-Y groups was statistically significant (P = 0.018, Table 3), which is similar to the observed trend for SMI. We believe that BMI cannot accurately distinguish between muscle and fat as a low BMI may mask too much fat and a high BMI can mask poor muscle conditions, leading to confusion in the analysis of BMI data.
Sarcopenia is a state of severe failure of skeletal muscle mass and function. Skeletal muscle parameters can be clearly determined by CT, and are closely related to increased mortality for many malignant tumors. In the present study, patients who underwent Billroth II anastomosis had a smaller reduction in skeletal muscle mass compared to Roux-en-Y anastomosis, and the P value was statistically significant (P = 0.001, Table 3). During postoperative recovery among gastric cancer patients, weight loss and decreased motor function often occur, which may be the result of various factors including the impact of surgery, the altered digestive tract pathway, and the postoperative adjuvant chemotherapy. Sarcopenia may cause a higher incidence of postoperative complications and is related to the toxicity of adjuvant chemotherapy, which may result in the discontinuation of chemotherapy [31,32,33]. We believe that our results demonstrate that Billroth II anastomosis has certain advantages for maintaining human skeletal muscle mass compared to Roux-en-Y anastomosis. These different anastomosis methods lead to different intestine anatomy. It has been reported that Roux-en-Y anastomosis can cause changes in nutrient utilization, such as more polysaccharide consumption and less fat consumption, which eventually result in reduced food intake [34]. Previous studies have also reported that, in open subtotal gastrectomy, Billroth II anastomosis and Roux-en-Y anastomosis show different developmental intestinal bacteria [35]. Our institution is conducting ongoing experiments on changes in intestinal bacteria after these two methods of anastomosis. Previous studies have shown that, during gastric bypass surgery, changes in the richness and diversity of intestinal bacteria may be associated with the utilization of nutrients and physiological regulation [36,37,38]. Therefore, it is believed that intestinal bacterial changes due to different anastomosis methods will lead to different human nutritional conditions and changes in body composition. Although the interactions between subtotal gastrectomy, anatomical changes caused by different anastomosis methods, and changes in metabolic methods are currently unknown, potential causes may include the following: (1) a reduced circulating level of lipopolysaccharides (LPS) [35, 39]; (2) changes in a patient’s enteroendocrine secretions due to changes in intestinal flora [40]; or (3) a bile acid disturbance [41].
Compared with open surgery and laparoscopic-assisted surgery, totally laparoscopic surgery has the well-known advantages of less postoperative pain and faster postoperative bowel function recovery. As Billroth II and Roux-en-Y are two commonly used anastomosis methods, previous research has focused on their safety, feasibility, and postoperative complications. Some studies have suggested that the incidence of complications can increase due to sarcopenia and poor nutritional status, and that loss of muscle mass and function can cause a decrease in voluntary activity and affect normal postoperative recovery, thereby increasing hospitalization time and hospitalization costs [9, 25]. After surgery, patients show special metabolic patterns and cannot participate in basic physical activity, which can cause early loss of muscle mass and lower food intake, leading to a loss of fat mass at a later time. Therefore, patients with postoperative complications should be given more nutrition education, have a comprehensive rehabilitation plan, and receive additional nutritional treatments. The present study has shown that there are no significant differences in the incidence or severity of postoperative complications between the two types of anastomosis, which is in line with previous research results. The choice of the anastomosis method is commonly based on the specific condition of the patient, including tumor size and location, and the preference of the surgeon. We believe that the nutritional status and body composition of the patient should also be used as a basis for selection of the anastomosis method.
Other biochemical and functional indicators used in this study, including hemoglobin, albumin, and lymphocyte count, were not significantly different between the two anastomosis groups. Lymphocyte count can reflect both the immune function and nutritional status of the human body. Patients with malnutrition or immune dysfunction often have a decreased lymphocyte count, which is associated with increased morbidity and mortality. At the same time, studies have shown that lymphopenia is often associated with poor prognosis [42]. These parameters, which are easily accessible, are helpful for identifying malnutrition, but often need to be comprehensively analyzed through a risk assessment score. As a result, analysis of a single indicator may not be statistically significant.
