Abstract
During post-occlusive reactive hyperemia (PORH) there is a temporary increase in the total hemoglobin + myoglobin (T[Hb+Mb]) signal as measured by near-infrared spectroscopy (NIRS). This transient increase predicts differences in the kinetic responses of deoxy[Hb+Mb] and oxy[Hb+Mb] during PORH. The purpose of this study was to determine whether sigmoidal (Gompertz or logistic) or exponential functions better describe these response curves during PORH. The fit of the three functions (exponential, Gompertz and logistic) to the NIRS responses, as determined from residual sum of squares, was compared using repeated measures ANOVA on Ranks. The Gompertz function provided a better fit to the oxy[Hb+Mb] response curve than did either the exponential or logistic function (χ 2 = 21.7, df = 2, p < 0.001). The logistic function provided a better fit for the deoxy[Hb+Mb] response (χ 2 = 22.9, df = 2, p < 0.001) than did either the Gompertz or exponential functions. For both NIRS signals, the better fitting sigmoidal functions fit the data well, with an average r value of 0.99 or greater. Adipose tissue thickness was correlated with parameters related to signal strength (amplitude, r = 0.86–0.89; baseline, r = 0.67–0.75; all p < 0.001) but was not related to kinetic parameters (time constant and inflection point; p > 0.05 for all comparisons). These results suggest that during PORH distinct sigmoidal mathematical functions best describe the responses of the oxy[Hb+Mb] (Gompertz) and deoxy[Hb+Mb] (logistic) as measured by NIRS. Further, differences in both the kinetic and amplitude aspects for the responses of oxy[Hb+Mb] and deoxy[Hb+Mb] predict the observed transient change in T[Hb+Mb]. Our methods provide a technique to evaluate and quantify NIRS responses during PORH, which may have clinical utility.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The magnitude of the hyperemic response to brachial artery occlusion, post-occlusive reactive hyperemia (PORH), has been used to evaluate the relative health of the vasculature in both diseased and apparently healthy populations (Rossi et al. 2008; Shechter et al. 2009; Suzuki et al. 2008; Takase et al. 2008). The limitation in these earlier studies is that the downstream hyperemia within skeletal muscle was not assessed; only the relationships between conduit artery and other easily accessible peripheral vascular beds (i.e., skin) were explored. An advantage of using near-infrared spectroscopy (NIRS) to assess PORH responses is the ability to detect changes in microvascular function within skeletal muscle itself, which could have significant clinical implications. For example, much of the insulin resistance found in obesity and type II diabetes mellitus is thought to occur in response to endothelial dysfunction within the capillaries of skeletal muscle (Bakker et al. 2009). The ability to compare information regarding microvascular function obtained from NIRS across patients or within the same patient over time could help inform treatment decisions. What is needed is a systematic process that facilitates the comparison of these microvascular responses as measured by NIRS; this project seeks to lay the groundwork for such a paradigm.
At near-infrared wavelengths the primary chromophores present in the human forearm are hemoglobin, myoglobin and cytochrome aa3 (Jobsis 1977). There is an overlap in the absorbance spectra of hemoglobin and myoglobin in the near-infrared spectra, rendering them almost indistinguishable with traditional commercial NIRS systems (but see Marcinek et al. 2007). Near-infrared spectroscopy (NIRS) allows for the non-invasive assessment of the concentration of oxygenated (oxy[Hb+Mb]) and deoxygenated hemoglobin + myoglobin (deoxy[Hb+Mb]) in skeletal muscle (Hamaoka et al. 2007). Total hemoglobin plus myoglobin (T[Hb + Mb]) is the sum of oxy[Hb+Mb] and deoxy[Hb+Mb]. Changes in the concentrations of these chromophores within skeletal muscle may provide information about microvascular function.
