Abstract
Peripheral fatigue results from multiple electrochemical and mechanical events in the cell body and the muscle–tendon complex. Combined force and surface electromyographic signal analysis is among the most widely used approaches to describe the behaviour of a fatigued muscle. Advances in technologies and methodological procedures (e.g. laser diffraction, 31P magnetic resonance spectroscopy, shear-wave elastography, tensiomyography, myotonometry, mechanomyography, and high-density surface electromyography) have expanded our knowledge of muscle behaviour before, during, and after a fatiguing task. This review gives an update on recent developments in technologies for investigating the effects of peripheral fatigue linked to skeletal muscle contraction and on mechanistic insights into the electrochemical and mechanical aspects of fatigue. The salient points from the literature analysis are: (1) the electrochemical and mechanical events in the cell (alterations in cross-bridge formation and function and in depolarization of the tubular membrane) precede the events taking place at the muscle–tendon complex (decrease in muscle–tendon unit stiffness); (2) the changes in the fatigued muscle are not homogenous along its length and width but rather reflect a functional compartmentalisation that counteracts the decline in performance; (3) fatigue induces changes in load sharing among adjacent/synergistic muscles. A focus of future studies is to observe how these regional differences occur within single muscle fibres. To do this, a combination of different approaches may yield new insights into the mechanisms underlying muscle fatigue and how the muscle counteracts fatigue.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Peripheral fatigue is defined as a reduction in force-generating capacity induced by skeletal muscle work (Gandevia 2001; Fitts 2008; Place et al. 2010). It has been extensively investigated: a literature search we conducted using the keywords “peripheral fatigue” or “skeletal muscle fatigue” retrieved 4584 studies in PubMed, 450 of which were reviews published over the last 10 years up to December 2018.
Peripheral fatigue affects the electrochemical and mechanical mechanisms downstream of the neuromuscular junction to the force transmission at the tendon insertion point (Allen et al. 2008; Fitts 2008; Westerblad 2016). Fatigue-induced alterations may occur locally at the cellular level and the muscle–tendon complex (MTC). Among electrochemical factors, the major mechanisms affected by the development of peripheral fatigue are alterations in (1) action potential synaptic transmission; (2) sarcolemmal action potential propagation properties, and Ca2+ release from the sarcoplasmic reticulum; and (3) excitation–contraction coupling, cross-bridge kinetics, and Ca2+ re-uptake. Among mechanical factors, peripheral fatigue seems to influence the force transmission from the muscle to the tendon insertion point by altering the mechanical properties of the MTC and other tissues surrounding a joint (e.g. altered viscoelasticity and stiffness of the system). These electrochemical and mechanical events concur to generate afferent feedback to the spinal cord, which then modulates motor drive and final force output.
The physiology of peripheral fatigue has been investigated mainly in isolated muscles or intact/skinned fibre models, which offer a very detailed description of the cellular aspects of peripheral fatigue (Fitts 1994; Allen et al. 2008), and in vivo, to investigate fatigue in an ecological environment (Kent-Braun et al. 2012). A widely used method to investigate peripheral fatigue in vivo is by a combined analysis of force and surface electromyographic (sEMG) signals (Scherrer and Bourguignon 1959; Asmussen 1979; Bigland-Ritchie 1979; Moritani et al. 1982; Petrofsky and Phillips 1985; Merletti et al. 1990; Goonetilleke et al. 1994). Advances in technologies and methodological procedures, including laser diffraction (Udaka et al. 2011; Nelson et al. 2014), 31P magnetic resonance spectroscopy (31P MRS) (Kent-Braun 1999; Bendahan et al. 2004), shear-wave elastography (SWE) (Bercoff et al. 2004; Gennisson et al. 2005; Hug et al. 2015), accelerometers [e.g. tensiomyography (TMG) (Valenčič and Knez 1997; Dahmane et al. 2005; Wang et al. 2017a), myotonometry (Bizzini and Mannion 2003; Wang et al. 2017a, b), mechanomyography (MMG) (Orizio et al. 2003; Cè et al. 2015a)], and high-density surface EMG (HD-sEMG) (Farina et al. 2008; Merletti et al. 2008; Gallina et al. 2011; Jordanic et al. 2016) have expanded our knowledge of the contribution of electrochemical and mechanical factors to force output impairment during and after a fatiguing task.
In light of the new understanding provided by these recent means, the present review gives an update on the effects of peripheral fatigue linked to skeletal muscle contraction and highlights mechanistic insights into the electrochemical and mechanical aspects underlying peripheral fatigue.
Methods
We searched the electronic databases PubMed, Scopus, Web of Science, Embase, and Google Scholar for relevant articles published until October 2018 using the following key words: laser diffraction, sarcomere; magnetic resonance spectroscopy, MRS, 31P MRS; shear-wave elastography, SWE; tensiomyography, TMG, myotonometry, myoton, mechanomyogram, mechanomyography, MMG; high-density surface EMG, EMG matrix array, electromyography matrix array, and HD-sEMG. All keywords were combined with: neuromuscular, skeletal muscle, stiffness, muscle, tendon, fatigue, peripheral fatigue, using #AND and #OR Boolean operators. Only articles (e.g. journal articles, conference proceedings, and clinical reports) published in English were selected and their bibliographies gleaned for potentially eligible studies (Fig. 1).
The following sections describe the recent technologies that have helped to define new mechanistic insights into peripheral muscle fatigue. The main parameters for each technology are detailed: how they are measured, their strengths and limitations, and the innovative contribution each has provided to advancing our knowledge of peripheral fatigue. The technologies will be presented starting from investigation at the level of the muscle fibre (laser diffraction, 31P MRS), followed by the mechanical events in the whole muscle and the MTC (SWE, TMG, myotonometry, MMG), and finally the changes in motor unit recruitment strategies induced by peripheral fatigue (MMG, HD-sEMG).
Laser diffraction
Description
Laser diffraction is a well-established technique that measures sarcomere length and its variability within a given volume (Patel et al. 2004). The technique dates to the late 1960s (Edman 1966; Kawai and Kuntz 1973) and was implemented in the late 1980s (Goldman 1987). Briefly, the repetitive pattern of actin and myosin filament arrays in skeletal muscle creates a periodic distribution of light and dark bands of different refractive indexes that can be detected using a photodiode array (Fig. 2). When the helium–neon laser beam emitted by the electronic microscope hits a muscle fibre sample, it is diffracted. The resulting periodical gradient of the refractive indexes produces a series of hyperbolic diffracted lines that align with the axis of the major fibres (Kawai and Kuntz 1973). The intensity and phase of the diffraction lines are analysed by an operational amplifier to obtain the average sarcomere length at a resolution of 50 Å (Kawai and Kuntz 1973). The diffracted intensity is a function of sarcomere length, angle of incidence, and fibre contraction (Baskin et al. 1979, 1981).

With permission from O’Connor et al. (2016)
Laser diffraction. a Motorized laser diffraction device. Two motors move a stage holding a slide-mounted muscle section through a laser beam path (red line). A photodiode array measures the diffraction pattern at each sample location. b Example diffraction signal (black line) of intensity (volts) as a function of position along the photodiode array. Custom software automatically identifies peak locations (red dots) and calculates sarcomere length (distance between two consecutive red dots). c Sagittal plane of longitudinal section of a rat’s tibialis anterior muscle transilluminated with visible light in which white light diffraction is largely apparent as a rainbow pattern
Generally, laser diffraction has been used to assess the relationship between sarcomere length and the force generated during fibre contraction (Edman et al. 1985; Rassier and Macintosh 2002; MacIntosh 2017) and relaxation phases (Edman and Flitney 1982). The length–tension relationship of the sarcomere was also the focus of studies investigating how and to what extent sarcomere length enhances contractile response (i.e. activity-dependent potentiation), a phenomenon that has been ascribed to increased [Ca2+] sensitivity due to the proximity of myofilaments (Rassier and Macintosh 2002; MacIntosh 2017).
Strengths and limitations
Recent advances in the technique have enabled researchers to investigate muscle behaviour through the integration of muscle structure and function, with the main advantage of obtaining information about active force production and stiffness in muscle fibre by measuring fibre length (O’Connor et al. 2016). However, although up to 2000 sarcomeres can be examined with this technique, it does not provide any information about the entire muscle length, particularly about the sarcomeres at the muscle extremities (O’Connor et al. 2016). To address this issue, a new automated laser device that measures sarcomere length over a large muscle area (O’Connor et al. 2016) generates a two-dimensional map of the muscle (tibialis anterior), revealing systematic differences in sarcomere length between the superficial and the deep muscles.
Contribution to peripheral fatigue
Laser diffraction can be used to determine relatively small changes in the segment length of a single muscle fibre and calculate its stiffness during fatiguing (e.g. tetanic) contractions (Edman and Lou 1990, 1992). When moderate fatigue was induced in isolated frog’s muscle fibres by tetanic stimulation every 15 s, tetanic force and stiffness (25% and 9%, respectively), as well as the rate of increase in force and stiffness were found to be differentially reduced. Because muscle fibre stiffness is proportional to the number of active cross-bridges, these results suggest that the fatigue-induced decrease in fibre force was only partially due to a reduction in the number of active cross-bridges. More likely, the major portion of the force decline could be ascribed to a reduced average force output for each individual cross-bridge (Edman and Lou 1990, 1992). However, when severe fatigue was induced by applying single twitches at 1–2-s intervals (Edman and Lou 1992), tetanic force and stiffness decreased to a greater extent compared to the moderate fatiguing protocol (− 60% and − 39%, respectively) (Edman and Lou 1990, 1992). In a high-frequency stimulation protocol, fatigue-induced reduction in maximum force was suggested to be largely due to a reduction in the number of active cross-bridges, together with a reduced average force output per single cross-bridge. A more recent study conducted in rat muscle fibres, in which a fatigued state was simulated by manipulating pH and Pi concentrations (6.2 and 30 mM, respectively) at 30 °C, observed that the reduction in muscle fibre peak power was not accompanied by any alteration in stiffness (Nelson et al. 2014). A possible explanation for the reduction in fibre peak power is that fatigue reduced the force per high-force cross-bridge and/or increased the number of low-force cross-bridges rather than altering the number of active cross-bridges (Nelson et al. 2014).
Magnetic resonance spectroscopy (MRS)
Description
MRS provides information on chemicals in tissue or metabolites based on the principle that the electron distribution around an atom causes the nuclei of molecules to slightly change magnetic orientation. This characteristic creates small resonant frequencies, which, in turn emit a slightly different signal. Metabolite peaks are identified by their position in the spectrum (i.e. their frequencies) and are expressed as a shift in frequency relative to a standard (Qayyum 2009). The nuclei commonly used for in vivo MRS are protons (1H), sodium (23Na), carbon (13Ca), and phosphorus (31P). When a radiofrequency pulse is emitted, the signal from the tissue is measured and then Fourier transformed. In MRS, the frequency (i.e. the position along the x-axis) is employed to separate and describe the actual metabolites or chemicals within a voxel (Qayyum 2009). Further chemical characteristics of the tissue are obtained from the signal intensity (i.e. the position along the y-axis) and the line width. The latter may be used to estimate the relative quantity of the chemical (Qayyum 2009). Among the nuclei employed in MRS analysis, 31P allows for non-invasive investigation of muscle metabolism (Hoult et al. 1974). It has been used for the evaluation of changes in high-energy phosphate compound concentrations and intracellular pH. When a typical 31P MRS spectrum at rest is examined, different peaks corresponding to Pi, PCr, and the three ATP phosphate groups (α-, β-, and γ-ATP) can be detected (Fig. 3). Phosphomonoester and phosphodiester peaks may be also visible, and intracellular pH can be measured from the chemical shift of the Pi peak (Moon and Richards 1973). Moreover, 31P MRS also allows estimation of ADP and AMP concentrations (Roth and Weiner 1991).

