Abstract
Purpose
This study investigated the physical fitness and oxygen uptake kinetics (\(\tau \dot{V}{\text{O}}_{2}\)) along with the exercise-onset O2 delivery (heart rate kinetics, τHR; changes in normalized deoxyhemoglobin/\(\dot{V}{\text{O}}_{2}\) ratio, Δ[HHb]/\(\dot{V}{\text{O}}_{2}\)) adaptations of individuals with different physical activity (PA) backgrounds responding to 4 weeks of high-intensity interval training (HIIT), and the possible effects of skeletal muscle mass (SMM) on training-induced adaptations.
Methods
Twenty subjects (10 high-PA level, HIIT-H; 10 moderate-PA level, HIIT-M) engaged in 4 weeks of treadmill HIIT. Ramp-incremental (RI) test and step-transitions to moderate-intensity exercise were performed. Cardiorespiratory fitness, body composition, muscle oxygenation status, VO2 and HR kinetics were assessed at baseline and post-training.
Results
HIIT improved fitness status for HIIT-H (\(\dot{V}{\text{O}}_{2\max }\), + 0.26 ± 0.07 L/min; SMM, + 0.66 ± 0.70 kg; body fat, − 1.52 ± 1.93 kg; \(\tau \dot{V}{\text{O}}_{2}\), − 7.11 ± 1.05 s, p < 0.05) and HIIT-M (\(\dot{V}{\text{O}}_{2\max }\), 0.24 ± 0.07 L/min, SMM, + 0.58 ± 0.61 kg; body fat, − 1.64 ± 1.37 kg; \(\tau \dot{V}{\text{O}}_{2}\), − 5.48 ± 1.05 s, p < 0.05) except for visceral fat area (p = 0.293) without between-group differences (p > 0.05). Oxygenated and deoxygenated hemoglobin amplitude during the RI test increased for both groups (p < 0.05) except for total hemoglobin (p = 0.179). The Δ[HHb]/\(\dot{V}{\text{O}}_{2}\) overshoot was attenuated for both groups (p < 0.05) but only eliminated in HIIT-H (1.05 ± 0.14 to 0.92 ± 0.11), and no change was observed in τHR (p = 0.144). Linear mixed-effect models presented positive effects of SMM on absolute \(\dot{V}{\text{O}}_{2\max }\) (p < 0.001) and ΔHHb (p = 0.034).
Conclusion
Four weeks of HIIT promoted positive adaptations in physical fitness and \(\dot{V}{\text{O}}_{2}\) kinetics, with the peripheral adaptations attributing to the observed improvements. The training effects are similar between groups suggesting that HIIT is effective for reaching higher physical fitness levels.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Exercise training promotes metabolic health and functional capacity through the skeletal muscle as an endocrine organ, regulating its own growth and regeneration for adaptation to exercise training (Hawley et al. 2014). As such, regular exercise training provides health benefits for different population groups. Healthy adults are encouraged to reach or exceed the recommended weekly volume of aerobic exercise (i.e., walking, running, swimming, and bicycling), which is referred to as aerobic physical activity (PA) in the current guideline, alongside with muscle-strengthening exercise to acquire maximal beneficial effects (Bull et al. 2020; Lee et al. 2022). Unfortunately, over a quarter of adults do not reach the recommended levels of aerobic PA globally (Guthold et al. 2018). Considering the lack of time is the critical barrier to consistent exercise adherence, a time-efficient strategy such as interval training is ideal for enhancing physical fitness levels and promoting public health.
High-intensity interval training (HIIT) is a training protocol involving short bursts of high-intensity exercise intercepted with periods of recovery or rest (MacInnis and Gibala 2017). This modality improves cardiorespiratory fitness (presenting as \(\dot{V}{\text{O}}_{2\max }\)), body fat reduction, and muscle metabolism (Rosenblat et al. 2022; Helgerud et al. 2007; Keating et al. 2017; Maillard et al. 2018; Milanović et al. 2015). Furthermore, HIIT possesses inherent similarities to resistance training (RT) and can be considered as an alternative modality to promote muscle growth (Callahan et al. 2021b). Skeletal muscle plays a modulating role in training-induced adaptations. The peripheral factors influencing \(\dot{V}{\text{O}}_{2\max }\) include muscular ability, such as the microvascular O2 distribution, utilization, and mitochondrial respiratory capacity (Rosenblat et al. 2022), which leads to the vital link between skeletal muscle mass (SMM) and \(\dot{V}{\text{O}}_{2\max }\) across age, sex, and BMI spectra since total body fat does not influence maximal aerobic capacity (Goran et al. 2000; Köhler et al. 2018; Krachler et al. 2015; Wittekind et al. 2020).
Another crucial component reflecting the regulation of cardiovascular adjustments and metabolic control of oxidative phosphorylation is the dynamic responses of \(\dot{V}{\text{O}}_{2}\) during exercise, referring to oxygen uptake kinetics (Murias et al. 2014; Hughson et al. 2001; Jones and Poole 2013). Individuals with training background have faster \(\dot{V}{\text{O}}_{2}\) kinetics compared to their untrained peers (George et al. 2018), and the speeding of oxygen uptake kinetics after training interventions has been reported in different population groups (Gildea et al. 2021; George et al. 2018; Hamasaki et al. 2018; Koschate et al. 2019; McCrudden et al. 2017; Murias et al. 2010; Berger et al. 2006). To directly observe the physiological adaptations associated with training, the biopsy was needed to analyze target markers within the muscle tissue (Granata et al. 2016; Callahan et al. 2021a). However, this invasive test is high-cost, laboratory-restricted, and technically demanding. Near-infrared spectroscopy (NIRS) can be a non-invasive substitute for assessing alterations in skeletal muscle oxidative capacity (Adami and Rossiter 2018; Lagerwaard et al. 2020; Grassi and Quaresima 2016). At the onset of exercise transition, indirect estimations of microvascular blood flow distribution using NIRS measurements of deoxygenated hemoglobin ([HHb]) combined with pulmonary O2 measurements (the normalized Δ[HHb]/\(\dot{V}{\text{O}}_{2}\) ratio) showed a more significant mismatch in local O2 delivery to utilization in untrained individuals compared with trained, observing as a transient “overshoot” phenomenon (Murias et al. 2012).
To date, although studies have proven that interval training promotes physical fitness status along with faster \(\dot{V}{\text{O}}_{2}\) kinetics and better matching of local O2 distribution and utilization at the onset of exercise in both trained and untrained individuals (Murias et al. 2010; Berger et al. 2006; Koschate et al. 2019; McCrudden et al. 2017), no study has directly compared the adaptations in adults with different physical activity backgrounds after HIIT training. There is no doubt that inactive/untrained individuals can benefit from HIIT, but to what extent for the non-endurance trained active individuals to gain from HIIT, and is it possible for this group to achieve a similar improvements in aerobic capacity to the inactive/untrained individuals remains unclear, considering the lesser impact of non-aerobic training (e.g., resistance training) on CRF compared to the aerobic exercise (Robinson et al. 2017). Since the skeletal muscle is directly linked to aerobic capacity, the coincident changes in \(\dot{V}{\text{O}}_{2\max }\) and muscular adaptations are observed consistently following short-term HIIT intervention (Granata et al. 2018; Callahan et al. 2021b; Robinson et al. 2017; van der Zwaard et al. 2016), but the potential effect of SMM levels on training-induced physiological changes has not been examined directly.
Accordingly, this study aimed to (1) examine and compare the adaptations in the cardiorespiratory fitness (CRF), body composition plus the dynamic responses of \(\dot{V}{\text{O}}_{2}\) during the moderate-intensity transition after 4 weeks of HIIT in individuals with different PA backgrounds and (2) explore the possible effects of SMM on training-induced adaptations of aerobic capacity. We hypothesized that HIIT would improve \(\dot{V}{\text{O}}_{2\max }\) and SMM, speed the \(\dot{V}{\text{O}}_{2}\) kinetics and reduce Δ[HHb]/\(\dot{V}{\text{O}}_{2}\) ratio (i.e., reflecting a better matching of O2 delivery to O2 utilization) for both group, and the lower PA background individuals, extending to lower fitness status at baseline, presenting more significant changes in all the variables (McKay et al. 2009; Raleigh et al. 2018; Astorino et al. 2017).
Method
Participants
Initially, 26 young, healthy, non-smoking students (age 18–30) were recruited from campus to assess eligibility. The exclusion criteria were clinically diagnosed cardiovascular, musculoskeletal diseases, or other clinical conditions that do not permit performing high-intensity exercise. After completing the physical activity readiness questionnaire (PAR-Q +), two participants were excluded due to musculoskeletal disorders. The remaining 24 participants provided informed consent before the baseline measurements and were divided into high-PA and moderate-PA groups depending on their International Physical Activity Questionnaire—Short form (IPAQ-S) results (The IPAQ group 2005; Lee et al. 2011) and the standardized scoring protocol provided by the IPAQ Research Committee (http://www.ipaq.ki.se). Participants in the high-PA group were recreational RT-trainers with no specialized or interval training experience. None of the participants in the moderate-PA group performed structured exercises (Table 1). This study conformed to the standards set by the latest revision of the Declaration of Helsinki and was approved by the Ethics Committee for Human Experiments at Beijing Sport University (No. 2022147H).