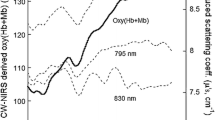
During PORH if oxy[Hb+Mb] and deoxy[Hb+Mb] change with the same amplitude and time course, we would expect T[Hb+Mb] to remain constant. In fact, a transient increase in T[Hb+Mb] is observed during PORH (Kragelj et al. 2001). Visual inspection of these responses following cuff release suggest that both have a sigmoidal shape, but that the oxy[Hb+Mb] signal changes more rapidly than does the deoxy[Hb+Mb] signal. This implies that oxy[Hb+Mb] and deoxy[Hb+Mb] change with different time courses and predicts that the oxy[Hb+Mb] and deoxy[Hb+Mb] response curves would be best fit by two distinct sigmoidal functions during PORH. A logistic function has a constant rate of change which is relatively sluggish early in the response, such as immediately post cuff release. This may make it a good descriptor of the changes in deoxy[Hb+Mb] response, but inappropriate to describe the rapid early changes observed in the oxy[Hb+Mb] response. On looking for an alternative function to characterize the rapid acceleration observed in the oxy[Hb+Mb] signal post cuff release, we chose the Gompertz function due to its earlier inflection point and resultant increased rate of change early in the response. Therefore, the purposes of this study were to: (a) Determine the best descriptor of the oxy[Hb+Mb] and deoxy[Hb+Mb] responses during PORH, and (b) determine which of the characteristics (kinetic vs. signal strength) of the oxy[Hb+Mb] and deoxy[Hb+Mb] responses contributed to the transient change in T[Hb+Mb] during PORH. Specifically, we tested these three hypotheses: (1) a logistic function would best describe the response of deoxy[Hb+Mb] during PORH, (2) a Gompertz function would best describe the response of oxy[Hb+Mb], and (3) the peak amplitude of change in oxy[Hb+Mb] and deoxy[Hb+Mb] would be similar, implying that the observed changes in T[Hb+Mb] would be related only to the distinct kinetics of each response.
Methods
Subjects
Twenty subjects between the ages of 18 and 26 (10 males, average age 21.6 years) were recruited from the general student body at Kansas State University. Informed consent was obtained after both written and verbal explanation about the possible risks and discomforts of the experimental protocol were given. The study was approved by the Institutional Review Board for Research Involving Human Subjects at Kansas State University. These subjects were apparently healthy and sedentary. Average adipose tissue thickness (ATT), measured with pulsed Doppler ultrasound, under the NIRS probe was 4.62 ± 1.45 mm. Average Body Mass Index was 23.6 ± 3.7 kg/m2, mean resting heart rate was 66 ± 8 beats/min, mean systolic blood pressure was 119 ± 9 mmHg and mean diastolic blood pressure was 72 ± 7 mmHg.
Protocol
All tests were conducted between 0700 and 0830 hours, and were performed in a quiet, temperature-controlled room. Participants were instructed to refrain from alcohol and exercise for 24 h and caffeine for 12 h and were fasted. Subjects were supine and the sampled arm was abducted to a 45° angle. The forearm was supinated to expose the sampling site. Skeletal muscle oxygenation was monitored continuously using an OxiplexTS, Model 96208 (ISS, Champaign, IL, USA) system with the probe placed over the brachialis, flexor digitorum superficialis and flexor carpi radialis by a trained anatomist. This placed the distal edge of the NIRS probe housing approximately halfway between the wrist and the anticubital fold, with the lateral edge of the probe approximately along the midline of the forearm. Characterization of the OxiplexTS probe has been previously described in detail (Ferreira et al. 2005). Briefly, the system operates at wavelengths of 692 and 834 nm with a modulation frequency of the light-source intensity of 110 MHz. The probe utilized source-detector separations of 2.0, 2.5, 3.0 and 3.5 cm. The probe was fixed in place with an elastic strap tightened to prevent movement. The entire forearm was covered with an opaque cloth to prevent ambient light from influencing the NIRS results. For each trial, NIRS measurements were collected continuously during 1 min of rest, 5 min of brachial artery occlusion and 150 s of reactive hyperemia. Total collection time per trial was 8.5 min. The protocol was repeated a total of three times with a 10 min rest period between trials. Occlusion of the brachial artery was accomplished by rapid (within 2 s) inflation, by hand, of a blood pressure cuff to >250 mmHg. The cuff was placed just proximal to the elbow. Complete occlusion of the brachial artery was assumed if the T[Hb+Mb] signal did not increase following cuff inflation. All NIRS data were time aligned to cuff release (t = 0) and averaged across trials.
Statistical analysis
All data were imported into SigmaPlot 10 with SigmaStat 3.5 (Systat Software, Inc., SanJose, CA, USA). Logistic (Eq. 1), Gompertz (Eq. 2) and exponential (eq. 3) functions were fit to both the oxy[Hb+Mb] and deoxy[Hb+Mb] responses producing six response curves per subject.