With permission from Bendahan et al. (2004)
Magnetic resonance spectroscopy. a The sample is placed in a magnetic field and the MRS signal is produced by excitation of the nuclei by radio waves and converted into nuclear magnetic resonance, which is detected by radio receivers. The intramolecular magnetic field surrounding an atom changes the resonance frequency, thus giving details on the electronic structure of a molecule and its individual functional groups. b Typical series of 31P magnetic resonance spectra recorded with a time resolution of 2 s at the end of an exercise (first spectrum, bottom) of the thigh muscles and during the following recovery period. Top spectrum: ref reference compound, PME phosphomonoesters, PDE phosphodiesters, Pi inorganic phosphate, PCr phosphocreatine, α, β, γ phosphate groups of ATP. Horizontal axis shows the chemical shifts expressed in Hz
Strengths and limitations
31P MRS can be helpful in assessing the role of bioenergetics in the development of muscle fatigue in health and disease in vivo (Bendahan et al. 2004; Tonon et al. 2012). One of its main limitations is that it does not allow estimation of single fibre-type contribution to peripheral fatigue. Indeed, 31P MRS provides values from a mixture of fibres with different metabolic characteristics. A further limitation is the difficulty to detect metabolite concentrations less than 0.5 mM. Moreover, the procedure can be long, the data analysis is time-consuming and requires expertise in physics and biochemistry. Lastly, due to the high equipment costs and cost of the exam itself, its availability is still limited.
Contribution to peripheral fatigue
One of the main contributions of 31P MRS to the study of peripheral fatigue is the description of time-dependent changes in Pi, PCr, and pH during a fatiguing protocol in vivo. Typically, the force decline observed during a maximum and a submaximum sustained isometric (Miller et al. 1987; Cady et al. 1989a, b; Kent-Braun 1999), an intermittent isometric (Miller et al. 1988), and a dynamic (Wilson et al. 1988) fatiguing protocol was accompanied by an accumulation of Pi and/or its diprotonated form H2PO4−, a decrease in PCr, and a decrease in pH (Miller et al. 1987, 1988; Nosek et al. 1987; Wilson et al. 1988; Cady et al. 1989a, b; Kent-Braun 1999). These findings posed the question of whether or not Pi accumulation and/or intracellular acidosis was responsible for the development of muscle fatigue. As far as Pi is concerned, 31P MRS revealed that its accumulation follows two phases: a rapid phase within the 1st min of contraction followed by a slow phase, during which [Pi] reaches a steady state (Degroot et al. 1993). It has been hypothesized that Pi accumulation may inhibit the transition from low-force to high-force cross-bridges, and/or reduce the force of high-force cross-bridges (Palmer and Kentish 1994; Debold 2006). This is thought to occur through disruption of ATP hydrolysis at myosin ATPase (Wilkie 1986; Nosek et al. 1987; Wilson et al. 1988; Westerblad and Allen 2003; Kent-Braun et al. 2012). However, despite acknowledging their important role, other studies in humans have questioned the direct effect of Pi and/or H2PO4− accumulation at the cross-bridge level on fatigue-induced force decline (Kent-Braun 1999; Rico-Sanz 2003). For instance, Kent-Braun (1999) showed a force decline even when [Pi] and [H2PO4−] reached a steady level. Furthermore, Rico-Sanz (2003) found that [Pi] decreased when the number of bouts increased during repeated, intense, dynamic exercise. These observations suggested that the role of Pi and/or H2PO4− accumulation may be mitigated by other factors that contribute to force inhibition (Kent-Braun 1999; Rico-Sanz 2003; Bendahan et al. 2004). Indeed, as demonstrated by previous in vitro studies, Pi may interfere also with transient Ca2+ released from the sarcoplasmic reticulum (Fryer et al. 1997; Allen and Westerblad 2001; Kent-Braun et al. 2002).
Regarding acidosis, 31P MRS has expanded our knowledge of the role of H+ accumulation in generating a fatigued state. The relationship between pH lowering during fatiguing contractions and force decline has been well established by in vitro studies (Fitts 1994, 2016; Kent-Braun et al. 2012). However, the question remains open whether or not the fatigue-induced acidosis in mammalian muscle fibres at physiological temperatures has a minor or a pivotal role in force decline during sustained or intermittent contractions (Fitts 1994, 2016; Kent-Braun 1999; Kent-Braun et al. 2012; Westerblad 2016). 31P MRS studies conducted in vivo in humans did not consistently highlight such a relationship, questioning the role of H+ accumulation in the aetiology of peripheral fatigue (Fitts 2016; Westerblad 2016). For example, some studies showed that fatigue-induced pH lowering (as low as ~ 6.2) during fatiguing contractions was correlated with force decline in both sustained and intermittent contractions (Taylor et al. 1986; Miller et al. 1988; Wilson et al. 1988). According to the in vitro studies, it seems that H+ accumulation could interfere at several sites involved in force generation (Fitts 2008). An exhaustive examination of these aspects is beyond the scope of the present review. Nonetheless, the main factors can be summarised as follows: (1) a reduction in the number of bridges and/or force per bridge generated from high-force cross-bridges (Nocella et al. 2011); (2) a reduction in the contraction velocity when pH is below 6.7 presumably due to a decrease in the rate of ADP release (Fitts 2008); and (3) reduced myofibrillar Ca2+ sensitivity (Fitts 1994, 2008, 2016). Furthermore, it is thought that pH provides feedback signals to the central nervous system and alters the central motor drive during the development of fatigue (Wilson et al. 1988; Kent-Braun 1999; Bendahan et al. 2004). Conversely, other 31P MRS studies conducted in humans demonstrated a dissociation between pH and changes in force during the development of fatigue and its recovery. It has been observed that minor intracellular acidification or transient alkalinisation may be present, and that the fatigue-induced force decline can recover without a recovery of pH (Sahlin and Ren 1989; Degroot et al. 1993; Kent-Braun 1999; Westerblad 2016). The role of acidosis in explaining the fatigue-induced effects on force decline in vivo in humans is still controversial. Lowering of pH might have only minor direct effects on the development of peripheral fatigue or act in an indirect manner together with other factors involved in the development of fatigue (Westerblad 2016).
Shear-wave elastography (SWE)
Description
Elastography is a recently developed ultrasound technology that started about 20 years ago. It is used for imaging tissue stiffness (e.g., muscle) in either passive or active condition (Fig. 4). Stiffness can be estimated from either analysis of the strain in a tissue under stress (quasi-static methods) or by imaging the mechanical waves (i.e., shear waves) propagated in the direction of the tissue stiffness (Gennisson et al. 2013).

With permission from Bouillard et al. (2014)
Shear-wave elastography. Typical maps of shear elastic modulus. The coloured region represents the shear elasticity map for the rectus femoris (a), vastus lateralis (b), and vastus medialis (c) muscles (blue denotes soft and red denotes stiff). To obtain a representative value, the shear elastic modulus (in kPa) is averaged over the greatest muscle area (white rectangles), avoiding hypoechoic regions and aponeurosis
Supersonic shear imaging, the most recent among elastography techniques (Gennisson et al. 2013; Bercoff et al. 2004), measures reliable quantitative passive and active tissue stiffness rapidly (Hug et al. 2015). Mechanical perturbations are created by a focused ultrasonic beam (i.e., “pushing beam”) to the tissue; muscle elasticity is then measured by calculating the velocity of the shear waves. The velocity is directly related to the shear modulus of the tissue, i.e., propagation of the shear wave will be faster in stiffer tissues than in more compliant tissues. Shear modulus is defined by the formula:
where \(\mu\) is the modulus in kPa, \(\rho\) is the muscle density (1000 kg m−3), and \(V_{\text{s}}\) is the shear-wave velocity in m s−1. The shear modulus is mapped onto a B-mode ultrasound image within a region of interest, with a coloured scale depicting its gradation from deep blue (most compliant) to dark red (most stiff). As a representative value, the shear modulus is averaged over the whole map.
The technique has been recently validated by directly comparing the shear modulus and Young’s modulus in animal muscles (Eby et al. 2013). The study also highlighted the sensitivity of SWE in characterising the anisotropic properties of muscle tissues (Eby et al. 2013). Besides muscle stiffness, shear-wave modulus is also linearly related to both passive (Maïsetti et al. 2012; Koo et al. 2013) and active forces (Bouillard et al. 2012). SWE can therefore be used to indirectly infer changes in force due to passive stretching and active muscle contraction.
Strengths and limitations
One of the main strengths of SWE is that the shear-wave modulus is a mechanical property; so it is less likely to be influenced by neuromuscular fatigue than sEMG, which is affected by electrophysiological parameters such as the velocity of motor unit action potential propagation. This characteristic makes SWE a very interesting technique for observing the changes in force, active muscle stiffness, and load sharing among synergistic muscles during and after fatiguing contractions, as well as passive muscle stiffness after a fatiguing task. Moreover, SWE allows investigators to separate the muscle contribution of MTC stiffness from the other components. One of the disadvantages of SWE is the high cost of the ultrasound equipment. From a methodological point of view, the modulus depends mainly on probe orientation and the pressure applied to the skin surface by the transducer; the technique requires experienced operators for reliable performance. A further limitation is that SWE does not directly quantify force but rather measures the changes in force.
Contribution to peripheral fatigue
SWE has been employed to observe possible fatigue-induced alterations in the shear-wave modulus of the muscle itself (Nordez et al. 2009; Bouillard et al. 2012, 2014; Nair et al. 2016; Akagi et al. 2017; Sadeghi et al. 2018). Some studies reported a decrease in modulus during (Bouillard et al. 2012, 2014) and immediately after (Nordez et al. 2009) a fatiguing protocol based on isometric contractions. Similar results were also found at 1 h (Andonian et al. 2016) and at 24 h (Sadeghi et al. 2018) after a prolonged running task. The authors hypothesized that the changes in modulus may reflect the changes in intrinsic mechanical properties of muscles and their ability to transmit force to the tendon (Nordez et al. 2009; Bouillard et al. 2012, 2014; Andonian et al. 2016; Sadeghi et al. 2018). However, when assessing fatigue-induced changes in shear wave in synergistic muscles, modulus did not follow a common pathway between the plantar flexors (Akagi et al. 2017) and the knee extensor muscles (Bouillard et al. 2012, 2014). For instance, gastrocnemius medialis shear-wave modulus was increased within 2 min after a prolonged, low-intensity isometric contraction, whereas the soleus and gastrocnemius lateralis were decreased and unchanged, respectively (Akagi et al. 2017). Similarly, changes in non-uniform shear-wave modulus direction were also observed in the vastus lateralis, vastus medialis, and rectus femoris muscles during low-intensity sustained (Bouillard et al. 2012) or intermittent (Bouillard et al. 2014) isometric contractions. These results provided evidence for fatigue-induced changes in load-sharing strategies between synergistic muscles. Nevertheless, since the technique is quite recent, further studies are needed to understand possible fatigue-induced changes in muscle mechanical properties during and after a fatiguing protocol.
Tensiomyography (TMG)
Description
TMG is a non-invasive technique developed in the last 15 years to assess the mechanical properties of skeletal muscle (Dahmane et al. 2001, 2005; Pišot et al. 2008; Carrasco et al. 2011; Hunter et al. 2012; de Paula Simola et al. 2015). It is based on the radial deformation of the muscle belly and the time that the deformation takes during a single twitch (Valenčič and Knez 1997). Two surface electrodes are placed proximal and distal to the sensor tip of a magnetic transducer pressed against the muscle belly perpendicularly to the skin. Incorporated in the transducer is a built-in spring (maximum backward force of 4.2 N) that provides initial controlled pressure on the surface of the muscle belly. After a single electrical stimulation, the muscle belly enlarges, and the sensor tip measures the radial displacement associated with the time of contraction (Fig. 5).