Measurement
Anthropometric measurements
Height was measured to the nearest 0.5 cm using equipment (GMCS-IV model, China). Body composition was assessed for skeletal muscle mass, body fat mass (BFM), percent body fat (BF%), and visceral fat area (VFA) using bioelectrical impedance analysis (Inbody720, South Korea). Details of this technology were described elsewhere (Nagai et al. 2010; Ling et al. 2011). All measurements were taken with participants in the fast overnight statement to avoid bias.
Ramp-incremental test
To determine their maximal oxygen consumption (\(\dot{V}{\text{O}}_{{{\text{2max}}}}\)) and gas-exchange estimated lactate threshold (GET), participants performed a ramp-incremental (RI) test to maximal voluntary exertion using an ergometer (100P; Cosmed, Rome, Italy) and wearing a facemask connecting to the gas mixed chamber. The polar belt was used to monitor heart rate, and Rating of Perceived Fatigue (RPE) was recorded every minute for all participants using the Borg 6–20 scale. Gas exchange and ventilatory variables were measured using a metabolic cart (CPET; Cosmed, Rome, Italy). Before each test, a trained staff calibrated the system following a standardized procedure. Participants were required to avoid vigorous PA, caffeinated drinks, and alcohol for at least 24 h and food intake for two h before testing and maintain a steady cadence of 50 rpm during the testing process. The test began with a 3-min warm-up at 0 W followed by a 30-W/min ramp for all participants until the following exhaustion criteria were reached: (1) RERmax > 1.13; (2) Maximal heart rate > (210-age)*96%; (3) RPE > 17; 4) Cadence dropped > 10 rpm below targeted value for > 10 s. (Wagner et al. 2020; Howley et al. 1995). Time to exhaustion (TTE) was recorded for the incremental test, and peak power output (PPO) was identified as that work rate coincident with volitional exhaustion.
Moderate-intensity transition
The second visit was separated 48 h from the RI test. Participants performed 4–6 step-transitions from 20 W for 6 min to 80–90% gas-exchange threshold (GET) workload acquired from the RI test for 6 min and another 6 min at 20 W (Murias et al. 2011a). The cadence requirement was similar to the previous incremental test.
NIRS-derived signal
Local muscle oxygenation profiles of the vastus lateralis muscle were measured with near-infrared spectroscopy (PortaLite MKIII, Artinis Medical Systems, The Netherlands) during the RI tests and moderate-intensity step-transitions. This device (Distance between receiver and transmitters: 30, 35, 40 mm; Wavelengths: 760 and 850 nm, others possible) consists of 1 channel to measure muscle O2 saturation percentage (SmO2%) and three channels to measure relative concentrations of oxygenated hemoglobin ([O2Hb]), deoxygenated hemoglobin ([HHb]) and total hemoglobin ([tHb] = [O2Hb] + [HHb]). Optodes were placed on the belly of the target muscle midway between the lateral epicondyle and inguinal crease of the right leg, with a 10-Hz sampling frequency per second by proprietary NIRS recording and analysis software (Oxysoft, Artinis Medical Systems, The Netherlands). A light-proof elastic strap was applied to secure the optodes in places and minimize the intrusion of external light. Skin and adipose tissue thickness was measured using a skin fold caliper at the probe placement. Before each test, a research staff calibrated the NIRS system according to the manufacturer’s instructions.
Training protocol
The training protocol adopted the 4 × 4 min interval treadmill running. Briefly, the protocol contained 4-min of running at 90–95% HRmax (acquired from RI test) intercepted with 3-min of active resting at 65–70% HRmax (Helgerud et al. 2007). A 5-min warm-up before and a 10-min resting phase after the training in the forms of dynamic stretching/light walking were applied for each session, and the RPE was recorded at the end of the training sessions. All participants were instructed to maintain their daily PA level and diet regime throughout the period. The training was three times per week, lasting 4 weeks.
Data analysis
The gas exchange profiles of RI tests were inspected to determine GET, which corresponded to the transition in the \(\dot{V}{\text{CO}}_{{2}}\)–\(\dot{V}{\text{O}}_{{2}}\) relationship slope (Keir et al. 2022). \(\dot{V}{\text{O}}_{{{\text{2max}}}}\) was defined as the highest 30 s average \(\dot{V}{\text{O}}_{{2}}\) at the end of the test.
Breath-by-breath \(\dot{V}{\text{O}}_{{2}}\) data from step-transitions tests were time-aligned and processed as follows: (1) the beginning of the transition corresponding to 0 s; (2) aberrant data laying ± 3SD from the local mean (i.e., 3–4 s) were removed; (3) all breaths from each trial were combined into a single data set, sorted by time and linearly interpolated on a second-by-second basis; (4) each profile was time-average into 5-s bins (Lamarra et al. 1987; Keir et al. 2014). The processed data were fitted with a mono-exponential model as follows:
In the presented model, Y(t) represents the \(\dot{V}{\text{O}}_{{2}}\) at any time (t) during the process; YBsl represents the steady-state baseline values of \(\dot{V}{\text{O}}_{{2}}\) before the moderate-intensity transition; Amp is the amplitude of the increase in \(\dot{V}{\text{O}}_{{2}}\) above the baseline value; τ is the time constant defined as the duration of time for \(\dot{V}{\text{O}}_{{2}}\) to increase to 63% of the steady-state level; TD represents the time delay of the response. To avoid the influence of the phase I component, the initial 20 s data were excluded from the fitting window. Data were modeled from the beginning of phase II (i.e., cardiodynamic phase) up to 240 s of the transition (Murias et al. 2011a). The model was estimated by least-squares nonlinear regression (Origin 2022, OriginLab, MA), and the best fit was defined by minimization of the residual sum of squares and minimal variation around the Y-axis (Y = 0). The 95% confidence interval for the parameter τ was determined after the preliminary fit of the data with YBsl, Amp, and TD constrained to the best-fit, and the τ was allowed to vary.
The NIRS-derived signal from RI tests was determined as follows: baseline equals 45-sec average starting at minute 2; peak equals an average of the highest/lowest 30-sec at the end of RI test. The process strategy for Δ[HHb] profile from the step-transitions was the same as \(\dot{V}{\text{O}}_{{2}}\) data. Considering the uncertainty of the optical path length at rest and during exercise, NIRS data from the incremental test are presented as delta (Δ) μmol/L (μM). The Δ[HHb] consists of a time delay (TD-Δ[HHb]) at the onset of exercise, followed by an increase in the signal with an exponential-like time course similar to \(\dot{V}{\text{O}}_{{{\text{2p}}}}\) (DeLorey et al. 2003). For each trial, the TD-Δ[HHb] was acquired from second-by-second data and corresponded to the time between the onset of exercise and the point of Δ[HHb] signal systematically increasing. Then, the Δ[HHb] data were modeled from the end of the TD-Δ[HHb] to 180 s of the transition with the same equation as phase II \(\dot{V}{\text{O}}_{{{\text{2p}}}}\). The τ[HHb] described the increase of Δ[HHb] and the overall time course of Δ[HHb] from the onset of transition was described by the τ′Δ[HHb] (τ′Δ[HHb] = TD-Δ[HHb] + τΔ [HHb]).
The original data sets were examined to determine the \(\dot{V}{\text{O}}_{{2}}\) and Δ[HHb] response. The second-by-second Δ[HHb] and \(\dot{V}{\text{O}}_{{2}}\) data were normalized for each trial (0% corresponding to the 20 W baseline; 100% corresponding to the steady-state response). Considering the \(\dot{V}{\text{O}}_{{2}}\) data start with the circulatory transit time delay caused by lung perfusion (i.e., phase I), the normalized \(\dot{V}{\text{O}}_{{2}}\) data were left-shifted by 20 s to account for the phase I duration as previous study (Murias et al. 2011a). Although the 20-s shift might result in underestimation/overestimation for the phase I duration, but the associated error extended to the overall Δ[HHb]/\(\dot{V}{\text{O}}_{{2}}\) ratio was < 8%, respectively (Murias et al. 2011b). To ensure the analytic approaches consistent with previous studies for comparability, the 20- s shift was applied in the current study.