The clinical significance of sarcopenia at predicting patient prognosis has been proven for gastric cancer as well as other malignancies [43,44,45]. CT is a commonly used tool throughout the diagnosis and treatment process for gastric cancer, both preoperatively and postoperatively [46]. In this study, we used CT data collected 8–12 months after surgery to evaluate changes in nutrition indicators. Adjuvant chemotherapy generally lasts about 6 months. The nutritional status of patients 8–12 months after surgery, which is thus less affected by chemotherapy and patients’ diets, are relatively stable. Considering the prognostic value of reduced body composition and the clinical role of early nutritional support, evaluation of body composition should be an indispensable step at specific points during the diagnosis and treatment of gastric cancer patients, and may be helpful for timely assessment of nutritional status and corresponding treatment [11, 25]. There are also reports that sarcopenia should be assessed at three levels (presarcopenia, sarcopenia, and severe sarcopenia) in order to more precisely predict postoperative complications and prognosis. However, it may be difficult to evaluate sarcopenia in stages due to the need for accurate evaluation of the patient’s muscle function and physical function as well as quantification of skeletal muscles [6].
A limitation of this study is that it is a single-center retrospective study and choice of anastomosis may lead to partially unknown bias, and more prospective randomized studies are worth considering. At the same time, the sample size is still small, and more data on patients from different centers can be considered to be collected and included in the follow-up study. Due to the short postoperative time for some patients, research related to survival analysis is not yet complete. Further study will continue, which will focus more on the long-term survival of patients and the long-term quality of life after surgery.
In summary, this study has shown that, compared with Roux-en-Y anastomosis, Billroth II anastomosis has advantages for maintaining body composition, especially SMI and BMI, and may be a useful reference for choosing the anastomosis method.
References
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A (2015) Global cancer statistics, 2012. CA Cancer J Clin 65(2):87–108
Ikeda O, Sakaguchi Y, Aoki Y, Harimoto N, Taomoto J, Masuda T, Ohga T, Adachi E, Toh Y, Okamura T, Baba H (2009) Advantages of totally laparoscopic distal gastrectomy over laparoscopically assisted distal gastrectomy for gastric cancer. Surg Endosc 23(10):2374–2379
Lee MS, Ahn SH, Lee JH, Park DJ, Lee HJ, Kim HH, Yang HK, Kim N, Lee WW (2012) What is the best reconstruction method after distal gastrectomy for gastric cancer? Surg Endosc 26(6):1539–1547
Tran TB, Worhunsky DJ, Squires MH, Jin LX, Spolverato G, Votanopoulos KI, Cho CS, Weber SM, Schmidt C, Levine EA, Bloomston M, Fields RC, Pawlik TM, Maithel SK, Norton JA, Poultsides GA (2016) To Roux or not to Roux: a comparison between Roux-en-Y and Billroth II reconstruction following partial gastrectomy for gastric cancer. Gastric Cancer 19(3):994–1001
Lee K, Kim KW, Lee JB, Shin Y, Jang JK, Yook JH, Kim BS, Lee IS (2019) Impact of remnant stomach volume and anastomosis on nutrition and body composition in gastric cancer patients. Surg Oncol 31:75–82
Kamarajah SK, Bundred J, Tan BHL (2019) Body composition assessment and sarcopenia in patients with gastric cancer: a systematic review and meta-analysis. Gastric Cancer 22(1):10–22
