Abstract
Purpose
Abdominal adipose distribution may be associated with tumor growth, but its impact on gastric carcinoma survival after neo-adjuvant therapies is uncertain. This retrospective study was to determine the association linking BMI and CT-measured fat parameters to the survival in advanced gastric cancer patients who underwent preoperative chemotherapy.
Methods
Eighty-four consecutive patients with locally advanced gastric cancer who received neo-adjuvant chemotherapy and following gastrectomy were identified between January 2005 and June 2008. CT parameters were measured retrospectively on the CT images obtained before chemotherapy initiation. Subcutaneous fat thicknesses of the anterior, lateral, and posterior abdominal wall (ASFT, LSFT, and PSFT) represented subcutaneous fat. Intraperitoneal fat thickness (IFT) and retro-renal fat thickness represented visceral fat. Association linking BMI and CT factors to overall survival was evaluated with survival analysis.
Results
ASFT and PSFT above the median value (i.e., high ASFT and PSFT) were associated with longer OS (P = 0.001; 0.003). Conversely, high IFT and high IFT/PSFT were associated with shorter OS (P = 0.003; 0.003) and DFS (P < 0.001; 0.004). By multivariate analysis, high IFT and PSFT were independently associated with OS (HR 2.94, 95 % CI 1.54–5.60; 0.38, 95 % CI 0.21–0.71) and DFS (HR 3.28, 95 % CI 1.55–6.93; 0.42, 95 % CI 0.21–0.82). BMI was not significant for OS and DFS.
Conclusions
This study provided the first evidence that IFT, ASFT, and PSFT measured before neo-adjuvant chemotherapy were likely to be useful predictive biomarkers for survival of advanced gastric cancer patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastric cancer is one of the most common malignancies worldwide, accounting for 986,600 new cases and 738,000 deaths each year. Its incidence and mortality rate are even higher in Asian countries (Jemal et al. 2011). Surgical resection is the main choice for gastric cancer treatment, and preoperative chemotherapy is commonly used for advanced gastric cancer (Ajani et al. 2013). Tumor stage, distant metastasis, treatment plan, and tumor-specific biomarker expressions are generally regarded as predictors for patient prognosis with gastric cancer (Grabsch and Tan 2013; Medina-Franco et al. 2004; Smith et al. 2005; Wagner et al. 2006).
In addition to these prognostic factors, gastric cancer patients’ health situations are also associated with prognosis (Piazuelo and Correa 2013). Obesity has become a research focus as a physical factor associated with tumor prognosis (Aleman et al. 2014; Bianchini et al. 2002; Calle et al. 2003; Demark-Wahnefried et al. 2012). Body mass index (BMI) has historically been used as a surrogate method for measuring an individual’s degree of obesity (Li et al. 2009; Renehan et al. 2008; Yuan et al. 2013). However, the BMI does not differentiate among differing body fat distributions; thus, it represents at best a poor and inexact parameter for obesity (House et al. 2008; Maurovich-Horvat et al. 2007). At present, several studies have used computed tomography (CT) images to quantitatively measure fat content on a specific body part to reflect the degree of obesity (Griggs and Sabel 2008; Guiu et al. 2010; Ladoire et al. 2011). In these studies, body fat measurements often included the measurements of subcutaneous fat (SF) and visceral fat (VF). CT images can obtain body fat distribution; thus, they probably provide more appropriate parameters than the BMI to define obesity.
Whether the BMI and SF and/or VF amounts at treatment initiation predict outcomes in patients with advanced gastric cancer has not been investigated. Therefore, we proposed a retrospective study into exploring the association linking BMI and fat parameters measured on CT images to the survival outcomes in patients with locally advanced gastric cancer. We also proposed to determine whether the CT parameters were better than the BMI in predicting prognosis.