Time-aligned data sets were further averaged into 5 s bins for comparison, and the overall ‘mean’ Δ[HHb]/\(\dot{V}{\text{O}}_{{2}}\) ratio during the transition was calculated for each participant as the average value from 20 to 120 s (i.e., from the start of the ratio overshoot to the steady-state for all groups). HR data were processed using the same strategy for the \(\dot{V}{\text{O}}_{{2}}\) data, with the fitting profiles starting at exercise onset (i.e., time zero).
Statistical analysis
Data are expressed as mean ± standard deviation (SD) and analyzed using SPSS (version 26; IBM, Armonk, NY). Shapiro-Will test was performed to verify the data normality before statistical analysis. An independent t test was used to compare variables between HIIT groups at baseline and post-training. Paired t test was used to determine within-group changes after training. To explore the effects of SMM on CRF-related adaptations, linear mixed-effect models (LME) were used with subjects for random intercept and fixed factors as (1) time; (2) group; (3) SMM. Two-way mixed ANOVA was used to assess training adaptations in groups, with PA level as the between-subjects variable and training as the within-subjects variable. If a significant group x time interaction appears, post hoc analysis with Bonferroni’s correction was used for pairwise comparisons. Pearson correlations were used to analyze the relationship between variables. The effect size for the F ratio was expressed as partial η2 (ηp2) with the alpha level fixed at p < 0.05 for statistical significance.
Result
Four out of 24 participants dropped out (3 due to personal reasons; 1 due to soft tissue injury unrelated to the present study) and were excluded from data analysis. The final results included 10 participants for each group (HIIT-H: male = 3, female = 7; HIIT-M: male = 2, female = 8), and baseline characteristics were presented in Table 1. Based on the IPAQ-S scoring protocol, the HIIT-H group fits in the “high” category with an average PA of 3404.0 ± 1039.8 MET-minutes/week. The PA background of the HIIT-M group was 1611.2 ± 913.9 MET-minutes/week, fitting in the “moderate” category and significantly lower than the HIIT-H group (p = 0.001). It should be noted that only the VPA was significantly different between groups (p = 0.001), and no difference exists in other types of PA.
Body composition
The HIIT-H and HIIT-M groups differed in weight, SMM, and BMI (p < 0.05). There were no statistical differences between groups for body fat mass, body fat percentage, and VFA (p = 0.978, p = 0.162, p = 0.239) at baseline. The training effects on the variables are presented in Table 2. A significant main effect of time was observed for SMM (F = 12.649, p < 0.01, ηp2 = 0.413), BF% (F = 13.37, p < 0.01, ηp2 = 0.426) and body fat mass (F = 17.754, p < 0.01, ηp2 = 0.497). After training, the SMM increased for HIIT-H (+ 0.66 ± 0.70 kg, p = 0.016) and HIIT-M (+ 0.58 ± 0.61 kg, p = 0.038). The change of BF% was significant in both groups (HIIT-H: − 3.08% ± 4.29%, p = 0.026; HIIT-M: − 2.58% ± 2.35%, p = 0.007) and the similar outcomes for the BFM (HIIT-H, − 1.52 ± 1.93 kg, p = 0.035; HIIT-M, − 1.64 ± 1.37 kg, p = 0.004). VFA and weight were unchanged after training without between-group differences.
Ramp-incremental test
All parameters were higher in HIIT-H than in HIIT-M (p < 0.05). A significant main effect of time was observed for TTE (F = 5.845, p < 0.05, ηp2 = 0.245) and PPO (F = 5.303, p < 0.05, ηp2 = 0.231) without between-group differences (p = 0.983, p = 0.935). A significant time main effect was observed for GET (F = 13.359, p < 0.01, ηp2 = 0.426) and GET-intensity power output (F = 18.646, p < 0.001, ηp2 = 0.509). After training, GET increased for HIIT-M (+ 0.21 ± 0.17 L/min, 25.05% ± 21.88%, p < 0.01) but not for HIIT-H (p = 0.179). The power output also increased for HIIT-M (+ 16.8 ± 10.4 W, 24.17% ± 16.05%, p = 0.001) but not for HIIT-H (p = 0.087).The absolute \(\dot{V}{\text{O}}_{{{\text{2max}}}}\) increased for both HIIT-H (+ 0.26 ± 0.07 L/min, 12.51% ± 6.5%, p = 0.025) and HIIT-M (+ 0.24 ± 0.07 L/min, 15.29% ± 7.95%, p < 0.001) with a significant time main effect (F = 23.302, p < 0.001, ηp2 = 0.564) after training. For relative \(\dot{V}{\text{O}}_{{{\text{2max}}}}\), the changes were significant for HIIT-H (4.96 ± 1.11 ml/kg/min, 15.07% ± 13.71%, p < 0.001) and HIIT-M (5.19 ± 1.12 ml/kg/min, 17.76% ± 6.43%, p < 0.001) with a significant time main effect (F = 42.006, p < 0.001, ηp2 = 0.7). The positive effect of SMM was significant for the absolute \(\dot{V}{\text{O}}_{{{\text{2max}}}}\) (β = 0.06 [0.04, 0.08], p < 0.001) but not for the relative \(\dot{V}{\text{O}}_{{{\text{2max}}}}\) (p = 0.558). SMM demonstrated a strong correlation with absolute \(\dot{V}{\text{O}}_{{{\text{2max}}}}\) combining two groups for regression (baseline: r = 0.841, p < 0.001; post-training: r = 0.851, p < 0.001), which was higher than body weight (baseline: r = 0.760, p < 0.001; post-training: r = 0.713, p < 0.001).
\({\dot{\text{V}}\text{O}}_{{2}}\) and HR kinetics
The parameters at baseline were different between the groups (p < 0.05) except for TD (p = 0.425). After training, the \(\tau \dot{V}{\text{O}}_{{2}}\) accelerated for HIIT-H (7.11 ± 1.05 s, 32.39% ± 20.64%, p < 0.001) and HIIT-M (5.48 ± 1.05 s, 18.72% ± 10.99%, p < 0.001), with a significant time main effect only (F = 72.274, p < 0.001, ηp2 = 0.801). The \(\dot{V}{\text{O}}_{{2}}\) baseline, TD, and τHR were unaffected by training (p = 0.374, p = 0.053, p = 0.144), but the \(\dot{V}{\text{O}}_{{2}}\) amplitude from baseline to steady-state improved for HIIT-H (0.16 ± 0.05 L/min, p < 0.05) and HIIT-M (0.16 ± 0.06 L/min, p < 0.05). The \(\dot{V}{\text{O}}_{{2}}\) steady-state was also changed for HIIT-H (0.12 ± 0.24 L/min, 11.93% ± 21.61%, p = 0.154) and HIIT-M (0.17 ± 0.16 L/min, 17.25% ± 13.7%, p < 0.01). A significant time main effect was observed for both \(\dot{V}{\text{O}}_{{2}}\) amplitude (F = 15.613, p < 0.01, ηp2 = 0.464) and \(\dot{V}{\text{O}}_{{2}}\) steady-state (F = 11.158, p < 0.01, ηp2 = 0.383). The LME result showed that SMM does not affect changes in \(\tau \dot{V}{\text{O}}_{{2}}\) (p = 0.280). Data for \(\dot{V}{\text{O}}_{{2}}\), τHR and the Δ[HHb]/\(\dot{V}{\text{O}}_{{2}}\) are presented in Table 3 and Fig. 1. \(\tau \dot{V}{\text{O}}_{{2}}\) presented a moderate negative correlation with absolute \(\dot{V}{\text{O}}_{{{\text{2max}}}}\) when groups were combined for the analysis (baseline: r = − 0.616, p = 0.004; post: r = − 0.625, p = 0.003). Separately speaking, the HIIT-H presented a moderate negative correlation without statistical significance (baseline: r = − 0.461, p = 0.180; post: r = − 0.429, p = 0.216) and the \(\tau \dot{V}{\text{O}}_{{2}}\) was significantly correlated with absolute \(\dot{V}{\text{O}}_{{{\text{2max}}}}\) in HIIT-M only at baseline level (baseline: r = − 0.667, p = 0.035; post: r = − 0.559, p = 0.093). However, the correlation between \(\tau \dot{V}{\text{O}}_{{2}}\) and relative \(\dot{V}{\text{O}}_{{{\text{2max}}}}\) was not significant for HIIT-H (baseline: r = − 0.120, p = 0.741; post: r = − 0.171, p = 0.636), HIIT-M (baseline: r = − 0.271, p = 0.448; post: r = − 0.084, p = 0.618) and combined analysis (baseline: r = − 0.420, p = 0.065; post: r = − 0.367, p = 0.112).