Zelnick R, Auguste LJ, Wise L (1989) Nutritional effects of postgastrectomy reconstruction: a clinical evaluation. J Surg Oncol 40(4):219–221
Kiyama T, Mizutani T, Okuda T, Fujita I, Tokunaga A, Tajiri T, Barbul A (2005) Postoperative changes in body composition after gastrectomy. J Gastrointest Surg 9(3):313–319
Park HS, Kim HS, Beom SH, Rha SY, Chung HC, Kim JH, Chun YJ, Lee SW, Choe EA, Heo SJ, Noh SH, Hyung WJ, Cheong JH, Kim HI, Son T, Lim JS, Baek SE, Jung M (2018) Marked loss of muscle, visceral fat, or subcutaneous fat after gastrectomy predicts poor survival in advanced gastric cancer: single-center study from the CLASSIC Trial. Ann Surg Oncol 25(11):3222–3230
Distrutti E, Monaldi L, Ricci P, Fiorucci S (2016) Gut microbiota role in irritable bowel syndrome: new therapeutic strategies. World J Gastroenterol 22(7):2219–2241
Shizgal HM (1985) Body composition of patients with malnutrition and cancer Summary of methods of assessment. Cancer 55(1 Suppl):250–253
Prado CM, Lieffers JR, McCargar LJ, Reiman T, Sawyer MB, Martin L, Baracos VE (2008) Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol 9(7):629–635
Yip C, Dinkel C, Mahajan A, Siddique M, Cook GJ, Goh V (2015) Imaging body composition in cancer patients: visceral obesity, sarcopenia and sarcopenic obesity may impact on clinical outcome. Insights Imaging 6(4):489–497
Yip C, Dinkel C, Mahajan A, Siddique M, Cook GJR, Goh V (2015) Imaging body composition in cancer patients: visceral obesity, sarcopenia and sarcopenic obesity may impact on clinical outcome. Insights Imaging 6(4):489–497
Seeruttun SR, Yuan S, Qiu H, Huang Y, Li Y, Liang Y, Guan Y, Zhan Y, Li W, Chen Y, Sun X, Xu D, Zhou Z (2017) A comprehensive analysis comparing the eighth AJCC gastric cancer pathological classification to the seventh, sixth, and fifth editions. Cancer Med 6(12):2804–2813
Yang D, He L, Tong WH, Jia ZF, Su TR, Wang Q (2017) Randomized controlled trial of uncut Roux-en-Y vs Billroth II reconstruction after distal gastrectomy for gastric cancer: which technique is better for avoiding biliary reflux and gastritis? World J Gastroenterol 23(34):6350–6356
Ma Z, Wang Z, Zhang J (2001) Carcinogenicity of duodenogastric reflux juice in patients undergoing gastrectomy. Zhonghua Wai Ke Za Zhi 39(10):764–766
Shimoda M, Kubota K, Katoh M, Kita J (2013) Effect of billroth II or Roux-en-Y reconstruction for the gastrojejunostomy on delayed gastric emptying after pancreaticoduodenectomy: a randomized controlled study. Ann Surg 257(5):938–942
So JB, Rao J, Wong AS, Chan YH, Pang NQ, Tay AYL, Yung MY, Su Z, Phua JNS, Shabbir A, Ng EKW (2018) Roux-en-Y or Billroth II reconstruction after radical distal gastrectomy for gastric cancer: a multicenter randomized controlled trial. Ann Surg 267(2):236–242
Johnsson F, Joelsson B, Gudmundsson K, Greiff L (1987) Symptoms and endoscopic findings in the diagnosis of gastroesophageal reflux disease. Scand J Gastroenterol 22(6):714–718
Xiong JJ, Altaf K, Javed MA, Nunes QM, Huang W, Mai G, Tan CL, Mukherjee R, Sutton R, Hu WM, Liu XB (2013) Roux-en-Y versus Billroth I reconstruction after distal gastrectomy for gastric cancer: a meta-analysis. World J Gastroenterol 19(7):1124–1134
Taylor PR, Mason RC, Filipe MI, Vaja S, Hanley DC, Murphy GM, Dowling RH, Mccoll I (1991) Gastric carcinogenesis in the rat induced by duodenogastric reflux without carcinogens - morphology, mucin histochemistry, polyamine metabolism, and labeling index. Gut 32(12):1447–1454