Materials and methods
Patients
This study retrospectively included consecutive patients with histopathologically proved locally advanced (>cT2 and/or cN+) gastric cancer who underwent preoperative chemotherapy in our hospital from January 2005 to June 2008. All eligible patients received abdominal CT examination before preoperative chemotherapy. We excluded patients with multiple tumors, patients who did not complete preoperative chemotherapy, patients with metastatic tumor verified by laparoscope, patients who received incomplete excision, patients who died within 30 days after surgery, and patients whose CT data could not be obtained. This study was approved by our institutional review board with an informed consent waiver.
In total, 112 patients were eligible for inclusion. We excluded 19 patients who had metastatic tumors that could not be surgically excised, four with incomplete excision, two with multiple tumors, two who could not tolerate preoperative chemotherapy, one patient suffering perioperative death. A total of 84 patients were thus included for analysis.
Image analysis
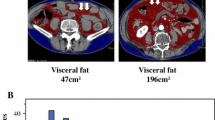
Fat measurements were taken independently by two experienced radiologists (Chen Y and Tang L, 8 and 10 years of experience) in the non-contrast phase of the available CT images obtained before treatment initiation. The fat parameters included intraperitoneal fat thickness (IFT, distance between the anterior peritoneum and retroperitoneum in the midline at the umbilicus level), retro-renal fat thickness (RFT, distance along renal axis between the perinephrium and retroperitoneum at the renal hilum level), subcutaneous fat thickness of anterior abdominal wall (ASFT, the largest paraumbilicus vertical distance from the skin to the muscle at the umbilicus level), lateral wall (LSFT, bilateral vertical distance from the skin to the muscle at the umbilicus level), and posterior wall (PSFT, the largest vertical distance from the skin to the iliopsoas muscle at the umbilicus level). The thickness of musculi psoas (PMT) was also measured on an axial image at the umbilicus level by measuring the maximum diameter. ASFT, LSFT, PSFT, and PMT were measured on both the left and right sides, and the mean values were used in analysis. IFT and RFT were considered parameters for visceral fat, and ASFT, LSFT, and PSFT were for subcutaneous fat. All parameters were recorded in cm. Figure 1 presents the fat parameter measurements on CT. The two radiologists were blind to patients’ clinical or prognostic information.
Measurements of fat parameters: IFT was measured as distance between the anterior peritoneum and retroperitoneum in the midline (the value of 56.6 mm on a), RFT as distance along renal axis between the perinephrium and retroperitoneum (the value of 10.6 mm on b), ASFT as the largest paraumbilicus vertical distance from the skin to the muscle (the value of 37.4 and 32.0 mm on a), LSFT as bilateral vertical distance from the skin to the muscle (the value of 27.3 and 22.3 mm on a), PSFT as the largest vertical distance from the skin to the iliopsoas muscle(the value of 58.9 and 59.8 mm on a). ASFT, LSFT and PSFT were measured on both the left and right sides
Therapeutic efficacy evaluation
Post-therapy CT images were also collected for each patient. The gastric wall thickness at tumor was measured and compared between pre-therapy and post-therapy. The change was calculated as (T Post-therapy − T pre-therapy)/T pre-therapy × 100 %. Patients with a decreased change of ≥30 % were regarded as good responders, those with change <30 % were as poor responders. In this study, there were 33 good responders and 42 poor responders; nine patients without post-therapy CT examination could not be judged for therapeutic efficacy.
Follow-up
Patients in this retrospective cohort were regularly followed up with after surgery. Follow-up consisted of an outpatient interview at 3-month interval for 1 year and then 6-month interval until death. Date of the last follow-up, data of recurrence, and date and cause of death were collected. Disease-free survival (DFS) was measured from the CT date until local recurrence or distant metastasis. Overall survival (OS) was measured from the CT date until all-cause death. Patients who were alive were censored at the last follow-up or death. The cutoff date for the follow-up in this study was July 31, 2014.