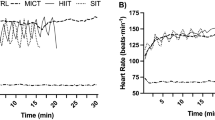
Group mean profiles for the adjustment of \(\tau \dot{V}{\text{O}}_{{2}}\), τHR during the moderate-intensity transitions. Black circle represents baseline; white circle represents post-training. Gray dashed line represents the average fit for the post-training statement, solid gray line represented the average fit for the baseline statement *Δ[HHb]/\(\dot{V}{\text{O}}_{{2}}\) significant different value between baseline and post-training at the time points (p < 0.05)
NIRS-derived parameters
At the baseline RI test, there was no difference between groups for SmO2peak%, ΔO2Hb, ΔtHb, and ΔSmO2%. However, the ΔHHb had a higher value in HIIT-H compared to HIIT-M (p < 0.05). In response to training, the ΔHHb improved for both groups (time main effect: F = 15.092, p < 0.01, ηp2 = 0.456), with significance for HIIT-H (+ 1.81 ± 0.43, p = 0.043) and HIIT-M (+ 1.49 ± 1.14, p = 0.003). Similar change was also showed in ΔO2Hb (time main effect: F = 11.127, p = 0.004. ηp2 = 0.382), with significance for HIIT-H (+ 2.15 ± 2.77, p = 0.037) and HIIT-M (+ 1.42 ± 1.94, p = 0.046). No change occurred in SmO2peak%, ΔtHb, and ΔSmO2%. Based on LME results, the SMM has a significantly positive effect on ΔHHb (β = 0.53 [0.42, 1.02], p = 0.034) but not on ΔO2Hb (p = 0.176).
The τ′ΔHHb was faster for HIIT-H compared to HIIT-M during the transition to moderate-intensity exercise, without differences in initial TD (p = 0.425). A significant time × group interaction effect was observed for τ′ΔHHb (F = 7.693, p = 0.013, ηp2 = 0.299). The post hoc analysis showed that τ′ΔHHb reduced significantly for both HIIT-H (− 3.9 ± 2.76, p = 0.01; time simple effect: F = 9.826, p < 0.001, ηp2 = 0.353) and HIIT-M (− 8.78 ± 4.09, p < 0.001; time simple effect: F = 49.803, p < 0.001, ηp2 = 0.735) after training, with distinct effect size. Similar results occurred in Δ[HHb]/\(\dot{V}{\text{O}}_{{2}}\) ratio (time main effect: F = 15.949, p = 0.001, ηp2 = 0.470), with significant reduction for HIIT-H (− 0.13 ± 0.08, p = 0.014) and HIIT-M (− 0.09 ± 0.11, p = 0.029). Correlations between \(\tau \dot{V}{\text{O}}_{{2}}\) and Δ[HHb]/\(\dot{V}{\text{O}}_{{2}}\) ratio was moderate at baseline (r = 0.451, p = 0.046) and post-training (r = 0.566, p = 0.009).
Discussion
In general, both groups showed improvements in body composition, cardiorespiratory fitness, \(\dot{V}{\text{O}}_{{2}}\) transport kinetics, and the matching of local O2 distribution and utilization. Although the high-PA group presented a more advanced whole-body physical fitness level, the training-induced adaptations were similar between groups without significant differences except for a few variables.
Body composition
According to IPAQ-S results, resistance training sided with a small amount of aerobic exercise (i.e., running/cycling) composed the majority of recreational training regimens for participants in the high-PA group. Considering the muscle hypertrophy adaptation mainly caused by structured resistance training (Francaux and Deldicque 2019; Seynnes et al. 2007), the distinct volume of habitual vigorous PA (i.e., resistance training, RT) between groups accounted for the baseline differences in SMM. Additionally, this RT-based regime partially explained the similar baseline BF% and VFA between groups since the maximal fat oxidative intensity exists at a lower intensity and weightlifting is mainly supported by muscular glycometabolism (Hargreaves and Spriet 2020; Brun et al. 2022).
After 4 weeks, the improvements in SMM and body fat (BF%, fat mass) were significant without group differences, which means HIIT can be effective in gaining muscle and losing fat for non-athletic healthy adults regardless of their PA backgrounds. In former studies with healthy adults, reduction in FM, BF%, and an increase in muscle mass were observed after short terms of HIIT (Schubert et al. 2017; Lan et al. 2022; Callahan et al. 2021a). In contrast, Astorino et al. (2011) reported unchanged BF% in physically active participants, which can be explained by a lower BF% value at baseline and fewer training sessions compared to this study. However, the VFA was unaffected by training for both groups, similar to previous reviews showing that HIIT did not affect visceral fat tissue when participants were overall normal-weight population (Maillard et al. 2018; Keating et al. 2017).
Robinson et al. (2017) reported muscle growth in untrained young adults presented as FFM, and protein in muscle increased after 12 weeks of HIIT. Despite the shorter training period, similar changes in SMM were observed in this study. The greater activation of type II muscle fibers during high-intensity exercise might contribute to HIIT-induced muscle growth (Edgett et al. 2013; Folland and Williams 2007). Interestingly, comparing to HIIT training, the \(\dot{V}{\text{O}}_{{{\text{2max}}}}\) and mitochondrial function were unchanged after RT in previous study (Robinson et al. 2017). This will be discussed in the following section (i.e., aerobic capacity).
Aerobic capacity
The circulatory and respiratory systems provide oxygen to skeletal muscle mitochondria for energy production during physical activity, referring as cardiorespiratory fitness (i.e., \(\dot{V}{\text{O}}_{{{\text{2max}}}}\)) (Raghuveer et al. 2020), and the dominant physiological pathways responsible for the training-induced \(\dot{V}{\text{O}}_{{{\text{2max}}}}\) improvement were wildly discussed (Lundby et al. 2017; Wagner 2015; van der Zwaard et al. 2016; Rosenblat et al. 2022). A stronger correlation exists between SMM and absolute \(\dot{V}{\text{O}}_{{{\text{2max}}}}\) than body weight and absolute \(\dot{V}{\text{O}}_{{{\text{2max}}}}\). This result linked the BIA-measured SMM to cardiorespiratory fitness as a better scaling variable than the traditional method of scaling to body mass, as the previous study described (Wittekind et al. 2020).
The training adaptation presented in this study suggests that HIIT can promote SMM gains for individuals with different fitness levels. Also, the amplitude of ΔHHb and ΔO2Hb increased during the RI test, supporting the capacity for microvascular O2 extraction has improved. Previous studies demonstrated that HIIT improves skeletal muscle oxidative capacity by stimulating mitochondrial biogenesis, mitochondrial respiration, and tissue angiogenesis, which might explain these adaptations (Granata et al. 2018; Rosenblat et al. 2022; Callahan et al. 2021b).
Participants were instructed to continue their daily PA level and diet regime, leading the intervention as concurrent strength and HIIT-based training for the high-PA group. The current evidence suggests minimal to no interference effects between the training modalities, suggesting that the gains from HIIT or RT will not be compromised through the process (Vechin et al. 2021; Schumann et al. 2022).
Through LME, the SMM positively affects absolute \(\dot{V}{\text{O}}_{{{\text{2max}}}}\), and ΔHHb increases. More specifically, the local microvascular O2 extraction for HIIT-H improved more than HIIT-M, and the absolute \(\dot{V}{\text{O}}_{{{\text{2max}}}}\) had higher overall increases for trained individuals since they possessed more SMM. These results were different from the original hypothesis, assuming the HIIT-M group adaptation would be more profound. Considering the RT has a relatively weak impact on \(\dot{V}{\text{O}}_{{{\text{2max}}}}\) and mitochondria function (Robinson et al. 2017; Callahan et al. 2021a; Ramírez-Vélez et al. 2020), the \(\dot{V}{\text{O}}_{{{\text{2max}}}}\) gap between groups at baseline can be explained by the SMM due to the link mentioned above, but the differences in mitochondrial respiration capacity, capillary density and other peripheral factors at the muscle tissue level between groups might not be significant.
This might explain the similar improvements in absolute \(\dot{V}{\text{O}}_{{{\text{2max}}}}\) values. Milanović et al. (2015) conducted a meta-analysis on the effectiveness of HIIT on \(\dot{V}{\text{O}}_{{{\text{2max}}}}\) improvements and detected a more remarkable improvement for subjects with lower baseline fitness. Similar results also occurred in other studies (Raleigh et al. 2018; Astorino et al. 2017). When normalized, the absolute \(\dot{V}{\text{O}}_{{{\text{2max}}}}\) changes to the percentage of the baseline value, and HIIT-M showed slightly more significant increases in \(\dot{V}{\text{O}}_{{{\text{2max}}}}\) following training.