With permission from de Paula Simola et al. (2015)
Tensiomyography. a Tensiomyography system positioning during rectus femoris measurement. b Signal analysis from the radial twitch responses as a function of time in muscle mechanical properties: (1) Dm, maximum radial deformation of the muscle belly; (2) Td, delay time calculated from the onset of electrical stimulus to 10% Dm; (3) Tc, contraction time calculated from 10 to 90% Dm; (4) Ts, sustained time calculated as the time between 50% Dm during muscle contraction and relaxation; and (5) Tr, relaxation time calculated from time from 90 to 50% Dm
The following parameters are retrieved from the signal analysis: displacement (mm), maximum radial deformation of the muscle belly during contraction; delay time (ms) calculated from the onset of the electrical stimulus to 10% of maximum radial displacement; contraction time (ms) calculated from 10 to 90% of maximum displacement; sustained time (ms) defined as the time between 50% of maximum displacement during a muscle contraction and 50% of maximum displacement during a muscle relaxation phase; and half-relaxation time (ms) calculated from the time from 90 to 50% of maximum displacement during muscle relaxation. The two most informative TMG parameters are displacement and contraction time. Displacement is commonly considered an indirect measure of active MTC stiffness (Šimunič et al. 2011; García-García et al. 2013, 2015) and muscle fibre activation (Carrasco et al. 2011; Hunter et al. 2012; de Paula Simola et al. 2015), whereas contraction time is an indirect marker of muscle fibre contraction time (de Paula Simola et al. 2015).
TMG has been used to investigate the effects of strength tasks (García-Manso et al. 2012; Hunter et al. 2012; de Paula Simola et al. 2015), endurance exercises (García-Manso et al. 2011), and bed rest (Pišot et al. 2008) on the mechanical properties of skeletal muscle. It has also been employed to indirectly estimate the proportion of fibre types within a muscle (Dahmane et al. 2005; Šimunič et al. 2011) and to monitor changes in passive tension associated with changes in muscle length (Ditroilo et al. 2011). Interestingly, TMG has also been used to measure changes in displacement and contraction time associated with peripheral fatigue as an index of changes in fatigue-induced muscle mechanical properties (García-Manso et al. 2011; Macgregor et al. 2016).
Strengths and limitations
TMG is a rapid, non-invasive technique operators can use to examine muscle contractile properties by means of a portable device (de Paula Simola et al. 2015). The superficial bellies of synergistic muscles can be assessed individually (García-García et al. 2015). TMG measurements do not require dedicated post-processing analysis or filtering (Križaj et al. 2008) and may be only marginally affected by longitudinal tendon, ligament, serial connective tissue compliance, joint friction, and extremity inertia (Dahmane et al. 2005). A limitation is that TMG parameters differ in reliability, i.e. displacement and contraction time are more reliable than the other parameters, depending on the testing condition (Tous-Fajardo et al. 2010; Šimunič 2012; Ditroilo et al. 2013; Macgregor et al. 2018). Moreover, TMG parameters seem to be less sensitive to fatigue detection after high-intensity interval (Wiewelhove et al. 2017) or intensive endurance training (de Paula Simola et al. 2016).
Contribution to peripheral fatigue
To date, the effects of peripheral fatigue evaluated by TMG have been described in four articles. Three studies reported an increase in maximum displacement of the muscle belly (Carrasco et al. 2011; García-Manso et al. 2011; Giovanelli et al. 2016), regardless of the muscle examined. An increased displacement was found in the biceps femoris (García-Manso et al. 2011) and the vastus lateralis (Giovanelli et al. 2016) within 15 min after ultra-endurance races, as well as in the rectus femoris immediately after a 2-min cycling trial at a power corresponding to maximum oxygen uptake (Carrasco et al. 2011). These results were explained by a fatigue-induced reduction in muscle stiffness (Carrasco et al. 2011; García-Manso et al. 2011; Giovanelli et al. 2016; Macgregor et al. 2018). However, when peripheral fatigue was induced by electrical stimulation of the gastrocnemius medialis (Macgregor et al. 2016), the maximum muscle belly displacement was decreased. The reasons for the discrepancy in previous results may be attributable to the type of fatiguing protocol and the possible contribution of central fatigue, which was not elicited in the study by Macgregor et al. (2016).
Myotonometry
Description
A myotonometer is a small, hand-held, non-invasive device that incorporates a triaxial accelerometer that indirectly measures muscle mechanical properties, such as tone, passive muscle stiffness and elasticity (Bizzini and Mannion 2003; Viir et al. 2007; Aird et al. 2012; Marusiak et al. 2012). The probe is held perpendicularly against the skin surface above the targeted muscle. Besides the controlled pre-load of 0.18 N, an additional mechanical force of 0.40 N lasting 15 ms is automatically generated that induces damped or decaying natural oscillation of the tissue. The time of the exerted mechanical force is operator set to avert neural reaction and non-elastic deformation of the tissue (Viir et al. 2007). The accelerometer measures the peak acceleration of the natural oscillation. Velocity is calculated by the first integration of the acceleration signal, while displacement is calculated by the second integration (Fig. 6). After signal acquisition, the mechanical parameters can be estimated. Analysis of oscillation frequency provides an indirect estimation of muscle tone (i.e., intrinsic tension), with high-frequency oscillation related to high muscle tone. Passive stiffness is the resistance opposing the applied external force. Passive stiffness can be calculated with the formula:
where m is the 0.18 N pre-load of the probe, a is the maximum amplitude of oscillation in the acceleration signal, and Δl is the amplitude of the displacement signal at the end of the impulse time. Elasticity refers to the ability of a muscle to recover its initial shape after a perturbation. It is estimated by calculating the logarithmic reduction in a muscle’s natural oscillation, with a small reduction associated with high muscle elasticity.
Strengths and limitations
Studies have reported high reliability intraclass correlation coefficient > 0.80) for myotonometric parameters in different muscles (Bizzini and Mannion 2003; Zinder and Padua 2011; Aird et al. 2012; Liu et al. 2018; Van Deun et al. 2018). The main advantages are its accessible costs, portability, and velocity of measurement. Conversely, a major limitation is the lack of depth of perturbation on the skin (2 cm) (Nair et al. 2016), which means that the mechanical properties can be assessed only in superficial muscles. Another limitation is the interference during measurements from the subcutaneous tissue over the muscle of interest (Kelly et al. 2018). In body areas with thick subcutaneous tissue, measurement validity and reliability may be reduced and the mechanical signal may be related more to the properties of the subcutaneous tissue than to the muscle.
Contribution to peripheral fatigue
Studies using myotonometry to evaluate fatigue-induced changes in passive stiffness (Wang et al. 2017a, b) have found muscle stiffness to be increased immediately after fatiguing protocols, such as prolonged and isokinetic concentric/eccentric contractions (Wang et al. 2017b), running (Wang et al. 2017b), and cycling (Wang et al. 2017a). The explanation was possible mechanisms linked to (1) acidification occurring during fatigue (Curtin and Edman 1989; Edman and Lou 1990; Zhang and Rymer 2001); (2) reduction in cross-bridge detachment rate causing slower muscle relaxation (Allen et al. 2008; Fitts 2008), possibly contributing to increased passive tension and resting muscle stiffness (Akagi et al. 2017); and (3) increase in motor drive and resultant rise in the firing rate and/or recruitment of motor units, possibly triggered by the accumulation of fatigue-induced intramuscular metabolites (Amann and Dempsey 2008; Decorte et al. 2012). The assessment approach (i.e., SWE vs. TMG vs. myotonometry) and the type of fatiguing protocol more likely explain the discrepancies in muscle stiffness changes across studies using different technologies to investigate this variable.
Mechanomyography (MMG)
Description
MMG is used to non-invasively study mechanical activity in skeletal muscle (Orizio 1993; Orizio et al. 2003; Cè et al. 2015a). The MMG signal is detectable at the skin surface via dimensional changes of active muscle fibres during contraction. The fibres generate pressure waves due to their cyclic shortening when voluntarily activated or electrically evoked. These waves can be detected superficially by specific transducers that record the muscle oscillations related to the mechanical activity of motor units (Orizio et al. 2003; Cè et al. 2015a). Several types of transducers can be used, such as piezo-electric contact sensors, microphones, laser distance sensors, electric condenser microphones, and accelerometers, which are the most widely used types of sensor for MMG.
The time-domain (MMG peak-to-peak, root mean square, and averaged rectified value) and the frequency-domain parameters (mean frequency, mean power–frequency, and median frequency) are calculated from analysis of the MMG signal (Fig. 7). MMG peak-to-peak is the maximal transverse oscillation of the muscle belly induced by shortening of the contractile elements during the on phase of a muscle contraction (Longo et al. 2014). MMG peak-to-peak is generally associated with whole muscle activation, i.e. the greater the muscle activation, the wider the MMG amplitude. The MMG root mean square and the averaged rectified value calculated from oscillation during the force plateau indirectly reflect both the number of active motor units (Orizio et al. 2003) and the mechanical characteristics of the contractile and viscoelastic components during muscle contraction (Longo et al. 2014). The MMG frequency characteristics indirectly mirror the mean firing rate of the pool of motor units recruited during muscle contraction (Orizio et al. 2003). Analysis of the MMG signal provides an indirect characterization of the neuromuscular strategies adopted by the contracting muscle to activate and modulate force output during muscle contraction (Orizio 1993; Orizio et al. 2003; Cè et al. 2015a).

With permission from Cè et al. (2015a)
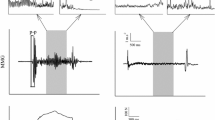
Mechanomyography. a Sensor (microphone/accelerometer) positioning for mechanomyographic signal detection. b Upper graph: time- and frequency-domain analysis of mechanomyographic signal during voluntary (left column) and electrically evoked contraction (right column). RMS root mean square, ARV averaged rectified value, p–p peak-to-peak, MF mean frequency, MPF mean power–frequency, MNF median frequency. (Lower graph) force signals during voluntary (left column) and electrically evoked contraction (right column)
Analysis of the MMG signal in combination with sEMG and force signal detection allows for a more detailed identification of the electromechanical delay (EMD) components (Cè et al. 2013, 2014, 2015b, 2017; Rampichini et al. 2014; Longo et al. 2016, 2017; Smith et al. 2017a, b). A delay exists between the onset of muscle activation and the beginning of force development, and between the end of muscle activation and the return of the force output toward pre-activation values. These time lags are termed EMD (Cavanagh and Komi 1979) and relaxation EMD (R-EMD), respectively (Viitasalo and Komi 1981; Ferris-Hood et al. 1996). They are calculated from the onset of the sEMG and of the force signal (for determining EMD) and from the offset of the sEMG signal to the return of the force signal to baseline (for determining R-EMD). Analysis of the MMG signal allows for partitioning of EMD and R-EMD into a “mainly” electrochemical component and a “mainly” mechanical component. In the EMD, the electrochemical component principally includes the events linked to excitation–contraction coupling and pressure wave transmission to the skin surface detected at the onset of the MMG signal. The mechanical component reflects the time needed to take up the muscle–tendon unit slack before force transmission becomes effective at the tendon insertion point (Hufschmidt 1985; Esposito et al. 2011; Sasaki et al. 2011; Cè et al. 2013) (Fig. 8). During the relaxation phase, the electrochemical component comprises mainly (1) cessation of action potential propagation of the motor unit; (2) Ca2+ re-uptake by the sarcoplasmic reticulum and blocking of acto-myosin interaction by troponin and tropomyosin; and (3) cross-bridge switch from a strongly to a weakly bound status. The mechanical component comprises the return of sarcomeres to their resting length and release of the series elastic components that were stretched during contraction.