Statistical analysis
Continuous variables were described as means ± standard deviations. Two raters’ agreements were assessed with the Bland–Altman plot and intraclass correlation coefficient (ICC). The percentage of difference between two raters was plotted on the Y-axis against the average of two raters on the X-axis. An ICC > 0.80 indicated almost perfect agreement, 0.61–0.80 substantial, 0.41–0.60 moderate, 0.21–0.40 fair, and 0.0–0.2 indicated poor or no agreement. The measurements from the two raters were averaged for the following analysis. The correlation between parameters was tested with Pearson’s correlation coefficient, and 0.81–1.0 indicated a very strong relationship, 0.61–0.8 strong, 0.41–0.60 moderate, 0.21–0.40 weak, 0.0–0.2 was very weak. CT measurements were converted into dichotomized variables by medians. The BMI was dichotomized as overweight (>25 kg/cm2) and standard weight (18–25 kg/cm2). Survival curves were estimated using the Kaplan–Meier method and compared using a log-rank test. Kaplan–Meier analyses were also conducted in sex, cStage, and/or therapeutic efficacy-stratified population. A multivariate Cox model was then constructed to discover independent prognostic factors and compute the adjusted hazard ratios (HRs) with their 95 % CIs. The multivariate model was internally validated using bootstrapping (200 replications). The difference of fat distribution between males and females was tested with an independent T test. P values <0.05 were considered statistically significant. Calculations were performed using the Statistical Package for Social Sciences Program, version 22.0 (SPSS, Chicago, IL).
Results
Patient characteristics and clinical outcome
Eighty-four patients were included in the analysis, and patient characteristics and CT parameters are listed in Table 1. No patient was lost to follow-up, and the median follow-up at the cutoff data was 33 months (6–102 months). The 3-year survival rate of all included patients was 48 % (95 % CI 37–59 %).
Measurement consistency
Substantial agreement was obtained measuring IFT (ICC = 0.65, Table 2). According to Bland–Altman plot (Fig. 2) of IFT, the average percentage of difference between two raters was 9.3 %, the percentage of difference between two raters was <20 % for 73 out of 84 cases, and the percentage of difference was beyond 95 % CI in only three cases. Perfect agreement was observed in the measurements of RFT, ASFT, LSFT, PSFT, and PMT (all ICC >0.80, Table 2). The Bland–Altman plots (Fig. 2) for these parameters also suggested good agreement, showing the average percentage of difference between two raters less than 3 %, the percentage of difference was <10 % for most cases. Therefore, the CT measurements in this study were accurate and reliable.
Correlation analysis
The CT-measured fat parameters showed weak to moderate positive correlation with the BMI. The three subcutaneous fat parameters of ASFT, LSFT, and PSFT showed strong or very strong positive correlation between either of the two parameters. IFT and RFT, two visceral fat parameters, showed moderate positive correlation. The SF parameters had moderate positive correlations with VF parameters. Both SF and VF parameters exhibited weak positive correlation with PMT (Table 3).
Survival analysis
The median OS of all included patients was 30 months (95 % CI 13–47 months), and the median DFS was 33 months (95 % CI 14–52 months). Table 4 summarizes the univariate survival analysis results of patient characteristics and fat parameters according to survival outcomes.
Poor responders, patients at Stage III, and patients with signet ring cell cancer presented statistically worse OS (P = 0.001 < 0.001 and 0.045, respectively, on univariate log-rank test). Poor responders and patients at Stage III still presented statistically worse DFS (P = 0.002 and 0.001, respectively). Patients with IFT >4.9 cm, ASFT ≤1.9 cm, or PSFT ≤4.16 cm presented statistically worse OS than patients with IFT ≤4.9 cm, ASFT >1.9 cm, or PSFT >4.16 cm (P = 0.003, 0.001 and 0.003, respectively). Patients with IFT/PSFT >1.2 also presented significantly worse OS (P = 0.003). IFT and IFT/PSFT were significant for DFS on univariate analysis (P was <0.001 and 0.003, respectively). ASFT and PSFT showed borderline significant association with DFS (P = 0.05 and 0.056, respectively). Overweight patients showed similar OS and DFS as standard weight patients (P = 0.814 and 0.56, respectively). Kaplan–Meier survival curves are shown in Figs. 3 and 4.