No change was observed in the amplitude of ΔtHb in addition to the increased ΔO2Hb, suggesting the local O2 availability was relatively stable before and after training. The increase in local O2 availability can be induced by capillary density improvement (Laughlin and Roseguini 2008), functional and structural adaptations in the conduit arteries (Green et al. 2017), and improvements in cardiac output (MacInnis and Gibala 2017). Parameters concerning the central adaptations were not measured in the present study, but there were no changes observed in maximal cardiac output after four weeks of HIIT (Raleigh et al. 2018), suggesting the improvements in \(\dot{V}{\text{O}}_{{{\text{2max}}}}\) are accomplished by peripheral adaptations involving changes in skeletal muscle oxidative capacity (Gillen et al. 2013; Scribbans et al. 2014). Meanwhile, increased maximal stroke volume and cardiac output have been reported after extended training periods (Astorino et al. 2017; Montero and Díaz-Cañestro 2016), suggesting the central adaptations may not contribute to the increases in \(\dot{V}{\text{O}}_{{{\text{2max}}}}\) during short-duration HIIT. Taken together, alterations in the peripheral system (skeletal muscle) can partially explain the \(\dot{V}{\text{O}}_{{{\text{2max}}}}\) improvement in the present study.
Another crucial factor was the method used for prescribing training intensity. The wildly-practiced method, which is based on a fixed percentage of maximal anchors, was applied in various settings and populations (Robinson et al. 2017; Callahan et al. 2021a; Ramírez-Vélez et al. 2020; Milanović et al. 2015; Raleigh et al. 2018; Astorino et al. 2011, 2017). However, this strategy ignored the variability in individual physiological responses (Jamnick et al. 2020). In the current study, training intensity was determined by percentage-HRmax for both groups, with HIIT-H possessing higher \(\dot{V}{\text{O}}_{{{\text{2max}}}}\) levels, leading to relatively higher training volume in the HIIT-H group. Since the SMM is responsible for the \(\dot{V}{\text{O}}_{{{\text{2max}}}}\) differences at baseline, critical power (i.e., the operational surrogate for maximal metabolic steady-state) did not change significantly after RT despite improved W′ (Sawyer et al. 2014). It is possible that the same training protocol based on fix-percentage anchor would result in higher depletion of W′ during training for the RT-based trained individuals, extending to prolonged time spent at \(\dot{V}{\text{O}}_{{{\text{2max}}}}\) (Vinetti et al. 2019). Therefore, the higher training stimulus in the HIIT-H group might lead to similar CRF improvements between groups rather than the HIIT-M group presenting more significant changes, as we hypothesized at first.
O2 kinetics of delivery and utilization
The training-induced speeding of \(\dot{V}{\text{O}}_{{2}}\) kinetics during the moderate-intensity transition has been reported in numerous studies (George et al. 2018; Hamasaki et al. 2018; Koschate et al. 2019; McCrudden et al. 2017; Murias et al. 2010; Corvino et al. 2019; Berger et al. 2006). For recreationally-trained individuals, \(\dot{V}{\text{O}}_{{2}}\) kinetics were significantly fast compared to their age-matched counterparts (Inglis et al. 2021; Berger et al. 2006), but the training background-matched individuals across the aging spectrum showed similar results, and the decreases in \(\tau \dot{V}{\text{O}}_{{2}}\) were significant in both older and young adults (Murias et al. 2010), suggesting that fitness level is the determinant for \(\tau \dot{V}{\text{O}}_{{2}}\) and regular training can eliminate the age-related slacken of \(\dot{V}{\text{O}}_{{2}}\) kinetics (George et al. 2018). The correlation between \(\tau \dot{V}{\text{O}}_{{2}}\) and \(\dot{V}{\text{O}}_{{{\text{2max}}}}\) was only significant for the absolute value but not for the relative value. This inconsistency with a previous study (Inglis et al. 2021) is partially due to the differences in fitness status. The untrained group in the previous study still had a higher \(\dot{V}{\text{O}}_{{{\text{2max}}}}\) level than the HIIT-H group in this study (2.46 ± 0.34 L/min; 39.41 ± 4.85 mL/kg). The differences in body fat percentage could be the other reason for this result. Since the participants in the previous study were all males and the participants in this study were primarily females, the relatively higher percentage of body fat in females might lead to insignificant correlations between the relative \(\dot{V}{\text{O}}_{{{\text{2max}}}}\) and \(\tau \dot{V}{\text{O}}_{{2}}\) since total body fat does not influence maximal aerobic capacity.
In the present study, participants in both groups showed the training-induced reduction in \(\tau \dot{V}{\text{O}}_{{2}}\) without between-group differences (Fig. 1). These results are inconsistent with the previous study, in which individuals with relatively fast baseline \(\tau \dot{V}{\text{O}}_{{2}}\) showed less reduction after training (McKay et al. 2009). However, the standard for fast \(\dot{V}{\text{O}}_{{2}}\) kinetics was \(\tau \dot{V}{\text{O}}_{{2}}\) < 25 s in the previous study, and the recreational trainers (i.e., faster \(\tau \dot{V}{\text{O}}_{{2}}\) group) from this study had an average 31.05 ± 2.52 s baseline value, considered as the “slower \(\dot{V}{\text{O}}_{{2}}\) kinetics” in the previous study (McKay et al. 2009).
The mismatch of faster Δ[HHb] kinetics than \(\tau \dot{V}{\text{O}}_{{2}}\) was presented as the ‘overshoot’ phenomenon in normalized Δ[HHb]/\(\dot{V}{\text{O}}_{{2}}\) ratio (Murias et al. 2011a), suggesting a lower O2 delivery relative to muscle O2 utilization compared with exercise steady-state (i.e., ratio = 1) and the matching of O2 delivery to O2 utilization was better for the trained individuals (Murias et al. 2010, 2011a; George et al. 2018). In response to training, the attenuated Δ[HHb]/\(\dot{V}{\text{O}}_{{2}}\) ratio in both groups meaning that local blood supply and O2 delivery were better matched to the muscular requirement. Adequate provision was considered as a key rate limiting mechanism for \(\dot{V}{\text{O}}_{{2}}\) adjustment rate across age groups besides intracellular factors (George et al. 2018) and the attenuation of Δ[HHb]/\(\dot{V}{\text{O}}_{{2}}\) in this study was aligned with this theory.
From the O2 delivery perspective, once the overshoot was eliminated (Fig. 2), the O2 delivery and utilization in active muscle remained “matched” after further training (Murias et al. 2010; 2014). The HIIT-M group showed a decreased Δ[HHb]/\(\dot{V}{\text{O}}_{{2}}\) after 4 weeks, but the overshoot was not eliminated. These results could explain the more prominent reduction for τ′Δ[HHb] in the HIIT-M compared to HIIT-H.
Group mean profiles for the adjustment of Δ[HHb] and \(\dot{V}{\text{O}}_{{2}}\) during the moderate-intensity transition. Black circles indicate time points with significant differences exist between the relative increase of Δ[HHb] (white circles) and the relative increase of \(\dot{V}{\text{O}}_{{2}}\) (triangles). Extended profiles for the adjustment of the Δ[HHb]\(\dot{V}{\text{/O}}_{{2}}\) are presented (gray circles) with the steady-state ratio of 1.0 marked (gray dotted line)
It should be noted that HR kinetics is used as the operational surrogate for the \(\dot{Q}\) kinetics considering the technicalities. The previous study demonstrated no significant differences between \(\tau \dot{Q}\) and τHR in participants with distinct training backgrounds (Zuccarelli et al. 2018; Inglis et al. 2021). There were no statistical differences in τHR between groups at the same time and within-group training adaptations. Consistent with the previous study (Inglis et al. 2021), this suggests that the central components of O2 transportation do not predominately affect the \(\dot{V}{\text{O}}_{{2}}\) kinetics during step-transition. The previous section discussed that the central adaptations do not respond significantly to short-term HIIT (Gillen et al. 2013; Scribbans et al. 2014; Astorino et al. 2017; Montero and Díaz-Cañestro 2016), and HR kinetics remained the same until after eight training sessions (McKay et al. 2009). Therefore, the possible differences in training-induced adaptations in O2 transportation between individuals with varied PA-background require further research.
Limitations
The PA level before commencing the study was determined with a questionnaire rather than an accelerometer. There is the possibility that the participants with habitual training regimes may overrate or underrate their daily activity intensity/volume, which could have influenced some of the measures. Besides training, the leisure time physical activity (i.e., non-exercise physical activity, NEPA) was not addressed adequately in the current study during the intervention, which may interfere with the training-induced responses. The current evidence indicates no significant changes in the NEPA and sedentary time during training for the previously inactive groups (Fedewa et al. 2017; Bruseghini et al. 2020) except for younger females (Lu et al. 2022). As for previously active groups, the impact of additional structured training on NEPA has yet to be reported in the previous literature. Future research should monitor NEPA to elaborate on the training-induced responses more rigorously.
While the gender ratio was somewhat imbalanced for both groups, the oxygen uptake kinetics to the onset of moderate-intensity exercise is independent of sex (Fawkner et al. 2002). Additionally, The gender difference in SMM explained the most variability in CRF between males and females (Köhler et al. 2018; Krachler et al. 2015; Wittekind et al. 2020). This gender ratio may be acceptable since the focus of this study was on the effects of skeletal muscle mass on cardiorespiratory fitness-related adaptations.