Gustavsson S, Ilstrup DM, Morrison P, Kelly KA (1988) Roux-Y stasis syndrome after gastrectomy. Am J Surg 155(3):490–494
Mathias JR, Fernandez A, Sninsky CA, Clench MH, Davis RH (1985) Nausea, vomiting, and abdominal pain after Roux-en-Y anastomosis: motility of the jejunal limb. Gastroenterology 88(1 Pt 1):101–107
Ongaro E, Buoro V, Cinausero M, Caccialanza R, Turri A, Fanotto V, Basile D, Vitale MG, Ermacora P, Cardellino GG, Nicoletti L, Fornaro L, Casadei-Gardini A, Aprile G (2017) Sarcopenia in gastric cancer: when the loss costs too much. Gastric Cancer 20(4):563–572
Lieffers JR, Bathe OF, Fassbender K, Winget M, Baracos VE (2012) Sarcopenia is associated with postoperative infection and delayed recovery from colorectal cancer resection surgery. Br J Cancer 107(6):931–936
Tegels JJ, van Vugt JL, Reisinger KW, Hulsewe KW, Hoofwijk AG, Derikx JP, Stoot JH (2015) Sarcopenia is highly prevalent in patients undergoing surgery for gastric cancer but not associated with worse outcomes. J Surg Oncol 112(4):403–407
Li XT, Tang L, Chen Y, Li YL, Zhang XP, Sun YS (2015) Visceral and subcutaneous fat as new independent predictive factors of survival in locally advanced gastric carcinoma patients treated with neo-adjuvant chemotherapy. J Cancer Res Clin Oncol 141(7):1237–1247
Antoun S, Bayar A, Ileana E, Laplanche A, Fizazi K, di Palma M, Escudier B, Albiges L, Massard C, Loriot Y (2015) High subcutaneous adipose tissue predicts the prognosis in metastatic castration-resistant prostate cancer patients in post chemotherapy setting. Eur J Cancer 51(17):2570–2577
Janssen I, Heymsfield SB, Allison DB, Kotler DP, Ross R (2002) Body mass index and waist circumference independently contribute to the prediction of nonabdominal, abdominal subcutaneous, and visceral fat. Am J Clin Nutr 75(4):683–688
Fukuda Y, Yamamoto K, Hirao M, Nishikawa K, Nagatsuma Y, Nakayama T, Tanikawa S, Maeda S, Uemura M, Miyake M, Hama N, Miyamoto A, Ikeda M, Nakamori S, Sekimoto M, Fujitani K, Tsujinaka T (2016) Sarcopenia is associated with severe postoperative complications in elderly gastric cancer patients undergoing gastrectomy. Gastric Cancer 19(3):986–993
Wang SL, Zhuang CL, Huang DD, Pang WY, Lou N, Chen FF, Zhou CJ, Shen X, Yu Z (2016) Sarcopenia adversely impacts postoperative clinical outcomes following gastrectomy in patients with gastric cancer: a prospective study. Ann Surg Oncol 23(2):556–564
Zhuang CL, Huang DD, Pang WY, Zhou CJ, Wang SL, Lou N, Ma LL, Yu Z, Shen X (2016) Sarcopenia is an independent predictor of severe postoperative complications and long-term survival after radical gastrectomy for gastric cancer: analysis from a large-scale cohort. Medicine (Baltimore) 95(13):e3164
Thirlby RC, Bahiraei F, Randall J, Drewnoski A (2006) Effect of Roux-en-Y gastric bypass on satiety and food likes: the role of genetics. J Gastrointest Surg 10(2):270–277
Lin XH, Huang KH, Chuang WH, Luo JC, Lin CC, Ting PH, Young SH, Fang WL, Hou MC, Lee FY (2018) The long term effect of metabolic profile and microbiota status in early gastric cancer patients after subtotal gastrectomy. PLoS One 13(11):e0206930
Furet JP, Kong LC, Tap J, Poitou C, Basdevant A, Bouillot JL, Mariat D, Corthier G, Dore J, Henegar C, Rizkalla S, Clement K (2010) Differential adaptation of human gut microbiota to bariatric surgery-induced weight loss: links with metabolic and low-grade inflammation markers. Diabetes 59(12):3049–3057