IFT and IFT/PSFT were still significant for OS and DFS in different sex, cStage, and/or therapeutic efficacy-stratified population (all P < 0.05). ASFT and PSFT were still significant for OS in different sex and/or cStage-stratified population (all P < 0.05). However, ASFT and PSFT were not significant for OS in good responders, P = 0.177 and 0.715, respectively (Fig. 5).
On multivariate analysis (Table 5), IFT (>4.9 cm) and PSFT (>4.16 cm) were independent prognostic fat factors for OS; adjusted HR were 2.94 (95 % CI 1.54–5.60) and 0.38 (95 % CI 0.21–0.71), respectively. IFT (>4.9 cm) and PSFT (>4.16 cm) were also independent prognostic DFS factors; adjusted HR were 3.28 (95 % CI 1.55–6.93) and 0.42 (95 % CI 0.21–0.82), respectively.
When bootstrapping was performed to check the multivariate Cox model validity, the association between IFT and PSFT and OS was close to statistical significance (P = 0.069 and 0.055, respectively), the association between IFT and DFS was statistically significant (P = 0.007), and the association between PSFT and DFS was close to statistical significance (P = 0.051).
Sex and fat distribution
Table 6 shows the fat parameter comparison between males and females. Females presented higher ASFT and LSFT than males in this study, but no statistical difference was detected between males and females in IFT, RFT, PMT, PSFT, and the BMI.
Discussion
Obesity is a well-established risk factor for developing gastric cancer (Renehan et al. 2008) and is associated with increased mortality from gastric cancer (Calle et al. 2003). However, its definition is controversial, and it is unclear whether the BMI is an appropriate measure of obesity (Moore et al. 2004). Several studies used waist circumference or a waist-to-hip circumference ratio instead of the BMI to predict the risk of developing colon or prostate cancer (Hsing et al. 2000; Pischon et al. 2006). However, these circumferences are crude measures of body fat distribution that fail to distinguish between deep abdominal or visceral fat and subcutaneous fat (Bazzocchi et al. 2012; Fox et al. 2007). As such, the patients who have almost identical sagittal abdominal diameters (supine abdominal height) and similar waist circumferences may have completely different abdominal fat distribution. CT can be used to obtain an accurate assessment of intra-abdominal fat by measuring specific areas or distances representative of visceral and subcutaneous fat (Tokunaga et al. 1983; Yoshizumi et al. 1999), mainly by applying specialized software. This study used specific distances measured from cross-sectional images, as referred to other literature (House et al. 2008). These parameters were surrogates for visceral and subcutaneous fat and were easily obtained with good reproducibility.
In this study, males showed significantly lower ASF and LSF thicknesses and tended toward lower PSF and higher IFT, RFT, and PMT, although statistically significant differences were not obtained. The general impression that female obesity is predominantly located in subcutaneous fat deposits, whereas male obesity is found more in visceral fat deposits, was to some extent confirmed by our analysis. A weak to moderate positive correlation was observed between the parameters measured on CT and the BMI, suggesting these CT parameters had both similarities and differences with the BMI as body fat surrogates. The practical CT parameters have advantages over the BMI in the quantitative assessment of the visceral and subcutaneous fat in certain body parts.
Adipose tissue is now recognized as an endocrine and paracrine organ that releases cytokine-like polypeptides responsible for widespread biological effects (Fox et al. 2007). In particular, adipocytes produce insulin-like growth factor and multiple angiogenic factors including VEGF and leptin, which exert direct angiogenic effects (Cao 2007). The cytokine production profile differs between subcutaneous and visceral fat (Cao 2007; Miyazawa-Hoshimoto et al. 2005), so different subcutaneous and visceral fat amounts and distributions may result in different disease progress, response to therapies, or prognosis.