Participants performed step-transitions corresponding to 80–90% GET workload, resulting in the different amplitude responses between groups (Table 3). However, the kinetics of \(\dot{V}{\text{O}}_{{2}}\) is relatively stable within the moderate intensity domain despite the differences in amplitude (Keir et al. 2016), suggesting that these differences had minimal effects on the temporal differences or between-group comparison. The HIIT protocol in this study was based on maximal anchor rather than CP/CS and W′ recovery model, which might cause deviations in training volume between two groups. Future study should aim to develop a standard HIIT protocol based on CP/CS and W′ recovery model.
Finally, it should be noted that although the sample size was in line with previous studies in the field (Murias et al. 2011a; McKay et al. 2009; Berger et al. 2006) and the statistical power was large enough to detect changes in parameters before and after training, the sample size was not large enough to provide enough power for all the possible interaction effects. The cluster analysis should be applied to assign individuals in order to achieve a more rigorous allocation of groups (Laudani et al. 2013). However, this study was unable to apply the cluster analysis with N = 10 for each cluster and provide sufficient statistical power due to the relatively small sample size (Rutterford et al. 2015; Dalmaijer et al. 2022). Caution should be used when interpreting these results.
Conclusion
Four weeks of HIIT significantly increased the aerobic capacity, skeletal muscle mass, and \(\dot{V}{\text{O}}_{{2}}\) kinetics of recreational trained and untrained participants. Additionally, an improved Δ[HHb]/\(\dot{V}{\text{O}}_{{2}}\) ratio reflects a training-induced better match of O2 distribution and utilization within the muscle tissue. Taken together, the peripheral adaptations response to 4 weeks of HIIT is attributed to the observed improvements. The SMM directly links to \(\dot{V}{\text{O}}_{{{\text{2max}}}}\) gains and the training effects are similar between groups, presenting HIIT as a powerful method to reach better physical fitness levels for RT-based trained and untrained individuals. However, the fitness gap between groups in this study was relatively moderate. Participants with a broader range of fitness levels/training backgrounds are needed in further research to explore the possible differences in adaptations.
Data availability
The datasets that support this study are available from the corresponding author upon reasonable request.
Abbreviations
- ANOVA:
-
Analysis of variance
- BF%:
-
Body fat percentage
- BFM:
-
Body fat mass
- BMI:
-
Body mass index
- CP:
-
Critical power
- CRF:
-
Cardiorespiratory fitness
- GET:
-
Gas-exchange threshold
- HIIT:
-
High-intensity interval training
- HR:
-
Heart rate
- IPAQ-S:
-
International physical activity questionnaire short form
- LME:
-
Linear mixed-effect models
- MET:
-
Maximal metabolic equivalent
- MPA:
-
Moderate- intensity physical activity
- NIRS:
-
Near-infrared spectroscopy
- O2 :
-
Oxygen
- PA:
-
Physical activity
- PARq:
-
Physical activity readiness questionnaire
- PPO:
-
Peak power output
- \(\dot{Q}\) :
-
Cardiac output
- RER:
-
Respiratory exchange ratio
- RPE:
-
Rating of perceived exertion
- RT:
-
Resistance training
- SD:
-
Standard deviation
- SMM:
-
Skeletal muscle mass
- SmO2%:
-
Muscle O2 saturation
- TTE:
-
Time to exhaustion
- \({\dot{\text{V}}\text{O}}_{{2}}\) :
-
Oxygen uptake
- \({\dot{\text{V}}\text{O}}_{{{\text{2max}}}}\) :
-
Maximal oxygen uptake
- VFA:
-
Visceral fat sera
- VPA:
-
Vigorous-intensity physical activity
- W:
-
Watts
- W' :
-
A finite amount of energy available for work performed above CP
- Δ[HHb]/\({\dot{\text{V}}\text{O}}_{{2}}\) :
-
Deoxygenated hemoglobin over oxygen uptake ratio
- [HHb]:
-
Deoxygenated hemoglobin
- [O2Hb]:
-
Oxygenated hemoglobin
- [tHb]:
-
Total hemoglobin
References
Adami A, Rossiter HB (2018) Principles, insights, and potential pitfalls of the noninvasive determination of muscle oxidative capacity by near-infrared spectroscopy. J Appl Physiol 124:245–248. https://doi.org/10.1152/japplphysiol.00445.2017
Astorino TA, Allen RP, Roberson DW, Jurancich M, Lewis R, McCarthy K, Trost E (2011) Adaptations to high-intensity training are independent of gender. Eur J Appl Physiol 111:1279–1286. https://doi.org/10.1007/s00421-010-1741-y
Astorino TA, Edmunds RM, Clark A, King L, Gallant RA, Namm S, Fischer A, Wood KM (2017) High-intensity interval training increases cardiac output and VO2max. Med Sci Sports Exerc 49:265–273. https://doi.org/10.1249/MSS.0000000000001099
Berger NJ, Tolfrey K, Williams AG, Jones AM (2006) Influence of continuous and interval training on oxygen uptake on-kinetics. Med Sci Sports Exerc 38:504–512. https://doi.org/10.1249/01.mss.0000191418.37709.81
Brun JF, Myzia J, Varlet-Marie E, Raynaud de Mauverger E, Mercier J (2022) Beyond the calorie paradigm: taking into account in practice the balance of fat and carbohydrate oxidation during exercise? Nutrients 14:1605. https://doi.org/10.3390/nu14081605
Bruseghini P, Tam E, Calabria E, Milanese C, Capelli C, Galvani C (2020) High intensity interval training does not have compensatory effects on physical activity levels in older adults. Int J Environ Res Public Health 17:1083. https://doi.org/10.3390/ijerph17031083
Bull FC, Al-Ansari SS, Biddle S et al (2020) World health organization 2020 guidelines on physical activity and sedentary behaviour. Br J Sports Med 54:1451–1462. https://doi.org/10.1136/bjsports-2020-102955
Callahan MJ, Parr EB, Hawley JA, Camera DM (2021a) Can high-intensity interval training promote skeletal muscle anabolism? Sports Med 51:405–421. https://doi.org/10.1007/s40279-020-01397-3
Callahan MJ, Parr EB, Snijders T et al (2021b) Skeletal muscle adaptive responses to different types of short-term exercise training and detraining in middle-age men. Med Sci Sports Exerc 53:2023–2036. https://doi.org/10.1249/MSS.0000000000002684
Corvino RB, Oliveira MFM, Denadai BS, Rossiter HB, Caputo F (2019) Speeding of oxygen uptake kinetics is not different following low-intensity blood-flow-restricted and high-intensity interval training. Exp Physiol 104:1858–1867. https://doi.org/10.1113/EP087727
Dalmaijer ES, Nord CL, Astle DE (2022) Statistical power for cluster analysis. BMC Bioinform 23:205. https://doi.org/10.1186/s12859-022-04675-1
DeLorey DS, Kowalchuk JM, Paterson DH (2003) Relationship between pulmonary O2 uptake kinetics and muscle deoxygenation during moderate-intensity exercise. J Appl Physiol 95:113–120. https://doi.org/10.1152/japplphysiol.00956.2002
Edgett BA, Foster WS, Hankinson PB et al (2013) Dissociation of increases in PGC-1α and its regulators from exercise intensity and muscle activation following acute exercise. PLoS ONE 8:e71623. https://doi.org/10.1371/journal.pone.0071623
Fawkner SG, Armstrong N, Potter CR, Welsman JR (2002) Oxygen uptake kinetics in children and adults after the onset of moderate-intensity exercise. J Sports Sci 20:319–326. https://doi.org/10.1080/026404102753576099
Fedewa MV, Hathaway ED, Williams TD, Schmidt MD (2017) Effect of exercise training on non-exercise physical activity: a systematic review and meta-analysis of randomized controlled trials. Sports Med 47:1171–1182. https://doi.org/10.1007/s40279-016-0649-z
Folland JP, Williams AG (2007) The adaptations to strength training: morphological and neurological contributions to increased strength. Sports Med 37:145–168. https://doi.org/10.2165/00007256-200737020-00004
Francaux M, Deldicque L (2019) Exercise and the control of muscle mass in human. Pflugers Arch Eur J Physiol 471:397–411. https://doi.org/10.1007/s00424-018-2217-x
George MA, McLay KM, Doyle-Baker PK, Reimer RA, Murias JM (2018) Fitness level and not aging per se, determines the oxygen uptake kinetics response. Front Physiol 9:277. https://doi.org/10.3389/fphys.2018.00277
Gildea N, McDermott A, Rocha J, O’Shea D, Green S, Egaña M (2021) Time-course of VO2 kinetics responses during moderate-intensity exercise subsequent to HIIT versus moderate-intensity continuous training in type 2 diabetes. J Appl Physiol 130:1646–1659. https://doi.org/10.1152/japplphysiol.00952.2020