With permission from Longo et al. (2017)
Electromechanical delay. a Diagram of the experimental setup for tibialis anterior (left panel) and vastus lateralis (right panel) muscle assessment. Positioning of the participant on the ergometer, the electromyographic (EMG) linear array electrodes, accelerometer, stimulation electrodes, and force transducer. With permission from Esposito et al. (2016). b EMG, mechanomyographic (MMG), force (F) signals and delays in a representative participant. Contraction phase (left panel): the solid, dotted, and short dashed lines indicate the onset of EMG, MMG, and F signals, respectively. Relaxation phase (right panel): the solid, dotted, short dashed, medium dashed, and dashed and dotted lines indicate the EMG signal offset, the beginning of F decay, the beginning of maximum displacement of the MMG signal (MMGp-p), the duration of MMGp-p and the return of F signal to baseline, respectively. Partitioning of the total electromechanical during contraction (DelayTOT): (1) Δt EMG–MMG, mainly electrochemical component, from the onset of EMG to the onset of the MMG complex; and (2) Δt MMG–F, mainly mechanical component, from the MMG complex to F onset. Partitioning of the total electromechanical during relaxation (R-DelayTOT): (1) R–Δt EMG-F, mainly electrochemical component spanning from EMG cessation to the beginning of F decay; (2) R–Δt F–MMGp-p, first the mainly mechanical component, from the beginning of F decay to the beginning of gross lateral movement of the muscle at the end of contraction (R–); (3) R–Δt MMGp-p, second mainly mechanical component from the beginning to the end of maximum displacement of the MMG signal; and (4) R–Δt MMG–Fend, third mainly mechanical component from the end of maximum displacement of the MMG signal to F return to baseline
Strengths and limitations
MMG adds information complementary to data from sEMG signal analysis during muscle contraction. The main advantages are that application of the MMG transducer is less sensitive to sweat, hydration, and skin temperature; moreover, no specific skin preparation is needed. A major limitation when interpreting the results of MMG signal analysis is that attention should be paid to possible confounding factors (e.g., intramuscular pressure, muscle temperature). Differences in intramuscular pressure and muscle temperature (cooling in particular) have been reported to significantly alter both MMG signal amplitude and frequency content (Yoshitake et al. 2001; Blangsted et al. 2005; Vedsted et al. 2006; Cè et al. 2013). Moreover, differences in MMG signal characteristics and relative behaviour of the amplitude and the frequency content during fatigue in the lumbar erector spinae, deltoideus anterior and medialis, and the upper trapezius were reported to depend on accelerometer location and/or the length at which the muscles were tested (Madeleine et al. 2002a, 2007; Mamaghani et al. 2002; Madeleine and Farina 2008; Madeleine 2010).
Contribution to peripheral fatigue
Early studies on MMG and peripheral fatigue date to the late 1980s (Orizio et al. 1989; Goldenberg et al. 1991; Zwarts and Keidel 1991; Orizio 1992; Orizio and Veicsteinas 1992; Rodriguez et al. 1996; Esposito et al. 1998).The authors drew their conclusions about fatigue-induced alterations in motor unit recruitment strategy from analysis of the MMG signal. During low-intensity exhaustive effort, the MMG amplitude and its frequency content increased continuously. This behaviour was related to the recruitment of additional motor units and the synchronization/grouping of active motor units (Shinohara et al. 1998; Madeleine et al. 2002b, 2006; Blangsted et al. 2005). During medium-intensity effort, the MMG signal initially increased in amplitude and frequency content until it plateaued, followed by a decrease in both parameters. This suggests that at the beginning of an exhaustive medium-intensity contraction, the number of active motor units and their mean firing rate increased in the attempt to maintain force output stable. Thereafter, the MMG amplitude diminished until exhaustion. This has been ascribed to enlargement of mechanical twitching, with better fusion of the mechanical events between one motor command and the next, and possibly attributable to an increase in the intramuscular pressure that impairs changes in active muscle fibre dimension. In addition, the MMG mean frequency was shown to decrease, suggesting an overall reduction in the mean firing rate of the pool of recruited motor units (Søgaard et al. 2003; Kimura et al. 2004; Beck et al. 2007, 2009; Jaskólski et al. 2007; Hendrix et al. 2010). During a high-intensity effort to exhaustion, the MMG amplitude diminished from the beginning of muscle contraction, while the mean frequency increased over the first few seconds. This was hypothesized to reflect the attempt to activate fast fatigable-type motor units. The MMG mean frequency then decreased, suggesting an overall reduction in the mean firing rate of the recruited motor units from here on (Esposito et al. 1998; Blangsted et al. 2005; Al-Zahrani et al. 2009; Yang et al. 2009; Limonta et al. 2016). Although with some exceptions (Xie et al. 2009; Yang et al. 2009; Stock et al. 2013), similar results were also found by studies that investigated post-fatigue muscle behaviour under isometric (Shinohara et al. 1998; Esposito et al. 1998, 2003; Kouzaki et al. 1999; Madeleine et al. 2002b, 2006; Orizio et al. 2003; Søgaard et al. 2003; Beck et al. 2004; Kimura et al. 2004; Blangsted et al. 2005; Jaskólski et al. 2007; Kawczyński et al. 2007; Al-Zahrani et al. 2009; Hendrix et al. 2010; Camic et al. 2013; Smith et al. 2016) or isokinetic (Perry-Rana et al. 2002; Kawczyński et al. 2007; Ebersole and Malek 2008; Camic et al. 2014) conditions, as well as during incremental aerobic cycling exercise (Shinohara et al. 1997; Bull et al. 2000; Housh et al. 2000; Kimura et al. 2008; Zuniga et al. 2010).
Regarding determination of the electromechanical delay, measurement of EMD and R-EMD before and after peripheral fatigue evidenced an overall lengthening of both the electrochemical and the mechanical components with a prevalent alteration of the latter. These findings suggest that after a fatiguing task the decrease in force output and the following lengthening in the time needed to return to baseline may have been influenced by the fatigue-induced mechanical alterations in the in-series elastic component characteristics (e.g., decreased stiffness). This was reported for electrically evoked (Cè et al. 2014, 2017; Rampichini et al. 2014; Esposito et al. 2016, 2017), isometric (Cè et al. 2013, 2015b; Esposito et al. 2016), and dynamic voluntary muscle contractions (Smith et al. 2017a, b). Interestingly, during a fatiguing protocol based on isometric contractions, some recent studies found an earlier alteration in the EMD and the electrochemical R-EMD (Cè et al. 2017; Esposito et al. 2017; Smith et al. 2017a), with postponed modifications in the mechanical components. In contrast, Smith et al. (2017a) reported earlier changes in the mechanical EMD compared to the electrochemical components during dynamic fatiguing contractions. In any case, the changes in the mechanical components had an important role in EMD and R-EMD lengthening during the fatiguing protocol. Moreover, during the recovery phase, the mechanical vs. electrochemical components took longer to be fully restored. Though the combination of sEMG, MMG, and force signal analysis does not accurately determine the event/s affected by ongoing peripheral fatigue, the findings suggest that the mechanical alterations occurring at the in-series elastic element could play an important role in the decrease in muscle force output during and after fatigue.
High-density surface EMG (HD-sEMG)
Description
sEMG is a non-invasive technique that measures electrical muscle activity by means of surface electrodes placed on the skin over a muscle or muscle group (Hermens et al. 1999; Drost et al. 2006). It uses a single bipolar signal from two electrodes placed on one muscle and extracts limited information about muscle excitation. With the development of sEMG equipment to record the input of multiple electrodes placed over a single muscle (i.e., HD-sEMG), the characteristics of single motor units can now be measured (Merletti et al. 2003; Zwarts and Stegeman 2003), according to new spatiotemporal parameters concerning the motor units area of excitation and recruitment strategies (Rau and Disselhorst-Klug 1997; Stegeman et al. 2000; Zwarts and Stegeman 2003; Drost et al. 2006; Kleine et al. 2007). HD-sEMG is used (Fig. 9): (1) to observe the position of motor points within the same muscle (Hermens et al. 1999; Merletti et al. 2003) and to identify the best sEMG signals from multiple muscle areas; (2) to check for differences in muscle excitation in areas within the same muscle and/or in synergistic/adjacent muscles (Farina et al. 2008); and (3) to estimate the number of recruited motor units during muscle contraction (i.e., HD-sEMG signal decomposition) (Kleine et al. 2007; Merletti et al. 2008).

With permission from Watanabe et al. (2013)
High-density surface EMG. a Multi-channel surface electromyography electrodes placed over the rectus femoris muscle. b Representative mean normalized root mean square of multi-channel surface electromyography as shown by the colour map for knee extension (upper panel) and hip flexion (lower panel). Root mean square values are normalized by the initial value of the contraction for each channel. Dark blue denotes the initial value of the contraction for each channel and dark red the higher value relative to the initial value
For example, analysis of HD-sEMG maps has shown that the spatial distribution of sEMG amplitude is inhomogeneous (Holtermann et al. 2005), underlining heterogeneity in either motor units distribution within a muscle or motor units recruitment strategy (Holtermann et al. 2005). This could be seen as a kind of functional muscle compartmentalization, a characteristic that seems particularly convenient in muscles with highly diversified biomechanical functions, like the upper trapezius (Farina et al. 2008; Troiano et al. 2008; Gerdle et al. 2010; Samani et al. 2017) and the erector spinae (Tucker et al. 2009; Hu et al. 2010; Abboud et al. 2014, 2016; Ringheim et al. 2014).
Strengths and limitations
This relatively new technique is under development. A very high-quality signal is needed to obtain valid and reliable data. This implies the recruitment of a high number of participants in a study and repetition of the test over time.
Contribution to peripheral fatigue
Studies using HD-sEMG have reported that, during sustained muscle contractions up to fatigue, a spatial adaptation of muscle excitability takes place, as seen in activity response by non-homogenous motor units in different areas within the same muscle and between synergistic/adjacent muscles (Gallina et al. 2011; Watanabe et al. 2013; Abboud et al. 2014; Mista et al. 2014; Ringheim et al. 2014). Two hypotheses have been proposed to explain these heterogeneous spatial muscle adaptations: (1) restricted location of the most fatigable motor units in different regions; and/or (2) a dissimilar increase in motor unit activity in multiple compartments of the same muscle (Gallina et al. 2011; Watanabe et al. 2013; Abboud et al. 2014; Mista et al. 2014; Ringheim et al. 2014). Type II fibres are known to be most susceptible to fatigue (Fitts 2008; Piitulainen et al. 2010). In addition, histologic findings revealed that type II fibres are homogeneously distributed throughout the cross-sectional area of a muscle (Dahmane et al. 2005). It follows then that fatigue-induced differences in spatial adaptations over time would most likely be found in a cranial–caudal rather than in a medial–lateral direction (Watanabe et al. 2013). Moreover, fatigue-induced alterations in the load sharing among synergistic muscles has been advanced as a possible explanation for spatial adaptation (Tucker et al. 2009; Hu et al. 2010; Abboud et al. 2014, 2016; Jordanic et al. 2016).
From a functional point of view, variation in the distribution of motor unit activity within the same muscle or group of related muscles (i.e., spatial distribution) is an important factor in modulation of motor output when muscle fatigue is present (Farina et al. 2008). This mechanism might avert overload of muscle fibres during prolonged contraction, especially in muscles commonly exposed to sustained excitation, such as the lumbar erector spinae (Hu et al. 2010; Ringheim et al. 2014; Abboud et al. 2016).