For each equation, “Y 0” represents the baseline (end-cuff occlusion) value, “a” represents the signal amplitude, “X” is time, “b” represents a time constant, and “X 0” represents the time at the inflection point of each curve (Eqs. 1, 2) or time delay (Eq. 3) (Fig. 1). Y 0 was determined from the last 20 s of the 5-minute cuff occlusion. Goodness of fit for each function was determined by examining residual sum of square (RSS) values. We had originally planned to use the ΔAkaike method to compare our response curves (since the three functions are not nested models), but since all three models had the same number (four) of parameters, the equation reduced to RSS times a similar constant across models and subjects. Repeated measures ANOVA on ranks was thus used to compare the RSS values from each function within each response.
Schematic showing Gompertz (a) and exponential (b) functions fit to the oxy[Hb+Mb] data in Fig. 2. Y 0 denotes baseline, a is the amplitude of the response, b is the time constant, and X 0 is the time of the inflection point (a) or time delay (b). Parameters have same meaning for logistic function as for Gompertz. See text and Eqs. 1–3 for more information
Parameter estimates from the best fitting function for each response were plotted against ATT for each subject. Pearson product moment correlation was then performed between ATT and the uncorrected parameter estimates. The slope of this line was used to correct each parameter estimate affected by ATT (amplitude a, and baseline Y 0). Due to unequal variance, for corrected and uncorrected amplitude (a) values a Kruskal–Wallis One Way Analysis of Variance on Ranks analysis was performed. One way ANOVA was performed on the corrected and uncorrected baseline (Y 0) values. Tukey’s post-hoc analysis was used to determine group differences for both variables. Kinetic parameters (time constant b and inflection point X 0) were not influenced by ATT and, therefore, correction for ATT was not necessary. These parameters were compared with paired t tests.
Results
Figure 2 shows the T[Hb+Mb], oxy[Hb+Mb] and deoxy[Hb+Mb] responses across the entire experiment for one representative subject through baseline data collection, cuff occlusion and PORH following cuff release. Upon cuff release, while the time course of changes in oxy[Hb+Mb] and deoxy[Hb+Mb] appeared similar, they were not identical as demonstrated in Fig. 3. Oxy[Hb+Mb] increased as soon as the cuff was released. However, the deoxy[Hb+Mb] response had a slower rate of change for approximately the first 20 s before the dynamic hyperemic response occurred. The result of these disparate kinetics was that T[Hb+Mb] shows a transient increase following cuff release (Fig. 1).
Oxy[Hb+Mb] and deoxy[Hb+Mb] response curves during PORH from the same subject as in Fig. 1. Deoxy[Hb+Mb] response has been inverted for comparison. Time point 0 indicates cuff release
Figure 4 shows combined deoxy[Hb+Mb] and oxy[Hb+Mb] response curves and residuals for the same subject as in Figs. 2 and 3 fit with Gompertz, logistic and exponential functions. Tables 1 and 2 show the RSS data for all subjects for the three functions fit to the deoxy[Hb+Mb] and oxy[Hb+Mb] responses, respectively. For both responses a sigmoidal curve provided the better fit on average compared to the exponential function (deoxy[Hb+Mb]: χ 2 = 22.9, df = 2, p < 0.001; oxy[Hb+Mb]: χ 2 = 21.7, df = 2, p < 0.001). Post-hoc analysis revealed that the logistic function provided the best fit of the three functions tested for the deoxy[Hb+Mb] response; there was no significant difference between the fit of the exponential and the Gompertz functions. Conversely, the Gompertz function provided the best fit for the oxy[Hb+Mb] response when compared with both the exponential and logistic functions; there was no significant difference between the RSS values for the logistic and exponential functions. Since the exponential function was not the best fit on average for either response, it was excluded from further analyses.
Amplitude (a) and baseline (Y 0), but not time constant (b) and time of inflection point (X 0) (data not shown) were significantly correlated with ATT for all response curves (Fig. 5). Table 3 gives the average parameter estimates, uncorrected- and corrected-for-ATT where appropriate, for the best fit model (logistic for the deoxy[Hb+Mb] response and Gompertz for the oxy[Hb+Mb] response) across all subjects. Correcting for ATT resulted in a significant increase in the baseline (Y 0) and amplitude (a) values for both the oxy[Hb+Mb] and deoxy[Hb+Mb] responses (F = 367.82, df = 3, p < 0.001). For the Gompertz function fit to the oxy[Hb+Mb] response, the time constant (b) was significantly greater, and the inflection point (X 0) was significantly smaller (both p < 0.001) compared to the corresponding value for the logistic function fit to the deoxy[Hb+Mb] (Table 3).