Recent research has studied the influence of subcutaneous and visceral fat on cancer patient survival, mainly focusing on pancreatic, colon, prostate, and renal cancer (Gaujoux et al. 2012; Guiu et al. 2010; House et al. 2008; Ladoire et al. 2011; Pischon et al. 2006). However, agreement about subcutaneous and visceral fat’s influence on cancer prognosis has not been reached. The amount of visceral fat or the visceral fat/subcutaneous fat ratio significantly predicted cancer-related survival in several studies (Griggs and Sabel 2008; Guiu et al. 2010), but studies by Gaujoux showed that visceral fat was not associated with overall survival in pancreatic cancer patients (Gaujoux et al. 2012). There were also studies focusing on the relationship between visceral and/or subcutaneous adiposity and postoperative complications in colorectal cancer patients, and they indicated that fat volumetric/area parameters and other BMI were independent predictor for postoperative outcomes (Jung et al. 2014; Cecchini et al. 2011; Sakai et al. 2009). However, the prognostic significance of visceral and subcutaneous fat in patients with gastric cancer has not been previously studied.
This study first suggested that high IFT was associated with poorer OS and DFS in gastric cancer patients. The data strongly supported the notion that visceral fat may induce protumorigenic factor accumulation (Ohki et al. 2009) and therefore may be associated with poorer survival outcomes. The stomach is a intraperitoneal organ, and the invasion and metastasis of gastric cancer may be more associated with the intraperitoneal adipose tissue than extraperitoneal adipose tissue; this is to some extent explains why RFT was not found associated with OS or DFS. Contrarily, OS and DFS were longer in patients with high ASFT or PSFT; this finding is difficult to explain. One possible explanation is that gastric cancer as a debilitating disease leads to a weak body, so chemotherapy and surgery makes the body even feebler. Subcutaneous fat, serving as the body’s normal reservation source, may promote body recovery and maintain a better status. However, LSFT was not associated with survival; this is most likely due to the small sample size; another explanation is that ASFT and PSFT better reflect the subcutaneous fat content than LSFT.
Conversely, the BMI was not independently associated with survival outcomes. Thus, IFT, ASFT, and PSFT may be more accurate than the BMI at predicting survival. Obese patients defined by a BMI >30 kg/m2 presented poor prognoses in previous studies. However, we justly divided patients into overweight (>25 kg/cm2) and standard weight (18–25 kg/cm2) categories since obese patients with gastric cancer are rare. This may be one reason that no statistical correlation between the BMI and survival was detected.
Limitations of our study include the small number of patients, single-center patient recruitment, and retrospective design. However, all patients with gastric cancer in our hospital received abdominal CT examination before undergoing treatment, and all CT images were intact and preserved. Difference in CT measurements was observed between two raters, but consistency analysis showed there was good inter-rater reliability for these CT parameters. A small sample size contributes to overfitting, so bootstrapping was performed to internally validate the results and prevent overfitting. The results obtained by bootstrapping highlighted that high IFT, ASFT, and PSFT remained major independent OS predictors with respective P values of 0.01, 0.04, and 0.05.
In this study, we used the change of gastric wall thickness at tumor before and after neo-adjuvant chemotherapy to define therapeutic efficacy. However, there is none widely accepted imaging criteria for evaluation. We used 30 % as cutoff value according to the RECIST criteria, which might not be suitable as many papers (Tuma et al. 2006) suggested. Thus, we did not present the analyses associating fat parameters with therapeutic efficacy (Supplementary Table 1) in the results of this paper, mainly because the evaluation for therapeutic efficacy was possibly inaccurate. Actually, the corresponding univariate and multivariate logistic regression showed that none of these fat parameters were predictors for therapeutic efficacy.
In conclusion, our study provided the first evidence that IFT, ASFT, and PSFT measured before neo-adjuvant chemotherapy were likely to be a useful predictive biomarker for overall survival in patients with advanced gastric cancer. This study also provided practical parameters for the quantitative measurement of body fat. Further studies are continuing to validate our findings in a different dataset and determine the optimal cutoff points for these fat parameters. Further studies may still help us determine whether high IFT and low ASFT and PSFT are correlated with the poor response to preoperative treatment. If the hypothesis was proven true, patients with high IFT and low ASFT and PSFT might not benefit from neo-adjuvant chemotherapy or require higher dosage. If further validation studies corroborate our results, IFT, ASFT, and PSFT measurements will have to be taken into account as host parameters in managing individualized treatment plans for advanced gastric cancer.