Conclusion and perspectives
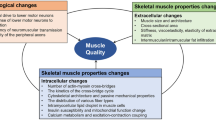
Peripheral fatigue involves changes at the cellular level and in the MTC. This review presents recent technologies and methodological procedures that researchers can apply to better understand the physiological mechanisms underlying peripheral fatigue (Fig. 10).
Diagram of the known mechanisms underlying peripheral fatigue without (Panel A) and with the contribution of recent technologies (Panel B). The black arrows indicate the mechanisms constituting afferent feedback, while the white arrows indicate the changes in motor drive. MTC muscle–tendon complex, MRS magnetic resonance spectroscopy, SWE shear-wave elastography, TMG tensiomyography, MMG mechanomyography, HD-sEMG high-density surface electromyography
Laser diffraction indirectly estimates the number of active cross-bridges by measuring muscle fibre stiffness during fatiguing contractions. When moderate fatigue was induced, the major portion of the decrease in force was ascribed to a reduced average force output of individual cross-bridges. In contrast, when severe fatigue occurred, the reduction in maximum force was largely attributed to a reduction in the number of active cross-bridges, together with a reduced average force output per single cross-bridge. More recently, it has been suggested that fatigue may reduce the force per high-force cross-bridge and/or increase the number of low-force cross-bridges, without influencing the total number of active cross-bridges.
The in vivo 31P MRS studies have corroborated some results of the in vitro investigations and expanded our knowledge about the metabolic aspects of peripheral fatigue via a more “ecological” approach. Within a muscle cell, the accumulation of Pi and/or H2PO4 and a decrease in pH seem to act directly or indirectly to: (1) reduce the number of cross-bridge formations and force per bridge; (2) slow the rate of ADP release with a likely reduction in cross-bridge dissociation velocity; (3) reduce myofibrillar Ca2+ sensitivity, Ca2+ flux release and re-uptake from and to the sarcoplasmic reticulum. However, the role of a reduction of intracellular pH induced by peripheral fatigue is still debated. While it is generally agreed that a lower pH could result in a decrease in muscle fibre force and power, in light of the role attributed to decrease in pH in skinned muscle fibres, the question remains open whether or not a reduction in pH could play a role in contractile function in intact muscle fibres at physiological temperatures.
The use of SWE, TMG, and myotonometry has revealed important alterations in muscle mechanical characteristics, which reflect muscle stiffness during and after a fatiguing protocol in response to voluntary or electrically elicited contractions. Some studies that used SWE and TMG reported a reduction in modulus and muscle belly displacement, respectively, indirectly mirroring a reduction in muscle stiffness. In contrast, others reported either no change or an increase in stiffness-related parameters. By means of SWE, a fatigue-induced redistribution of modulus (i.e., load sharing) among synergistic muscles was also shown. Additionally, investigations using myotonometry reported an increase in muscle belly stiffness after fatiguing protocols. So, the procedures need to be improved to accurately detect fatigue-induced changes in muscle mechanical characteristics.
MMG signal analysis in the time and the frequency domain allows for making inferences about the changes in motor unit recruitment strategies during fatiguing protocols at different intensities and paradigms. At low intensity, both MMG signal amplitude and frequency content increase continuously, possibly reflecting the recruitment of additional motor units and their synchronization. At medium intensity, MMG amplitude and frequency content begin to increase before plateauing, and then decrease. This was associated with initial attempts to maintain force output stable, followed by a reduction in motor drive to the fatigued muscle. At high intensity, MMG amplitude diminishes starting from the beginning of muscle contraction, while the mean frequency increases in the first few seconds, before decreasing subsequently. This behaviour was hypothesized to reflect the attempt to activate fast fatigable motor units followed by a reduction in the mean firing rate of recruited motor units. Additionally, when the MMG signal was detected in combination with the sEMG and force signals, fatigue-induced alterations in both EMD and R-EMD were reported. These alterations indicate that the decrease in force output and the longer time that the force output needs to return to baseline are likely influenced by fatigue-induced mechanical alterations in the series elastic component characteristics (e.g., a possible decrease in stiffness).
Finally, studies investigating fatigue-induced alterations in myoelectric activity by means of HD-sEMG reported a non-homogenous response in motor unit activity in different areas within the same muscle and between synergistic/adjacent muscles. Possible explanations are a restricted location of the most fatigable motor units in different regions, a dissimilar increase in motor unit activity within different muscle compartments, and alterations in load sharing among synergistic muscles.
From a technical and methodological point of view, some technologies now provide a detailed description of fatigue-induced changes in muscle fibres (e.g., laser diffraction). Others offer a wider view at the regional and whole muscle level (e.g., 31P MRS, SWE, TMG, myotonometry, MMG, HD-sEMG) and depict inhomogeneous behaviour of muscle portions during peripheral fatigue. A future area of focus would be to combine various approaches and then observe how these regional differences are reflected at the level of single muscle fibres. This could advance our understanding of the mechanisms underpinning muscle fatigue and the strategies adopted by the neuromuscular system to counteract it.
Abbreviations
- EMD:
-
Electromechanical delay
- HD-sEMG:
-
High-density surface electromyography
- MF:
-
Mean frequency
- MMG:
-
Mechanomyography
- MRS:
-
Magnetic resonance spectroscopy
- MTC:
-
Muscle–tendon complex
- PCr:
-
Phosphocreatine
- Pi:
-
Inorganic phosphate
- R-EMD:
-
Electromechanical delay during relaxation
- RMS:
-
Root mean square
- sEMG:
-
Surface electromyography
- SWE:
-
Shear-wave elastography
- TMG:
-
Tensiomyography
References
Abboud J, Nougarou F, Pagé I, Cantin V, Massicotte D, Descarreaux M (2014) Trunk motor variability in patients with non-specific chronic low back pain. Eur J Appl Physiol 114:2645–2654. https://doi.org/10.1007/s00421-014-2985-8
Abboud J, Nougarou F, Lardon A, Dugas C, Descarreaux M (2016) Influence of lumbar muscle fatigue on trunk adaptations during sudden external perturbations. Front Hum Neurosci 10:1–16. https://doi.org/10.3389/fnhum.2016.00576
Aird L, Samuel D, Stokes M (2012) Quadriceps muscle tone, elasticity and stiffness in older males: reliability and symmetry using the MyotonPRO. Arch Gerontol Geriatr 55:e31–e39. https://doi.org/10.1016/j.archger.2012.03.005
Akagi R, Fukui T, Kubota M, Nakamura M, Ema R (2017) Muscle shear moduli changes and frequency of alternate muscle activity of plantar flexor synergists induced by prolonged low-level contraction. Front Physiol 8:708. https://doi.org/10.3389/fphys.2017.00708
Allen DG, Westerblad H (2001) Role of phosphate and calcium stores in muscle fatigue. J Physiol 536:657–665
Allen DG, Lamb GD, Westerblad H (2008) Skeletal muscle fatigue: cellular mechanisms. Physiol Rev 88:287–332. https://doi.org/10.1152/physrev.00015.2007
Al-Zahrani E, Gunasekaran C, Callaghan M, Gaydecki P, Benitez D, Oldham J (2009) Within-day and between-days reliability of quadriceps isometric muscle fatigue using mechanomyography on healthy subjects. J Electromyogr Kinesiol 19:695–703. https://doi.org/10.1016/j.jelekin.2007.12.007
Amann M, Dempsey JA (2008) Locomotor muscle fatigue modifies central motor drive in healthy humans and imposes a limitation to exercise performance. J Physiol 586:161–173. https://doi.org/10.1113/jphysiol.2007.141838
Andonian P, Viallon M, Le Goff C, De Bourguignon C, Tourel C, Morel J, Giardini G, Gergelé L, Millet GP, Croisille P (2016) Shear-wave elastography assessments of quadriceps stiffness changes prior to, during and after prolonged exercise: a longitudinal study during an extreme mountain ultra-marathon. PLoS One 11:1–21. https://doi.org/10.2146/ajhp180163
Asmussen E (1979) Muscle fatigue. Med Sci Sports 11:313–321
Baskin RJ, Roos KP, Yeh Y (1979) Light diffraction study of single skeletal muscle fibres. Biophys J 28:45–64. https://doi.org/10.1016/S0006-3495(79)85158-9
Baskin R, Lieber R, Oba T, Yeh Y (1981) Intensity of light diffraction from striated muscle as a function of incident angle. Biophys J 36:759–773. https://doi.org/10.1016/S0006-3495(81)84764-9
Beck TW, Housh TJ, Johnson GO, Weir JP, Cramer JT, Coburn JW, Malek MH (2004) Mechanomyographic and electromyographic time and frequency domain responses during submaximal to maximal isokinetic muscle actions of the biceps brachii. Eur J Appl Physiol 92:352–359. https://doi.org/10.1007/s00421-004-1110-9
Beck TW, Housh TJ, Fry AC, Cramer JT, Weir JP, Schilling BK, Falvo MJ, Moore CA (2007) The influence of muscle fiber type composition on the patterns of responses for electromyographic and mechanomyographic amplitude and mean power frequency during a fatiguing submaximal isometric muscle action. Electromyogr Clin Neurophysiol 47:221–232
Beck TW, Housh T, Fry AC, Cramer JT, Weir J, Schilling B, Falvo M, Moore C (2009) MMG-EMG cross spectrum and muscle fiber type. Int J Sports Med 30:538–544. https://doi.org/10.1055/s-0029-1202349
Bendahan D, Giannesini B, Cozzone PJ (2004) Functional investigations of exercising muscle: a noninvasive magnetic resonance spectroscopy-magnetic resonance imaging approach. Cell Mol Life Sci 61:1001–1015. https://doi.org/10.1007/s00018-004-3345-3
Bercoff J, Tanter M, Fink M (2004) Supersonic shear imaging: a new technique for soft tissue elasticity mapping. IEEE Trans Ultrason Ferroelectr Freq Control 51:396–409
Bigland-Ritchie B (1979) Factors contributing to quantitative surface electromyographic recording and how they are affected by fatigue. Am Rev Respir Dis 119:95–97. https://doi.org/10.1164/arrd.1979.119.2P2.95
Bizzini M, Mannion AF (2003) Reliability of a new, hand-held device for assessing skeletal muscle stiffness. Clin Biomech 18:459–461. https://doi.org/10.1016/S0268-0033(03)00042-1