The relationship between the amplitude for deoxy[Hb+Mb] and oxy[Hb+Mb], both for the uncorrected and corrected-for-ATT values, for all subjects is further illustrated in Fig. 6. Note that while there was a strong relationship between the amplitudes of the two responses across subjects, most of the amplitudes for oxy[Hb+Mb] fell below the line of identity (i.e., were greater than the corresponding amplitude of deoxy[Hb+Mb]), for both uncorrected and corrected data. Figure 7 shows the relationships for the best fit model (oxy[Hb+Mb] fit with the Gompertz function and deoxy[Hb+Mb] fit with the logistic function) for both kinetic parameters (b and X 0) across all subjects. While both b and X 0 were consistently above the line of identity (i.e., deoxy[Hb+Mb] values smaller than the corresponding oxy[Hb+Mb] for b, but greater for X 0), nonetheless there were strong correlations across the range of values (i.e., subjects with faster responses (smaller b and X 0) in one response also showed faster responses in the other).
Discussion
The main findings of this investigation are that, consistent with hypotheses 1 and 2, a Gompertz function provided a better fit, on average, for the oxy[Hb+Mb], while a logistic function fit the deoxy[Hb+Mb] responses best, during PORH. Further, in contrast to hypothesis 3, while the amplitudes of change during PORH for both oxy- and deoxy[Hb+Mb] were similar, they were not identical. This difference in amplitudes, combined with the difference in kinetics of adjustment between the Gompertz and logistic functions, would predictably lead to a transient change in the T[Hb+Mb] signal, as was seen in the present study.
Exponential functions have typically been used to describe NIRS responses during dynamic exercise (DeLorey et al. 2004; Grassi et al. 2003; Koga et al. 2007), following maximal 3 s contractions in the human forearm and during 120 s of 1 Hz twitch contractions at various intensities in the rat hindlimb preparation (Maguire et al. 2007; Takakura et al. 2010; Masuda et al. 2010). In contrast, we found that the sigmoidal functions provided a better fit to NIRS data during PORH than the exponential function in 85% (34 of 40) of the responses modeled here (17 of 20 responses each for oxy[Hb+Mb] and deoxy[Hb+Mb]). Interestingly, two of the three exceptions occurred in the same subjects (subjects 5 and 13 in Tables 1, 2) (i.e., both oxy[Hb+Mb] and deoxy[Hb+Mb] responses were initially best described as exponential in these two subjects). However, when these exponential fits were examined more closely, it was noted that the amplitudes of the best fit function greatly exceeded the actual amplitude of the response (e.g., see Fig. 1). When the amplitude of the exponential function was constrained to the real amplitude of the responses, the resulting RSS was greater than that of the corresponding best fit sigmoidal function. We, thus, conclude that our overall observations that the logistic function best fit the deoxy[Hb+Mb] data, while the oxy[Hb+Mb] data were best described by the Gompertz function, was also true for these subjects (#5, 13, 14 and 16). These results demonstrate that modeling techniques that are appropriate for data obtained during or in recovery from exercise may not provide an appropriate description of the NIRS data obtained following 5 min of ischemia during PORH. Taken together, these findings suggest that care must be taken when selecting a function for mathematical modeling of NIRS response data.
To our knowledge, only Kragelj et al. (Kragelj et al. 2001) have previously examined tissue oxygenation during PORH using NIRS. Their model was tissue oxygenation of the foot during and after cuff occlusion of the popliteal artery. While their data analysis was model-independent, their overall observations were similar to those of the present study, i.e., that the kinetics of the oxy[Hb+Mb] response were faster than those of deoxy[Hb+Mb] during PORH.
Amplitude and baseline values which are related to signal strength, were significantly correlated with ATT, while the kinetic parameters, slope and inflection point, were not. These results suggest that ATT does not affect the kinetic characteristics of the response, only the signal strength, and that correction for ATT is not required when performing a purely temporal analysis.