Graessler J, Qin Y, Zhong H, Zhang J, Licinio J, Wong ML, Xu A, Chavakis T, Bornstein AB, Ehrhart-Bornstein M, Lamounier-Zepter V, Lohmann T, Wolf T, Bornstein SR (2013) Metagenomic sequencing of the human gut microbiome before and after bariatric surgery in obese patients with type 2 diabetes: correlation with inflammatory and metabolic parameters. Pharmacogenomics J 13(6):514–522
Kong LC, Tap J, Aron-Wisnewsky J, Pelloux V, Basdevant A, Bouillot JL, Zucker JD, Dore J, Clement K (2013) Gut microbiota after gastric bypass in human obesity: increased richness and associations of bacterial genera with adipose tissue genes. Am J Clin Nutr 98(1):16–24
Clemente-Postigo M, Roca-Rodriguez Mdel M, Camargo A, Ocana-Wilhelmi L, Cardona F, Tinahones FJ (2015) Lipopolysaccharide and lipopolysaccharide-binding protein levels and their relationship to early metabolic improvement after bariatric surgery. Surg Obes Relat Dis 11(4):933–939
Cani PD, Everard A, Duparc T (2013) Gut microbiota, enteroendocrine functions and metabolism. Curr Opin Pharmacol 13(6):935–940
Long SL, Gahan CGM, Joyce SA (2017) Interactions between gut bacteria and bile in health and disease. Mol Aspects Med 56:54–65
Lin JX, Lin JP, Xie JW, Wang JB, Lu J, Chen QY, Cao LL, Lin M, Tu R, Zheng CH, Huang CM, Li P (2019) Prognostic value and association of sarcopenia and systemic inflammation for patients with gastric cancer following radical gastrectomy. Oncologist 24(11):e1091–e1101
Fukuda T, Seto Y, Yamada K, Hiki N, Fukunaga T, Oyama S, Yamaguchi T (2008) Can immune-enhancing nutrients reduce postoperative complications in patients undergoing esophageal surgery? Dis Esophagus 21(8):708–711
Harada K, Ida S, Baba Y, Ishimoto T, Kosumi K, Tokunaga R, Izumi D, Ohuchi M, Nakamura K, Kiyozumi Y, Imamura Y, Iwatsuki M, Iwagami S, Miyamoto Y, Sakamoto Y, Yoshida N, Watanabe M, Baba H (2016) Prognostic and clinical impact of sarcopenia in esophageal squamous cell carcinoma. Dis Esophagus 29(6):627–633
Wagner D, DeMarco MM, Amini N, Buttner S, Segev D, Gani F, Pawlik TM (2016) Role of frailty and sarcopenia in predicting outcomes among patients undergoing gastrointestinal surgery. World J Gastrointest Surg 8(1):27–40
Heymsfield SB (2008) Development of imaging methods to assess adiposity and metabolism. Int J Obes (Lond) 32(Suppl 7):S76-82
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This study was not funded. Author Jiawen Zhang declares that he has no conflict of interest. Author Linhua Jiang declares that he has no conflict of interest. Author Xinguo Zhu declares that he has no conflict of interest. The study protocol was approved by the Ethics Committee of the First Affiliated Hospital of Soochow University. This project was conducted as a retrospective observational study; the study protocol involved minimal risk and did not threaten the health of the subjects. And, all participating patients signed informed consents. This project was conducted in compliance with the spirit of the “Declaration of Helsinki, Ethical Principles for Medical Research Involving Human Subjects” (as amended in Fortaleza, October 2013).
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jiang, L., Zhang, J. & Zhu, X. Billroth II anastomosis maintains SMI and BMI better than Roux-en-Y anastomosis following totally laparoscopic distal gastrectomy: a propensity score-matched study. Langenbecks Arch Surg 407, 1441–1450 (2022). https://doi.org/10.1007/s00423-022-02459-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00423-022-02459-y