References
Ajani JA et al (2013) Gastric cancer, version 2.2013: featured updates to the NCCN Guidelines. J Natl Compr Cancer Netw JNCCN 11:531–546
Aleman JO, Eusebi LH, Ricciardiello L, Patidar K, Sanyal AJ, Holt PR (2014) Mechanisms of obesity-induced gastrointestinal neoplasia. Gastroenterology 146:357–373. doi:10.1053/j.gastro.2013.11.051
Bazzocchi A, Diano D, Battista G (2012) How fat is fat? Lancet 380:e1. doi:10.1016/S0140-6736(11)61925-9
Bianchini F, Kaaks R, Vainio H (2002) Overweight, obesity, and cancer risk. Lancet Oncol 3:565–574
Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ (2003) Overweight, obesity, and mortality from cancer in a prospectively studied cohort of US adults. N Engl J Med 348:1625–1638. doi:10.1056/NEJMoa021423
Cao Y (2007) Angiogenesis modulates adipogenesis and obesity. J Clin Investig 117:2362–2368. doi:10.1172/JCI32239
Cecchini S, Cavazzini E, Marchesi F, Sarli L, Roncoroni L (2011) Computed tomography volumetric fat parameters versus body mass index for predicting short-term outcomes of colon surgery. World J Surg 35:415–423. doi:10.1007/s00268-010-0888-3
Demark-Wahnefried W et al (2012) The role of obesity in cancer survival and recurrence. Cancer Epidemiol Biomarkers Prev 21:1244–1259. doi:10.1158/1055-9965.EPI-12-0485
Fox CS et al (2007) Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation 116:39–48. doi:10.1161/CIRCULATIONAHA.106.675355
Gaujoux S et al (2012) Impact of obesity and body fat distribution on survival after pancreaticoduodenectomy for pancreatic adenocarcinoma. Ann Surg Oncol 19:2908–2916. doi:10.1245/s10434-012-2301-y
Grabsch HI, Tan P (2013) Gastric cancer pathology and underlying molecular mechanisms. Dig Surg 30:150–158. doi:10.1159/000350876
Griggs JJ, Sabel MS (2008) Obesity and cancer treatment: weighing the evidence. J Clin Oncol 26:4060–4062. doi:10.1200/JCO.2008.17.4250
Guiu B et al (2010) Visceral fat area is an independent predictive biomarker of outcome after first-line bevacizumab-based treatment in metastatic colorectal cancer. Gut 59:341–347. doi:10.1136/gut.2009.188946
House MG et al (2008) Preoperative predictors for complications after pancreaticoduodenectomy: impact of BMI and body fat distribution. J Gastrointest Surg 12:270–278. doi:10.1007/s11605-007-0421-7
Hsing AW et al (2000) Body size and prostate cancer: a population-based case–control study in China. Cancer Epidemiol Biomarkers Prev 9:1335–1341
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D (2011) Global cancer statistics. CA Cancer J Clin 61:69–90. doi:10.3322/caac.20107
Jung M et al (2014) Perirenal fat surface area as a risk factor for morbidity after elective colorectal surgery. Dis Colon Rectum 57:201–209. doi:10.1097/DCR.0000000000000029
Ladoire S et al (2011) Visceral fat area as a new independent predictive factor of survival in patients with metastatic renal cell carcinoma treated with antiangiogenic agents. Oncologist 16:71–81. doi:10.1634/theoncologist.2010-0227
Li D, Morris JS, Liu J, Hassan MM, Day RS, Bondy ML, Abbruzzese JL (2009) Body mass index and risk, age of onset, and survival in patients with pancreatic cancer. JAMA 301:2553–2562. doi:10.1001/jama.2009.886
Maurovich-Horvat P, Massaro J, Fox CS, Moselewski F, O’Donnell CJ, Hoffmann U (2007) Comparison of anthropometric, area- and volume-based assessment of abdominal subcutaneous and visceral adipose tissue volumes using multi-detector computed tomography. Int J Obes 31:500–506. doi:10.1038/sj.ijo.0803454