Blangsted AK, Sjøgaard G, Madeleine P, Olsen HB, Søgaard K (2005) Voluntary low-force contraction elicits prolonged low-frequency fatigue and changes in surface electromyography and mechanomyography. J Electromyogr Kinesiol 15:138–148. https://doi.org/10.1016/j.jelekin.2004.10.004
Bouillard K, Hug F, Guevel A, Nordez A (2012) Shear elastic modulus can be used to estimate an index of individual muscle force during a submaximal isometric fatiguing contraction. J Appl Physiol 113:1353–1361. https://doi.org/10.1152/japplphysiol.00858.2012
Bouillard K, Jubeau M, Nordez A, Hug F (2014) Effect of vastus lateralis fatigue on load sharing between quadriceps femoris muscles during isometric knee extensions. J Neurophysiol 111:768–776. https://doi.org/10.1152/jn.00595.2013
Bull AJ, Housh TJ, Johnson GO, Perry SR (2000) Electromyographic and mechanomyographic responses at critical power. Can J Appl Physiol 25:262–270. https://doi.org/10.1139/h00-020
Cady EB, Elshove H, Jones DA, Moll A (1989a) The metabolic causes of slow relaxation in fatigued human skeletal muscle. J Physiol 418:327–337. https://doi.org/10.1113/jphysiol.1989.sp017843
Cady EB, Jones DA, Lynn J, Newham DJ (1989b) Changes in force and intracellular metabolites during fatigue of human skeletal muscle. J Physiol 418:311–325
Camic CL, Housh TJ, Zuniga JM, Russell Hendrix C, Bergstrom HC, Traylor DA, Schmidt RJ, Johnson GO (2013) Electromyographic and mechanomyographic responses across repeated maximal isometric and concentric muscle actions of the leg extensors. J Electromyogr Kinesiol 23:342–348. https://doi.org/10.1016/j.jelekin.2012.09.010
Camic CL, Housh TJ, Zuniga JM, Bergstrom HC, Schmidt RJ, Johnson GO (2014) Mechanomyographic and electromyographic responses during fatiguing eccentric muscle actions of the leg extensors. J Appl Biomech 30:255–261. https://doi.org/10.1123/jab.2013-0178
Carrasco L, Sañudo B, De Hoyo M, Pradas F, Da Silva ME (2011) Effectiveness of low-frequency vibration recovery method on blood lactate removal, muscle contractile properties and on time to exhaustion during cycling at VO2max power output. Eur J Appl Physiol 111:2271–2279. https://doi.org/10.1007/s00421-011-1848-9
Cavanagh PR, Komi PV (1979) Electromechanical delay in human skeletal muscle under concentric and eccentric contractions. Eur J Appl Physiol Occup Physiol 42:159–163
Cè E, Rampichini S, Agnello L, Limonta E, Veicsteinas A, Esposito F (2013) Effects of temperature and fatigue on the electromechanical delay components. Muscle Nerve 47:566–576. https://doi.org/10.1002/mus.23627
Cè E, Rampichini S, Limonta E, Esposito F (2014) Fatigue effects on the electromechanical delay components during the relaxation phase after isometric contraction. Acta Physiol 211:82–96. https://doi.org/10.1111/apha.12212
Cè E, Rampichini S, Esposito F (2015a) Novel insights into skeletal muscle function by mechanomyography: from the laboratory to the field. Sport Sci Health 11:1–28. https://doi.org/10.1007/s11332-015-0219-z
Cè E, Rampichini S, Venturelli M, Limonta E, Veicsteinas A, Esposito F (2015b) Electromechanical delay components during relaxation after voluntary contraction: reliability and effects of fatigue. Muscle Nerve 51:907–915. https://doi.org/10.1002/mus.24466
Cè E, Rampichini S, Monti E, Venturelli M, Limonta E, Esposito F (2017) Changes in the electromechanical delay components during a fatiguing stimulation in human skeletal muscle: an EMG, MMG and force combined approach. Eur J Appl Physiol 117:95–107. https://doi.org/10.1007/s00421-016-3502-z
Curtin NA, Edman KAP (1989) Effects of fatigue and reduced intracellular pH on segment dynamics in “isometric” relaxation of frog muscle fibres. J Physiol 413:159–174
Dahmane R, Valenčič V, Knez N, Eržen I (2001) Evaluation of the ability to make non-invasive estimation of muscle contractile properties on the basis of the muscle belly response. Med Biol Eng Comput 39:51–55. https://doi.org/10.1007/BF02345266
Dahmane R, Djordjevič S, Šimunič B, Valenčič V (2005) Spatial fiber type distribution in normal human muscle: histochemical and tensiomyographical evaluation. J Biomech 38:2451–2459. https://doi.org/10.1016/j.jbiomech.2004.10.020
de Paula Simola RÁ, Harms N, Raeder C, Kellmann M, Meyer T, Pfeiffer M, Ferrauti A (2015) Tensiomyography reliability and prediction of changes in muscle force following heavy eccentric strength exercise using muscle mechanical properties. Sport Technol 8:58–66. https://doi.org/10.1080/19346182.2015.1117475
de Paula Simola RÁ, Raeder C, Wiewelhove T, Kellmann M, Meyer T, Pfeiffer M, Ferrauti A (2016) Muscle mechanical properties of strength and endurance athletes and changes after one week of intensive training. J Electromyogr Kinesiol 30:73–80. https://doi.org/10.1016/j.jelekin.2016.05.005
Debold EP (2006) The depressive effect of Pi on the force-pCa relationship in skinned single muscle fibers is temperature dependent. AJP Cell Physiol 290:C1041–C1050. https://doi.org/10.1152/ajpcell.00342.2005
Decorte N, Lafaix PA, Millet GY, Wuyam B, Verges S (2012) Central and peripheral fatigue kinetics during exhaustive constant-load cycling. Scand J Med Sci Sport 22:381–391. https://doi.org/10.1111/j.1600-0838.2010.01167.x
Degroot M, Massie BM, Boska M, Gober J, Miller RG, Weiner MW (1993) Dissociation of [H+] from fatigue in human muscle detected by high time resolution 31P-NMR. Muscle Nerve 16:91–98. https://doi.org/10.1002/mus.880160115
Ditroilo M, Hunter AM, Haslam S, De Vito G (2011) The effectiveness of two novel techniques in establishing the mechanical and contractile responses of biceps femoris. Physiol Meas 32:1315–1326. https://doi.org/10.1088/0967-3334/32/8/020
Ditroilo M, Smith IJ, Fairweather MM, Hunter AM (2013) Long-term stability of tensiomyography measured under different muscle conditions. J Electromyogr Kinesiol 23:558–563. https://doi.org/10.1016/j.jelekin.2013.01.014
Drost G, Stegeman DF, van Engelen BGM, Zwarts MJ (2006) Clinical applications of high-density surface EMG: a systematic review. J Electromyogr Kinesiol 16:586–602
Ebersole KT, Malek DM (2008) Fatigue and the electromechanical efficiency of the vastus medialis and vastus lateralis muscles. J Athl Train 43:152–156. https://doi.org/10.4085/1062-6050-43.2.152
Eby SF, Song P, Chen S, Chen Q, Greenleaf JF, An KN (2013) Validation of shear wave elastography in skeletal muscle. J Biomech 46:2381–2387. https://doi.org/10.1016/j.jbiomech.2013.07.033
Edman KAP (1966) The relation between sarcomere length and active tension in isolated semitendinosus fibres of the frog. J Physiol 183:407–417. https://doi.org/10.1113/jphysiol.1966.sp007873
Edman KAP, Flitney FW (1982) Laser diffraction studies of sarcomere dynamics during ‘isometric’ relaxation in isolated muscle fibres of the frog. J Physiol 329:1–20. https://doi.org/10.1113/jphysiol.1982.sp014287
Edman KAP, Lou F (1990) Changes in force and stiffness induced by fatigue and intracellular acidification in frog muscle fibres. J Physiol 424:133–149. https://doi.org/10.1113/jphysiol.1990.sp018059
Edman KAP, Lou F (1992) Myofibrillar fatigue versus failure of activation during repetitive stimulation of frog muscle fibres. J Physiol 457:655–673
Edman KAP, Reggiani C, te Kronnie G (1985) Differences in maximum velocity of shortening along single muscle fibres of the frog. J Physiol 365:147–163
Esposito F, Orizio C, Veicsteinas A (1998) Electromyogram and mechanomyogram changes in fresh and fatigued muscle during sustained contraction in men. Eur J Appl Physiol Occup Physiol 78:494–501. https://doi.org/10.1007/s004210050451
Esposito F, Orizio C, Parrinello G, Veicsteinas A (2003) Chronic hypobaric hypoxia does not affect electro-mechanical muscle activities during sustained maximal isometric contractions. Eur J Appl Physiol 90:337–343. https://doi.org/10.1007/s00421-003-0922-3
Esposito F, Limonta E, Cè E (2011) Passive stretching effects on electromechanical delay and time course of recovery in human skeletal muscle: new insights from an electromyographic and mechanomyographic combined approach. Eur J Appl Physiol 111:485–495. https://doi.org/10.1007/s00421-010-1659-4
Esposito F, Cè E, Rampichini S, Limonta E, Venturelli M, Monti E, Bet L, Fossati B, Meola G (2016) Electromechanical delay components during skeletal muscle contraction and relaxation in patients with myotonic dystrophy type 1. Neuromuscul Disord 26:60–72. https://doi.org/10.1016/j.nmd.2015.09.013
Esposito F, Cè E, Rampichini S, Monti E, Limonta E, Fossati B, Meola G (2017) Electromechanical delays during a fatiguing exercise and recovery in patients with myotonic dystrophy type 1. Eur J Appl Physiol 117:551–566. https://doi.org/10.1007/s00421-017-3558-4
Farina D, Leclerc F, Arendt-Nielsen L, Buttelli O, Madeleine P (2008) The change in spatial distribution of upper trapezius muscle activity is correlated to contraction duration. J Electromyogr Kinesiol 18:16–25. https://doi.org/10.1016/j.jelekin.2006.08.005
Ferris-Hood K, Threlkeld AJ, Horn TS, Shapiro R (1996) Relaxation electromechanical delay of the quadriceps during selected movement velocities. Electromyogr Clin Neurophysiol 36:157–170
Fitts RH (1994) Cellular mechanisms of muscle fatigue. Physiol Rev 74:49–94
Fitts RH (2008) The cross-bridge cycle and skeletal muscle fatigue. J Appl Physiol 104:551–558. https://doi.org/10.1152/japplphysiol.01200.2007
Fitts RH (2016) The role of acidosis in fatigue: pro perspective. Med Sci Sports Exerc 48:2335–2338. https://doi.org/10.1249/MSS.0000000000001043
Fryer MW, West JM, Stephenson DG (1997) Phosphate transport into the sarcoplasmic reticulum of skinned fibres from rat skeletal muscle. J Muscle Res Cell Motil 18:161–167. https://doi.org/10.1023/A:1018605605757
Gallina A, Merletti R, Vieira TMM (2011) Are the myoelectric manifestations of fatigue distributed regionally in the human medial gastrocnemius muscle? J Electromyogr Kinesiol 21:929–938. https://doi.org/10.1016/j.jelekin.2011.08.006
Gandevia SC (2001) Spinal and supraspinal factors in human muscle fatigue. Physiol Rev 81:1725–1789
García-García O, Cancela-Carral JM, Martínez-Trigo R, Serrano-Gómez V (2013) Differences in the contractile properties of the knee extensor and flexor muscles in professional road cyclists during the season. J Strength Cond Res 27:2760–2767. https://doi.org/10.1519/JSC.0b013e31828155cd
García-García O, Cancela-Carral JM, Huelin-Trillo F (2015) Neuromuscular profile of top-level women kayakers assessed through tensiomyography. J Strength Cond Res 29:844–853. https://doi.org/10.1519/JSC.0000000000000702