The NIRS signal primarily emanates from skeletal muscle [Mb] and the microvasculature [Hb] (Liu et al. 1995). Assuming all of the transient increase in T[Hb+Mb] is due to increased [Hb] (with skeletal muscle [Mb] remaining constant) the increased T[Hb+Mb] signal likely represents increased microvascular hematocrit (Boushel et al. 2001; Kindig et al. 2002; Klitzman and Duling 1979). However, the underlying reasons for the temporal differences observed in the oxy- and deoxy[Hb+Mb] responses during PORH are not known. One potential mechanism for the delayed response of deoxy[Hb+Mb] compared to oxy[Hb+Mb] during PORH may involve additional unloading of oxygen during the early recovery phase of PORH to refill tissue Mb stores utilized during the ischemic period. Tran et al. (1999) demonstrated that during cuff ischemia of the lower leg, Mb desaturation by NMR reached a maximum value by ~4.5 min. Given that Mb content of human skeletal muscle is greater than previously thought (Marcinek et al. 2007; Davis 2009), the dynamics of Mb de/resaturation may contribute significantly to the overall kinetics of the NIRS responses during PORH. Consistent with this, the observed time to the inflection point (X 0) for deoxy[Hb+Mb] in the present study (16.9 s) is similar to the time constants for Mb resaturation reported by Duteil et al. (2004).
It should be noted that one limitation of the present analysis is that only 3 models (exponential, logistic and Gompertz) were compared. There could be another, unidentified model which may provide an even better fit to the response curve data. In addition, our subjects were a relatively young, healthy cohort. It is currently unclear to what extent our findings and approach to data analysis may be applicable to clinical populations. We expect any pathological condition affecting vascular function, such as diabetes, heart failure and peripheral vascular disease, would alter the kinetics and possibly amplitude of the observed deoxy[Hb+Mb] and oxy[Hb+Mb] responses, which in turn might alter the T[Hb+Mb] response. In fact, Kragelj et al. (2001) observed slowing of the NIRS responses in patients with peripheral vascular disease. Many of the signs of microvascular dysfunction predate a clinical diagnosis and may, therefore, be useful as novel markers of disease risk, disease progression and treatment efficacy (Bakker et al. 2009). Evaluation of the impact of diseases which affect microvascular circulation must await future studies.
Conclusion
Our findings support our initial hypotheses that during PORH, sigmoidal functions do fit the NIRS data better than an exponential function and specifically that the oxy[Hb+Mb] response is best described by a Gompertz function, while the logistic function provides a better fit for the deoxy[Hb+Mb] response. The distinct time courses of these responses in combination with their different response amplitudes, leads to a transient increase in T[Hb+Mb]. While the exact mechanism for the delayed response of deoxy[Hb+Mb] relative to that of oxy[Hb+Mb] is currently unknown, we speculate that it likely relates to sustained unloading of O2 from Hb during the early phase of PORH to restore tissue MbO2 stores.
References
Bakker W, Eringa EC, Sipkema P, van Hinsbergh VW (2009) Endothelial dysfunction and diabetes: roles of hyperglycemia, impaired insulin signaling and obesity. Cell Tissue Res 335:165–189
Boushel R, Langberg H, Olesen J, Gonzales-Alonzo J, Bulow J, Kjaer M (2001) Monitoring tissue oxygen availability with near infrared spectroscopy (NIRS) in health and disease. Scand J Med Sci Sports 11:213–222
Davis M (2009) Estimated contribution of hemoglobin and myoglobin to near infrared spectroscopy. M.S. Thesis, Department of Kinesiology. Kansas State University, Manhattan, KS
DeLorey DS, Kowalchuk JM, Paterson DH (2004) Effect of age on O(2) uptake kinetics and the adaptation of muscle deoxygenation at the onset of moderate-intensity cycling exercise. J Appl Physiol 97:165–172
Duteil S, Bourrilhon C, Raynaud JS, Wary C, Richardson RS, Leroy-Willig A, Jouanin JC, Guezennec CY, Carlier PG (2004) Metabolic and vascular support for the role of myoglobin in humans: a multiparametric NMR study. Am J Physiol Regul Integr Comp Physiol 287:R1441–R1449