Medina-Franco H, Contreras-Saldivar A, Ramos-De La Medina A, Palacios-Sanchez P, Cortes-Gonzalez R, Ugarte JA (2004) Surgery for stage IV gastric cancer. Am J Surg 187:543–546. doi:10.1016/j.amjsurg.2003.12.045
Miyazawa-Hoshimoto S, Takahashi K, Bujo H, Hashimoto N, Yagui K, Saito Y (2005) Roles of degree of fat deposition and its localization on VEGF expression in adipocytes. Am J Physiol Endocrinol Metab 288:E1128–E1136. doi:10.1152/ajpendo.00003.2004
Moore LL, Bradlee ML, Singer MR, Splansky GL, Proctor MH, Ellison RC, Kreger BE (2004) BMI and waist circumference as predictors of lifetime colon cancer risk in Framingham Study adults. Int J Obes Relat Metab Disord 28:559–567. doi:10.1038/sj.ijo.0802606
Ohki T et al (2009) Visceral fat accumulation is an independent risk factor for hepatocellular carcinoma recurrence after curative treatment in patients with suspected NASH. Gut 58:839–844. doi:10.1136/gut.2008.164053
Piazuelo MB, Correa P (2013) Gastric cancer: overview. Colomb Med 44:192–201
Pischon T et al (2006) Body size and risk of colon and rectal cancer in the European Prospective Investigation Into Cancer and Nutrition (EPIC). J Natl Cancer Inst 98:920–931. doi:10.1093/jnci/djj246
Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M (2008) Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet 371:569–578. doi:10.1016/S0140-6736(08)60269-X
Sakai T, Maekawa T, Mikami K, Kuramochi H, Noda S (2009) Short-term outcomes of colon surgery are better predicted by fat volumetric parameters than by the BMI. This study has provided new elements for discussion on the impact of visceral and subcutaneous adiposity in laparoscopic and traditional colon surgery. Int Surg 94:370–372. doi:10.1007/s00268-010-0888-3
Smith DD, Schwarz RR, Schwarz RE (2005) Impact of total lymph node count on staging and survival after gastrectomy for gastric cancer: data from a large US-population database. J Clin Oncol 23:7114–7124. doi:10.1200/JCO.2005.14.621
Tokunaga K, Matsuzawa Y, Ishikawa K, Tarui S (1983) A novel technique for the determination of body fat by computed tomography. Int J Obes 7:437–445
Tuma RS et al (2006) Sometimes size doesn’t matter: reevaluating RECIST and tumor response rate endpoints. J Natl Cancer Inst 98:1272–1274
Wagner AD, Grothe W, Haerting J, Kleber G, Grothey A, Fleig WE (2006) Chemotherapy in advanced gastric cancer: a systematic review and meta-analysis based on aggregate data. J Clin Oncol 24:2903–2909. doi:10.1200/JCO.2005.05.0245
Yoshizumi T et al (1999) Abdominal fat: standardized technique for measurement at CT. Radiology 211:283–286. doi:10.1148/radiology.211.1.r99ap15283
Yuan C et al (2013) Prediagnostic body mass index and pancreatic cancer survival. J Clin Oncol 31:4229–4234. doi:10.1200/JCO.2013.51.7532
Acknowledgments
This work was supported by the National Basic Research Program of China (973 Program) (Grant No. 2011CB707705), National Natural Science Foundation of China (Grant Nos. 81471640, 81371715), the Capital Health Research and Development of Special Foundation (Grant No. 2011-2015-02), Beijing Health System High Level Health Technical Personnel Training Plan (Grant No. 2013-3-083), and the Capital Special Project for Featured Clinical Application (Grant No. Z121107001012115).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, XT., Tang, L., Chen, Y. et al. Visceral and subcutaneous fat as new independent predictive factors of survival in locally advanced gastric carcinoma patients treated with neo-adjuvant chemotherapy. J Cancer Res Clin Oncol 141, 1237–1247 (2015). https://doi.org/10.1007/s00432-014-1893-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-014-1893-y