García-Manso JM, Rodríguez-Ruiz D, Rodríguez-Matoso D, de Yves S, Sarmiento S, Quiroga M (2011) Assessment of muscle fatigue after an ultra-endurance triathlon using tensiomyography (TMG). J Sports Sci 29:619–625. https://doi.org/10.1080/02640414.2010.548822
García-Manso JM, Rodríguez-Matoso D, Sarmiento S, de Saa Y, Vaamonde D, Rodríguez-Ruiz D, Da Silva-Grigoletto ME (2012) Effect of high-load and high-volume resistance exercise on the tensiomyographic twitch response of biceps brachii. J Electromyogr Kinesiol 22:612–619. https://doi.org/10.1016/j.jelekin.2012.01.005
Gennisson JL, Cornu C, Catheline S, Fink M, Portero P (2005) Human muscle hardness assessment during incremental isometric contraction using transient elastography. J Biomech 38:1543–1550. https://doi.org/10.1016/j.jbiomech.2004.07.013
Gennisson JL, Deffieux T, Fink M, Tanter M (2013) Ultrasound elastography: principles and techniques. Diagn Interv Imaging 94:487–495. https://doi.org/10.1016/j.diii.2013.01.022
Gerdle B, Grönlund C, Karlsson SJ, Holtermann A, Roeleveld K (2010) Altered neuromuscular control mechanisms of the trapezius muscle in fibromyalgia. BMC Musculoskelet Disord 11:42. https://doi.org/10.1186/1471-2474-11-42
Giovanelli N, Taboga P, Rejc E, Simunic B, Antonutto G, Lazzer S (2016) Effects of an uphill marathon on running mechanics and lower-limb muscle fatigue. Int J Sports Physiol Perform 11:522–529. https://doi.org/10.1123/ijspp.2014-0602
Goldenberg M, Yack H, Cerny F, Burton H (1991) Acoustic myography as an indicator of force during sustained contractions of a small hand muscle. J Appl Physiol 70:87–91
Goldman YE (1987) Measurement of sarcomere shortening in skinned fibers from frog muscle by white light diffraction. Biophys J 52:57–68. https://doi.org/10.1016/S0006-3495(87)83188-0
Goonetilleke A, Modarres-Sadeghi H, Guiloff RJ (1994) Accuracy, reproducibility, and variability of hand-held dynamometry in motor neuron disease. J Neurol Neurosurg Psychiatry 57:326–332. https://doi.org/10.1136/jnnp.57.3.326
Hendrix CR, Housh TJ, Camic CL, Zuniga JM, Johnson GO, Schmidt RJ (2010) Comparing electromyographic and mechanomyographic frequency-based fatigue thresholds to critical torque during isometric forearm flexion. J Neurosci Methods 194:64–72. https://doi.org/10.1016/j.jneumeth.2010.07.006
Hermens HJ, Freriks B, Merletti R, Stegeman D, Blok J, Rau G, Disselhorst-Klug C, Hägg G (1999) European recommendations for surface electromyography. Roessingh Res Dev. https://doi.org/10.1016/S1050-6411(00)00027-4
Holtermann A, Roeleveld K, Karlsson JS (2005) Inhomogeneities in muscle activation reveal motor unit recruitment. J Electromyogr Kinesiol 15:131–137. https://doi.org/10.1016/j.jelekin.2004.09.003
Hoult DI, Busby SJW, Gadian DG, Radda GK, Richards RE, Seeley PJ (1974) Observation of tissue metabolites using 31P nuclear magnetic resonance. Nature 252:285–287. https://doi.org/10.1038/252285a0
Housh TJ, Perry SR, Bull AJ, Johnson GO, Ebersole KT, Housh DJ, de Vries HA (2000) Mechanomyographic and electromyographic responses during submaximal cycle ergometry. Eur J Appl Physiol 83:381–387. https://doi.org/10.1007/s004210000315
Hu Y, Siu SH, Mak JN, Luk KD (2010) Lumbar muscle electromyographic dynamic topography during flexion-extension. J Electromyogr Kinesiol 20:246–255. https://doi.org/10.1016/j.jelekin.2009.05.002
Hufschmidt A (1985) Acoustic phenomena in the latent period of skeletal muscle: a simple method for in vivo measurement of the electro-mechanic latency (EML). Pflugers Arch 404:162–165
Hug F, Tucker K, Gennisson JL, Tanter M, Nordez A (2015) Elastography for muscle biomechanics: toward the estimation of individual muscle force. Exerc Sport Sci Rev 43:125–133. https://doi.org/10.1249/JES.0000000000000049
Hunter AM, Galloway SDR, Smith IJ, Tallent J, Ditroilo M, Fairweather MM, Howatson G (2012) Assessment of eccentric exercise-induced muscle damage of the elbow flexors by tensiomyography. J Electromyogr Kinesiol 22:334–341. https://doi.org/10.1016/j.jelekin.2012.01.009
Jaskólski A, Andrzejewska R, Marusiak J, Kisiel-Sajewicz K, Jaskólska A (2007) Similar response of agonist and antagonist muscles after eccentric exercise revealed by electromyography and mechanomyography. J Electromyogr Kinesiol 17:568–577. https://doi.org/10.1016/j.jelekin.2006.05.002
Jordanic M, Rojas-Martínez M, Mañanas MA, Alonso JF (2016) Spatial distribution of HD-EMG improves identification of task and force in patients with incomplete spinal cord injury. J Neuroeng Rehabil 13:41. https://doi.org/10.1186/s12984-016-0151-8
Kawai M, Kuntz ID (1973) Optical diffraction studies of muscle fibers. Biophys J 13:857–876. https://doi.org/10.1016/S0006-3495(73)86031-X
Kawczyński A, Nie H, Jaskólska A, Jaskólski A, Arendt-Nielsen L, Madeleine P (2007) Mechanomyography and electromyography during and after fatiguing shoulder eccentric contractions in males and females. Scand J Med Sci Sport 17:172–179. https://doi.org/10.1111/j.1600-0838.2006.00551.x
Kelly JP, Koppenhaver SL, Michener LA, Proulx L, Bisagni F, Cleland JA (2018) Characterization of tissue stiffness of the infraspinatus, erector spinae, and gastrocnemius muscle using ultrasound shear wave elastography and superficial mechanical deformation. J Electromyogr Kinesiol 38:73–80. https://doi.org/10.1016/j.jelekin.2017.11.001
Kent-Braun JA (1999) Central and peripheral contributions to muscle fatigue in humans during sustained maximal effort. Eur J Appl Physiol Occup Physiol 80:57–63. https://doi.org/10.1007/s004210050558
Kent-Braun JA, Ng AV, Doyle JW, Towse TF (2002) Human skeletal muscle responses vary with age and gender during fatigue due to incremental isometric exercise. J Appl Physiol 93:1813–1823. https://doi.org/10.1152/japplphysiol.00091.2002
Kent-Braun JA, Fitts RH, Christie A (2012) Skeletal muscle fatigue. Compr Physiol 2:997–1044. https://doi.org/10.1002/cphy.c110029
Kimura T, Hamada T, Watanabe T, Maeda A, Oya T, Moritani T (2004) Mechanomyographic responses in human biceps brachii and soleus during sustained isometric contraction. Eur J Appl Physiol 92:533–539. https://doi.org/10.1007/s00421-004-1147-9
Kimura T, Fujibayashi M, Tanaka S, Moritani T (2008) Mechanomyographic responses in quadriceps muscles during fatigue by continuous cycle exercise. Eur J Appl Physiol 104:651–656. https://doi.org/10.1007/s00421-008-0816-5
Kleine BU, van Dijk JP, Lapatki BG, Zwarts MJ, Stegeman DF (2007) Using two-dimensional spatial information in decomposition of surface EMG signals. J Electromyogr Kinesiol 17:535–548. https://doi.org/10.1016/j.jelekin.2006.05.003
Koo TK, Guo JY, Cohen JH, Parker KJ (2013) Relationship between shear elastic modulus and passive muscle force: an ex vivo study. J Biomech 46:2053–2059. https://doi.org/10.1016/j.jbiomech.2013.05.016
Kouzaki M, Shinohara M, Fukunaga T (1999) Non-uniform mechanical activity of quadriceps muscle during fatigue by repeated maximal voluntary contraction in humans. Eur J Appl Physiol Occup Physiol 80:9–15. https://doi.org/10.1007/s004210050551
Križaj D, Šimunič B, Žagar T (2008) Short-term repeatability of parameters extracted from radial displacement of muscle belly. J Electromyogr Kinesiol 18:645–651. https://doi.org/10.1016/j.jelekin.2007.01.008
Limonta E, Cè E, Gobbo M, Veicsteinas A, Orizio C, Esposito F (2016) Motor unit activation strategy during a sustained isometric contraction of finger flexor muscles in elite climbers. J Sports Sci 34:133–142. https://doi.org/10.1080/02640414.2015.1035738
Liu CL, Feng YN, Zhang HQ, Li YP, Zhu Y, Zhang ZJ (2018) Assessing the viscoelastic properties of upper trapezius muscle: intra- and inter-tester reliability and the effect of shoulder elevation. J Electromyogr Kinesiol 43:226–229. https://doi.org/10.1016/j.jelekin.2017.09.007
Longo S, Cè E, Rampichini S, Devoto M, Limonta E, Esposito F (2014) Mechanomyogram amplitude correlates with human gastrocnemius medialis muscle and tendon stiffness both before and after acute passive stretching. Exp Physiol 99:1359–1369. https://doi.org/10.1113/expphysiol.2014.080366
Longo S, Devoto M, Monti E, Venturelli M, Limonta E, Rampichini S, Valentina A (2016) Acute effects of static stretching on skeletal muscle relaxation at different ankle joint angles. Sport Sci Health 12:429–436. https://doi.org/10.1007/s11332-016-0309-6
Longo S, Cè E, Rampichini S, Devoto M, Venturelli M, Limonta E, Esposito F (2017) Correlation between stiffness and electromechanical delay components during muscle contraction and relaxation before and after static stretching. J Electromyogr Kinesiol 33:83–93. https://doi.org/10.1016/j.jelekin.2017.02.001
Macgregor LJ, Ditroilo M, Smith IJ, Fairweather MM, Hunter AM (2016) Reduced radial displacement of the gastrocnemius medialis muscle after electrically elicited fatigue. J Sport Rehabil 25:241–247. https://doi.org/10.1123/jsr.2014-0325
Macgregor LJ, Hunter AM, Orizio C, Fairweather MM, Ditroilo M (2018) Assessment of skeletal muscle contractile properties by radial displacement: the case for tensiomyography. Sport Med 48:1607–1620
MacIntosh BR (2017) Recent developments in understanding the length dependence of contractile response of skeletal muscle. Eur J Appl Physiol 117:1059–1071. https://doi.org/10.1007/s00421-017-3591-3
Madeleine P (2010) On functional motor adaptations: from the quantification of motor strategies to the prevention of musculoskeletal disorders in the neck-shoulder region. Acta Physiol 199:1–46. https://doi.org/10.1111/j.1748-1716.2010.02145.x
Madeleine P, Farina D (2008) Time to task failure in shoulder elevation is associated to increase in amplitude and to spatial heterogeneity of upper trapezius mechanomyographic signals. Eur J Appl Physiol 102:325–333. https://doi.org/10.1007/s00421-007-0589-2
Madeleine P, Farina D, Merletti R, Arendt-Nielsen L (2002a) Upper trapezius muscle mechanomyographic and electromyographic activity in humans during low force fatiguing and non-fatiguing contractions. Eur J Appl Physiol 87:327–336. https://doi.org/10.1007/s00421-002-0655-8
Madeleine P, Jørgensen LV, Søgaard K, Arendt-Nielsen L, Sjøgaard G (2002b) Development of muscle fatigue as assessed by electromyography and mechanomyography during continuous and intermittent low-force contractions: effects of the feedback mode. Eur J Appl Physiol 87:28–37. https://doi.org/10.1007/s00421-002-0578-4
Madeleine P, Ge HY, Jaskólska A, Farina D, Jaskölski A, Arendt-Nielsen L (2006) Spectral moments of mechanomyographic signals recorded with accelerometer and microphone during sustained fatiguing contractions. Med Biol Eng Comput 44:290–297. https://doi.org/10.1007/s11517-006-0036-2