Gillen JB, Percival ME, Ludzki A, Tarnopolsky MA, Gibala MJ (2013) Interval training in the fed or fasted state improves body composition and muscle oxidative capacity in overweight women. Obesity (Silver Spring) 21:2249–2255. https://doi.org/10.1002/oby.20379
Goran M, Fields DA, Hunter GR, Herd SL, Weinsier RL (2000) Total body fat does not influence maximal aerobic capacity. Int J Obes Relat Metab Disord 24:841–848. https://doi.org/10.1038/sj.ijo.0801241
Granata C, Oliveira RS, Little JP, Renner K, Bishop DJ (2016) Mitochondrial adaptations to high-volume exercise training are rapidly reversed after a reduction in training volume in human skeletal muscle. FASEB J 30:3413–3423. https://doi.org/10.1096/fj.201500100R
Granata C, Jamnick NA, Bishop DJ (2018) Training-induced changes in mitochondrial content and respiratory function in human skeletal muscle. Sports Med 48:1809–1828. https://doi.org/10.1007/s40279-018-0936-y
Grassi B, Quaresima V (2016) Near-infrared spectroscopy and skeletal muscle oxidative function in vivo in health and disease: a review from an exercise physiology perspective. J Biomed Opt 21:091313. https://doi.org/10.1117/1.JBO.21.9.091313
Green DJ, Hopman MT, Padilla J, Laughlin MH, Thijssen DH (2017) Vascular adaptation to exercise in humans: role of hemodynamic stimuli. Physiol Rev 97:495–528. https://doi.org/10.1152/physrev.00014.2016
Guthold R, Stevens GA, Riley LM, Bull FC (2018) Worldwide trends in insufficient physical activity from 2001 to 2016: a pooled analysis of 358 population-based surveys with 1·9 million participants. Lancet Glob Health 6:e1077–e1086. https://doi.org/10.1016/S2214-109X(18)30357-7
Hamasaki A, Arima S, Hirakoba K (2018) Changes in pulmonary oxygen uptake and muscle deoxygenation kinetics during cycling exercise in older women performing walking training for 12 weeks. Eur J Appl Physiol 118:2179–2188. https://doi.org/10.1007/s00421-018-3946-4
Hargreaves M, Spriet LL (2020) Skeletal muscle energy metabolism during exercise. Nat Metab 2:817–828. https://doi.org/10.1038/s42255-020-0251-4
Hawley JA, Hargreaves M, Joyner MJ, Zierath JR (2014) Integrative biology of exercise. Cell 159:738–749. https://doi.org/10.1016/j.cell.2014.10.029
Helgerud J, Høydal K, Wang E et al (2007) Aerobic high-intensity intervals improve VO2max more than moderate training. Med Sci Sports Exerc 39:665–671. https://doi.org/10.1249/mss.0b013e3180304570
Howley ET, Bassett DR, Welch HG (1995) Criteria for maximal oxygen uptake: review and commentary. Med Sci Sports Exerc 27:1292–1301
Hughson RL, Tschakovsky ME, Houston ME (2001) Regulation of oxygen consumption at the onset of exercise. Exerc Sport Sci Rev 29:129–133. https://doi.org/10.1097/00003677-200107000-00008
Inglis EC, Iannetta D, Murias JM (2021) Association between VO2 kinetics and VO2max in groups differing in fitness status. Eur J Appl Physiol 121:1921–1931. https://doi.org/10.1007/s00421-021-04623-6
Jamnick NA, Pettitt RW, Granata C, Pyne DB, Bishop DJ (2020) An examination and critique of current methods to determine exercise intensity. Sports Med 50:1729–1756. https://doi.org/10.1007/s40279-020-01322-8
Jones AM, Poole DC (2013) Oxygen uptake kinetics in sport, exercise and medicine. Taylor & Francis Inc., Routledge, New York
Keating SE, Johnson NA, Mielke GI, Coombes JS (2017) A systematic review and meta-analysis of interval training versus moderate-intensity continuous training on body adiposity: exercise for body fat reduction. Obes Rev 18:943–964. https://doi.org/10.1111/obr.12536
Keir DA, Murias JM, Paterson DH, Kowalchuk JM (2014) Breath-by-breath pulmonary O2 uptake kinetics: effect of data processing on confidence in estimating model parameters. Exp Physiol 99:1511–1522. https://doi.org/10.1113/expphysiol.2014.080812
Keir DA, Robertson TC, Benson AP, Rossiter HB, Kowalchuk JM (2016) The influence of metabolic and circulatory heterogeneity on the expression of pulmonary oxygen uptake kinetics in humans. Exp Physiol 101:176–192. https://doi.org/10.1113/EP085338
Keir DA, Iannetta D, MattioniMaturana F, Kowalchuk JM, Murias JM (2022) Identification of non-invasive exercise thresholds: methods, strategies, and an online app. Sports Med 52:237–255. https://doi.org/10.1007/s40279-021-01581-z
Köhler A, King R, Bahls M et al (2018) Cardiopulmonary fitness is strongly associated with body cell mass and fat-free mass: the study of health in Pomerania (SHIP). Scand J Med Sci Sports 28:1628–1635. https://doi.org/10.1111/sms.13057
Koschate J, Gerlich L, Wirtz V, Thieschäfer L, Drescher U, Hoffmann U (2019) Cardiorespiratory kinetics: comparisons between athletes with different training habits. Eur J Appl Physiol 119:1875–1883. https://doi.org/10.1007/s00421-019-04176-9
Krachler B, Savonen K, Komulainen P, Hassinen M, Lakka TA, Rauramaa R (2015) Cardiopulmonary fitness is a function of lean mass, not total body weight: the DR’s EXTRA study. Eur J Prev Cardiol 22:1171–1179. https://doi.org/10.1177/2047487314557962
Lagerwaard B, Nieuwenhuizen AG, de Boer VCJ, Keijer J (2020) In vivo assessment of mitochondrial capacity using NIRS in locomotor muscles of young and elderly males with similar physical activity levels. GeroScience 42:299–310. https://doi.org/10.1007/s11357-019-00145-4
Lamarra N, Whipp BJ, Ward SA, Wasserman K (1987) Effect of interbreath fluctuations on characterizing exercise gas exchange kinetics. J Appl Physiol 62:2003–2012. https://doi.org/10.1152/jappl.1987.62.5.2003
Lan C, Liu Y, Wang Y (2022) Effects of different exercise programs on cardiorespiratory fitness and body composition in college students. J Exerc Sci Fit 20:62–69. https://doi.org/10.1016/j.jesf.2021.12.004
Laudani L, Vannozzi G, Sawacha Z, Della Croce U, Cereatti A, Macaluso A (2013) Association between physical activity levels and physiological factors underlying mobility in young, middle-aged and older individuals living in a city district. PLoS ONE 8:e74227. https://doi.org/10.1371/journal.pone.0074227
Laughlin MH, Roseguini B (2008) Mechanisms for exercise training-induced increases in skeletal muscle blood flow capacity: differences with interval sprint training versus aerobic endurance training. J Physiol Pharmacol 59(Suppl 7):71–88
Lee PH, Macfarlane DJ, Lam TH, Stewart SM (2011) Validity of the international physical activity questionnaire short form (IPAQ-SF): a systematic review. Int J Behav Nutr Phys Act 8:115. https://doi.org/10.1186/1479-5868-8-115
Lee DH, Rezende LFM, Joh HK et al (2022) Long-term leisure-time physical activity intensity and all-cause and cause-specific mortality: a prospective cohort of US adults. Circulation 146:523–534. https://doi.org/10.1161/CIRCULATIONAHA.121.058162
Ling CH, de Craen AJ, Slagboom PE et al (2011) Accuracy of direct segmental multi-frequency bioimpedance analysis in the assessment of total body and segmental body composition in middle-aged adult population. Clin Nutr 30:610–615. https://doi.org/10.1016/j.clnu.2011.04.001
Lu Y, Wiltshire HD, Baker JS, Wang Q (2022) Effects of low-volume high-intensity interval exercise on 24 h movement behaviors in inactive female university students. Int J Environ Res Public Health 19:7177. https://doi.org/10.3390/ijerph1912717
Lundby C, Montero D, Joyner M (2017) Biology of VO2max: looking under the physiology lamp. Acta Physiol (Oxf) 220:218–228. https://doi.org/10.1111/apha.12827
MacInnis MJ, Gibala MJ (2017) Physiological adaptations to interval training and the role of exercise intensity. J Physiol 595:2915–2930. https://doi.org/10.1113/JP273196
Maillard F, Pereira B, Boisseau N (2018) Effect of high-intensity interval training on total, abdominal and visceral fat mass: a meta-analysis. Sports Med 48:269–288. https://doi.org/10.1007/s40279-017-0807-Z
McCrudden MC, Keir DA, Belfry GR (2017) The effects of short work vs. longer work periods within intermittent exercise on VO2p kinetics, muscle deoxygenation, and energy system contribution. J Appl Physiol 122:1435–1444. https://doi.org/10.1152/japplphysiol.00514.2016