Ferreira LF, Townsend DK, Lutjemeier BJ, Barstow TJ (2005) Muscle capillary blood flow kinetics estimated from pulmonary O2 uptake and near-infrared spectroscopy. J Appl Physiol 98:1820–1828
Grassi B, Pogliaghi S, Rampichini S, Quaresima V, Ferrari M, Marconi C, Cerretelli P (2003) Muscle oxygenation and pulmonary gas exchange kinetics during cycling exercise on-transitions in humans. J Appl Physiol 95:149–158
Hamaoka T, McCully KK, Quaresima V, Yamamoto K, Chance B (2007) Near-infrared spectroscopy/imaging for monitoring muscle oxygenation and oxidative metabolism in healthy and diseased humans. J Biomed Opt 12(6):062105
Jobsis FF (1977) Noninvasive, infrared monitoring of cerebral and myocardial oxygen sufficiency and circulatory parameters. Science 198:1264–1267
Kindig CA, Richardson TE, Poole DC (2002) Skeletal muscle capillary hemodynamics from rest to contractions: implications for oxygen transfer. J Appl Physiol 92:2513–2520
Klitzman B, Duling BR (1979) Microvascular hematocrit and red cell flow in resting and contracting striated muscle. Am J Physiol 237:H481–H490
Koga S, Poole DC, Ferreira LF, Whipp BJ, Kondo N, Saitoh T, Ohmae E, Barstow TJ (2007) Spatial heterogeneity of quadriceps muscle deoxygenation kinetics during cycle exercise. J Appl Physiol 103:2049–2056
Kragelj R, Jarm T, Erjavec T, Presern-Strukelj M, Miklavcic D (2001) Parameters of postocclusive reactive hyperemia measured by near infrared spectroscopy in patients with peripheral vascular disease and in healthy volunteers. Ann Biomed Eng 29:311–320
Liu H, Boas DA, Zhang Y, Yodh AG, Chance B (1995) Determination of optical properties and blood oxygenation in tissue using continuous NIR light. Phys Med Biol 40:1983–1993
Maguire MA, Weaver TW, Damon BM (2007) Delayed blood reoxygenation following maximum voluntary contraction. Med Sci Sports Exerc 39:257–267
Marcinek DJ, Amara CE, Matz K, Conley KE, Schenkman KA (2007) Wavelength shift analysis: a simple method to determine the contribution of hemoglobin and myoglobin to in vivo optical spectra. Appl Spectrosc 61:665–669
Masuda K, Takakura H, Furuichi Y, Iwase S, Jue T (2010) NIRS measurement of O(2) dynamics in contracting blood and buffer perfused hindlimb muscle. Adv Exp Med Biol 662:323–328
Rossi R, Nuzzo A, Origliani G, Modena MG (2008) Prognostic role of flow-mediated dilation and cardiac risk factors in post-menopausal women. J Am Coll Cardiol 51:997–1002
Shechter M, Issachar A, Marai I, Koren-Morag N, Freinark D, Shahar Y, Shechter A, Feinberg MS (2009) Long-term association of brachial artery flow-mediated vasodilation and cardiovascular events in middle-aged subjects with no apparent heart disease. Int J Cardiol 134:52–58
Suzuki T, Hirata K, Elkind MS, Jin Z, Rundek T, Miyake Y, Boden-Albala B, Di Tullio MR, Sacco R, Homma S (2008) Metabolic syndrome, endothelial dysfunction, and risk of cardiovascular events: the Northern Manhattan Study (NOMAS). Am Heart J 156:405–410
Takakura H, Masuda K, Hashimoto T, Iwase S, Jue T (2010) Quantification of myoglobin deoxygenation and intracellular partial pressure of O2 during muscle contraction during haemoglobin-free medium perfusion. Exp Physiol 95:630–640
Takase B, Matsushima Y, Uehata A, Ishihara M, Kurita A (2008) Endothelial dysfunction, carotid artery plaque burden, and conventional exercise-induced myocardial ischemia as predictors of coronary artery disease prognosis. Cardiovasc Ultrasound 6:61. doi:10.1186/1476-7120-6-61
Tran T-K, Sailasuta N, Kreutzer U, Hurd R, Chung Y, Mole’ P, Kuno S, Jue T (1999) Comparative analysis of NMR and NIRS measurements of intracellular PO 2 in human skeletal muscle. Am J Physiol Regul Integr Comp Physiol 276:R1682–R1690
Acknowledgments
The authors thank ISS, Inc. for the use of the OxiplexTS system for the duration of this investigation. The assistance of Carl Ade was greatly appreciated. This study was funded in part by a University Small Research Grant, Office of Sponsored Research, Kansas State University, awarded to TJB.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Susan A. Ward.
Rights and permissions
About this article
Cite this article
Bopp, C.M., Townsend, D.K. & Barstow, T.J. Characterizing near-infrared spectroscopy responses to forearm post-occlusive reactive hyperemia in healthy subjects. Eur J Appl Physiol 111, 2753–2761 (2011). https://doi.org/10.1007/s00421-011-1898-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-011-1898-z