McKay BR, Paterson DH, Kowalchuk JM (2009) Effect of short-term high-intensity interval training vs. continuous training on O2 uptake kinetics, muscle deoxygenation, and exercise performance. J Appl Physiol 107:128–138. https://doi.org/10.1152/japplphysiol.90828.2008
Milanović Z, Sporiš G, Weston M (2015) Effectiveness of high-intensity interval training (HIT) and continuous endurance training for VO2max improvements: a systematic review and meta-analysis of controlled trials. Sports Med 45:1469–1481. https://doi.org/10.1007/s40279-015-0365-0
Montero D, Díaz-Cañestro C (2016) Endurance training and maximal oxygen consumption with ageing: role of maximal cardiac output and oxygen extraction. Eur J Prev Cardiol 23:733–743. https://doi.org/10.1177/2047487315617118
Murias JM, Kowalchuk JM, Paterson DH (2010) Speeding of VO2 kinetics with endurance training in old and young men is associated with improved matching of local O2 delivery to muscle O2 utilization. J Appl Physiol 108:913–922. https://doi.org/10.1152/japplphysiol.01355.2009
Murias JM, Spencer MD, Kowalchuk JM, Paterson DH (2011a) Muscle deoxygenation to VO2 relationship differs in young subjects with varying τVO2. Eur J Appl Physiol 111:3107–3118. https://doi.org/10.1007/s00421-011-1937-9
Murias JM, Spencer MD, Delorey DS, Gurd BJ, Kowalchuk JM, Paterson DH (2011b) Speeding of VO2 kinetics during moderate-intensity exercise subsequent to heavy-intensity exercise is associated with improved local O2 distribution. J Appl Physiol 111:1410–1415. https://doi.org/10.1152/japplphysiol.00607.2011
Murias JM, Spencer MD, Pogliaghi S, Paterson DH (2012) Noninvasive estimation of microvascular O2 provision during exercise on-transients in healthy young males. Am J Physiol Regul Integr Comp Physiol 303:R815–R823. https://doi.org/10.1152/ajpregu.00306.2012
Murias JM, Spencer MD, Paterson DH (2014) The critical role of O2 provision in the dynamic adjustment of oxidative phosphorylation. Exerc Sport Sci Rev 42:4–11. https://doi.org/10.1249/JES.0000000000000005
Nagai M, Komiya H, Mori Y, Ohta T, Kasahara Y, Ikeda Y (2010) Estimating visceral fat area by multifrequency bioelectrical impedance. Diabetes Care 33:1077–1079. https://doi.org/10.2337/dc09-1099
Raghuveer G, Hartz J, Lubans DR et al (2020) Cardiorespiratory fitness in youth: an important marker of health: a scientific statement from the american heart association. Circulation 142:e101–e118. https://doi.org/10.1161/CIR.0000000000000866
Raleigh JP, Giles MD, Islam H et al (2018) Contribution of central and peripheral adaptations to changes in maximal oxygen uptake following 4 weeks of sprint interval training. Appl Physiol Nutr Metab 43:1059–1068. https://doi.org/10.1139/apnm-2017-0864
Ramírez-Vélez R, Castro-Astudillo K, Correa-Bautista JE et al (2020) The effect of 12 weeks of different exercise training modalities or nutritional guidance on cardiometabolic risk factors, vascular parameters, and physical fitness in overweight adults: cardiometabolic high-intensity interval training-resistance training randomized controlled study. J Strength Cond Res 34:2178–2188. https://doi.org/10.1519/JSC.0000000000003533
Robinson MM, Dasari S, Konopka AR et al (2017) Enhanced protein translation underlies improved metabolic and physical adaptations to different exercise training modes in young and old humans. Cell Metab 25:581–592. https://doi.org/10.1016/j.cmet.2017.02.009
Rosenblat MA, Granata C, Thomas SG (2022) Effect of interval training on the factors influencing maximal oxygen consumption: a systematic review and meta-analysis. Sports Med 52:1329–1352. https://doi.org/10.1007/s40279-021-01624-5
Rutterford C, Copas A, Eldridge S (2015) Methods for sample size determination in cluster randomized trials. Int J Epidemiol 44:1051–1067. https://doi.org/10.1093/ije/dyv113
Sawyer BJ, Stokes DG, Womack CJ, Morton RH, Weltman A, Gaesser GA (2014) Strength training increases endurance time to exhaustion during high-intensity exercise despite no change in critical power. J Strength Cond Res 28:601–609. https://doi.org/10.1519/JSC.0b013e31829e113b
Schubert MM, Clarke HE, Seay RF, Spain KK (2017) Impact of 4 weeks of interval training on resting metabolic rate, fitness, and health-related outcomes. Appl Physiol Nutr Metab 42:1073–1081. https://doi.org/10.1139/apnm-2017-0268
Schumann M, Feuerbacher JF, Sünkeler M et al (2022) Compatibility of concurrent aerobic and strength training for skeletal muscle size and function: an updated systematic review and meta-analysis. Sports Med 52:601–612. https://doi.org/10.1007/s40279-021-01587-7
Scribbans TD, Ma JK, Edgett BA et al (2014) Resveratrol supplementation does not augment performance adaptations or fibre-type-specific responses to high-intensity interval training in humans. Appl Physiol Nutr Metab 39:1305–1313. https://doi.org/10.1139/apnm-2014-0070
Seynnes OR, de Boer M, Narici MV (2007) Early skeletal muscle hypertrophy and architectural changes in response to high-intensity resistance training. Journal of applied physiology. J Appl Physiol 102:368–373. https://doi.org/10.1152/japplphysiol.00789.2006
The IPAQ Group (2005) Guidelines for data processing analysis of the international physical activity questionnaire (IPAQ)—short and long forms. Available online: https://www.sites.google.com/site/theipaq/. Accessed 15 Nov 2022
van der Zwaard S, de Ruiter CJ, Noordhof DA et al (2016) Maximal oxygen uptake is proportional to muscle fiber oxidative capacity, from chronic heart failure patients to professional cyclists. J Appl Physiol 121:636–645. https://doi.org/10.1152/japplphysiol.00355.2016
Vechin FC, Conceição MS, Telles GD, Libardi CA, Ugrinowitsch C (2021) Interference phenomenon with concurrent strength and high-intensity interval training-based aerobic training: an updated model. Sports Med 51:599–605. https://doi.org/10.1007/s40279-020-01421-6
Vinetti G, Taboni A, Bruseghini P et al (2019) Experimental validation of the 3-parameter critical power model in cycling. Eur J Appl Physiol 119:941–949. https://doi.org/10.1007/s00421-019-04083-z
Wagner PD (2015) CrossTalk proposal: Diffusion limitation of O2 from microvessels into muscle does contribute to the limitation of VO2max. J Physiol 593:3757–3758. https://doi.org/10.1113/JP270551
Wagner J, Niemeyer M, Infanger D et al (2020) New data-based cutoffs for maximal exercise criteria across the lifespan. Med Sci Sports Exerc 52:1915–1923. https://doi.org/10.1249/MSS.0000000000002344
Wittekind SG, Powell AW, Opotowsky AR et al (2020) Skeletal muscle mass is linked to cardiorespiratory fitness in youth. Med Sci Sports Exerc 52:2574–2580. https://doi.org/10.1249/MSS.0000000000002424
Zuccarelli L, Porcelli S, Rasica L, Marzorati M, Grassi B (2018) Comparison between slow components of HR and VO2 kinetics: functional significance. Med Sci Sports Exerc 50:1649–1657. https://doi.org/10.1249/MSS.0000000000001612
Acknowledgements
The authors would like to express our gratitude to the participants in this study. We would also extend our gratitude to the technical guidance provided by Miss. Xi Zhang and to the experimental design assistance provided by Mr. Chao Lan.
Author information
Authors and Affiliations
Contributions
YJL and YW conceived and designed the research; YJL, YCX and FYL performed experiments; XXZ, AYZ and TY analyzed data; YJL, YCX and XXZ interpreted results of experiments; XXZ, YBY and YHZ prepared figures and tables; YJL, FYL and TY drafted manuscript; YJL, YCX and YW edited and revised manuscript; All authors approved final version of manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest relevant to this article.
Additional information
Communicated by Guido Ferretti.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, Y., Xia, Y., Yue, T. et al. Adaptations to 4 weeks of high-intensity interval training in healthy adults with different training backgrounds. Eur J Appl Physiol 123, 1283–1297 (2023). https://doi.org/10.1007/s00421-023-05152-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-023-05152-0