Madeleine P, Tuker K, Arendt-Nielsen L, Farina D (2007) Heterogeneous mechanomyographic absolute activation of paraspinal muscles assessed by a two-dimensional array during short and sustained contractions. J Biomech 40:2663–2671. https://doi.org/10.1016/j.jbiomech.2006.12.011
Maïsetti O, Hug F, Bouillard K, Nordez A (2012) Characterization of passive elastic properties of the human medial gastrocnemius muscle belly using supersonic shear imaging. J Biomech 45:978–984. https://doi.org/10.1016/j.jbiomech.2012.01.009
Mamaghani NK, Shimomura Y, Iwanaga K, Katsuura T (2002) Mechanomyogram and electromyogram responses of upper limb during sustained isometric fatigue with varying shoulder and elbow postures. J Physiol Anthropol Appl Hum Sci 21:29–43. https://doi.org/10.2114/jpa.21.29
Marusiak J, Jaskólska A, Koszewicz M, Budrewicz S, Jaskólski A (2012) Myometry revealed medication-induced decrease in resting skeletal muscle stiffness in Parkinson’s disease patients. Clin Biomech 27:632–635. https://doi.org/10.1016/j.clinbiomech.2012.02.001
Merletti R, Knaflitz M, De Luca CJ (1990) Myoelectric manifestations of fatigue in voluntary and electrically elicited contractions. J Appl Physiol 69:1810–1820. https://doi.org/10.1152/jappl.1990.69.5.1810
Merletti R, Farina D, Gazzoni M (2003) The linear electrode array: a useful tool with many applications. J Electromyogr Kinesiol 13:37–47. https://doi.org/10.1016/S1050-6411(02)00082-2
Merletti R, Holobar A, Farina D (2008) Analysis of motor units with high-density surface electromyography. J Electromyogr Kinesiol 18:879–890. https://doi.org/10.1016/j.jelekin.2008.09.002
Miller RG, Giannini D, Milner-Brown HS, Layzer RB, Koretsky AP, Hooper D, Weiner MW (1987) Effects of fatiguing exercise on high-energy phosphates, force, and EMG: evidence for three phases of recovery. Muscle Nerve 10:810–821. https://doi.org/10.1002/mus.880100906
Miller RG, Boska MD, Moussavi RS, Carson PJ, Weiner MW (1988) 31P nuclear magnetic resonance studies of high energy phosphates and pH in human muscle fatigue. Comparison of aerobic and anaerobic exercise. J Clin Investig 81:1190–1196. https://doi.org/10.1172/JCI113434
Mista CA, Salomoni SE, Graven-Nielsen T (2014) Spatial reorganisation of muscle activity correlates with change in tangential force variability during isometric contractions. J Electromyogr Kinesiol 24:37–45. https://doi.org/10.1016/j.jelekin.2013.10.014
Moon RB, Richards JH (1973) Determination of intracellular pH by 31P magnetic resonance. J Biol Chem 248:7276–7278
Moritani T, Nagata A, Muro M (1982) Electromyographic manifestations of muscular fatigue. Med Sci Sports Exerc 14:198–202
Nair K, Masi AT, Andonian BJ, Barry AJ, Coates BA, Dougherty J, Schaefer E, Henderson J, Kelly J (2016) Stiffness of resting lumbar myofascia in healthy young subjects quantified using a handheld myotonometer and concurrently with surface electromyography monitoring. J Bodyw Mov Ther 20:388–396. https://doi.org/10.1016/j.jbmt.2015.12.005
Nelson CR, Debold EP, Fitts RH (2014) Phosphate and acidosis act synergistically to depress peak power in rat muscle fibers. AJP Cell Physiol 307:C939–C950. https://doi.org/10.1152/ajpcell.00206.2014
Nocella M, Colombini B, Benelli G, Cecchi G, Bagni MA, Bruton J (2011) Force decline during fatigue is due to both a decrease in the force per individual cross-bridge and the number of cross-bridges. J Physiol 589:3371–3381. https://doi.org/10.1113/jphysiol.2011.209874
Nordez A, Guével A, Casari P, Catheline S, Cornu C (2009) Assessment of muscle hardness changes induced by a submaximal fatiguing isometric contraction. J Electromyogr Kinesiol 19:484–491. https://doi.org/10.1016/j.jelekin.2007.11.005
Nosek TM, Fender KY, Godt RE (1987) It is diprotonated inorganic phosphate that depresses force in skinned skeletal muscle fibers. Science (80-) 236:191–193
O’Connor SM, Cheng EJ, Young KW, Ward SR, Lieber RL (2016) Quantification of sarcomere length distribution in whole muscle frozen sections. J Exp Biol 219:1432–1436. https://doi.org/10.1242/jeb.132084
Orizio C (1992) Soundmyogram and EMG cross-spectrum during exhausting isometric contractions in humans. J Electromyogr Kinesiol 2:141–149. https://doi.org/10.1016/1050-6411(92)90011-7
Orizio C (1993) Muscle sound: bases for the introduction of a mechanomyographic signal in muscle studies. Crit Rev Biomed Eng 21:201–243
Orizio C, Veicsteinas A (1992) Soundmyogram analysis during sustained maximal voluntary contraction in sprinters and long distance runners. Int J Sports Med 13:594–599. https://doi.org/10.1055/s-2007-1024572
Orizio C, Perini R, Veicsteinas A (1989) Changes of muscular sound during sustained isometric contraction up to exhaustion. J Appl Physiol 66:1593–1598. https://doi.org/10.1152/jappl.1989.66.4.1593
Orizio C, Gobbo M, Diemont B, Esposito F, Veicsteinas A (2003) The surface mechanomyogram as a tool to describe the influence of fatigue on biceps brachii motor unit activation strategy. Historical basis and novel evidence. Eur J Appl Physiol 90:326–336. https://doi.org/10.1007/s00421-003-0924-1
Palmer S, Kentish JC (1994) The role of troponin C in modulating the Ca2+ sensitivity of mammalian skinned cardiac and skeletal muscle fibres. J Physiol 480(Pt 1):45–60
Patel TJ, Das R, Fridén J, Lutz GJ, Lieber RL (2004) Sarcomere strain and heterogeneity correlate with injury to frog skeletal muscle fiber bundles. J Appl Physiol 97:1803–1813
Perry-Rana SR, Housh TJ, Johnson GO, Bull AJ, Berning JM, Cramer JT (2002) MMG and EMG responses during fatiguing isokinetic muscle contractions at different velocities. Muscle Nerve 26:367–373. https://doi.org/10.1002/mus.10214
Petrofsky JS, Phillips CA (1985) Closed-loop control of movement of skeletal muscle. Crit Rev Biomed Eng 13:35–96
Piitulainen H, Bottas R, Komi P, Linnamo V, Avela J (2010) Impaired action potential conduction at high force levels after eccentric exercise. J Electromyogr Kinesiol 20:879–887. https://doi.org/10.1016/j.jelekin.2009.10.001
Pišot R, Narici MV, Šimunič B, De Boer M, Seynnes O, Jurdana M, Biolo G, Mekjavič IB (2008) Whole muscle contractile parameters and thickness loss during 35-day bed rest. Eur J Appl Physiol 104:409–414. https://doi.org/10.1007/s00421-008-0698-6
Place N, Yamada T, Bruton JD, Westerblad H (2010) Muscle fatigue: from observations in humans to underlying mechanisms studied in intact single muscle fibres. Eur J Appl Physiol 110:1–15. https://doi.org/10.1007/s00421-010-1480-0
Qayyum A (2009) MR spectroscopy of the liver: principles and clinical applications. RadioGraphics 29:1653–1664. https://doi.org/10.1148/rg.296095520
Rampichini S, Cè E, Limonta E, Esposito F (2014) Effects of fatigue on the electromechanical delay components in gastrocnemius medialis muscle. Eur J Appl Physiol 114:639–651. https://doi.org/10.1007/s00421-013-2790-9
Rassier DE, Macintosh BR (2002) Potentiation in mouse skeletal muscle. BMC Physiol 8:1–8
Rau G, Disselhorst-Klug C (1997) Principles of high-spatial-resolution surface EMG (HSR-EMG): single motor unit detection and application in the diagnosis of neuromuscular disorders. J Electromyogr Kinesiol 7:233–239. https://doi.org/10.1016/S1050-6411(97)00007-2
Rico-Sanz J (2003) Progressive decrease of intramyocellular accumulation of H+ and Pi in human skeletal muscle during repeated isotonic exercise. AJP Cell Physiol 284:C1490–C1496. https://doi.org/10.1152/ajpcell.00419.2002
Ringheim I, Indahl A, Roeleveld K (2014) Alternating activation is related to fatigue in lumbar muscles during sustained sitting. J Electromyogr Kinesiol 24:380–386. https://doi.org/10.1016/j.jelekin.2014.01.011
Rodriguez AA, Agre JC, Franke TM, Swiggum ER, Curt JT (1996) Acoustic myography during isometric fatigue in postpolio and control subjects. Muscle Nerve 19:384–387
Roth K, Weiner MW (1991) Determination of cytosolic ADP and AMP concentrations and the free energy of ATP hydrolysis in human muscle and brain tissues with 31P NMR spectroscopy. Magn Reson Med 22:505–511 [Erratum appears in Magn Reson Med 1995;33(2):282]
Sadeghi S, Newman C, Cortes DH (2018) Change in skeletal muscle stiffness after running competition is dependent on both running distance and recovery time: a pilot study. PeerJ 6:e4469. https://doi.org/10.7717/peerj.4469
Sahlin K, Ren JM (1989) Relationship of contraction capacity to metabolic changes during recovery from a fatiguing contraction. J Appl Physiol 67:648–654. https://doi.org/10.1152/jappl.1989.67.2.648
Samani A, Srinivasan D, Mathiassen SE, Madeleine P (2017) Variability in spatio-temporal pattern of trapezius activity and coordination of hand-arm muscles during a sustained repetitive dynamic task. Exp Brain Res 235:389–400. https://doi.org/10.1007/s00221-016-4798-y
Sasaki K, Sasaki T, Ishii N (2011) Acceleration and force reveal different mechanisms of electromechanical delay. Med Sci Sport Exerc 43:1200–1206. https://doi.org/10.1249/MSS.0b013e318209312c
Scherrer J, Bourguignon A (1959) Changes in the electromyogram produced by fatigue in man. Am J Phys Med 38:148–158
Shinohara M, Kouzaki M, Yoshihisa T, Fukunaga T (1997) Mechanomyography of the human quadriceps muscle during incremental cycle ergometry. Eur J Appl Physiol Occup Physiol 76:314–319. https://doi.org/10.1007/s004210050254
Shinohara M, Kouzaki M, Yoshihisa T, Fukunaga T (1998) Mechanomyogram from the different heads of the quadriceps muscle during incremental knee extension. Eur J Appl Physiol Occup Physiol 78:289–295. https://doi.org/10.1007/s004210050422
Šimunič B (2012) Between-day reliability of a method for non-invasive estimation of muscle composition. J Electromyogr Kinesiol 22:527–530. https://doi.org/10.1016/j.jelekin.2012.04.003
Šimunič B, Degens H, Rittweger J, Narici M, Mekjavić IB, Pišot R (2011) Noninvasive estimation of myosin heavy chain composition in human skeletal muscle. Med Sci Sport Exerc 43:1619–1625. https://doi.org/10.1249/MSS.0b013e31821522d0
Smith C, Housh T, Jenkins N, Hill E, Cochrane K, Miramonti A, Schmidt R, Johnson G (2016) Combining regression and mean comparisons to identify the time course of changes in neuromuscular responses during the process of fatigue. Physiol Meas 37:1993–2002. https://doi.org/10.1088/0967-3334/37/11/1993
Smith CM, Housh TJ, Hill EC, Johnson GO, Schmidt RJ (2017a) Changes in electromechanical delay during fatiguing dynamic muscle actions. Muscle Nerve 56:315–320. https://doi.org/10.1002/mus.25502
Smith CM, Housh TJ, Hill EC, Johnson GO, Schmidt RJ (2017b) Dynamic versus isometric electromechanical delay in non-fatigued and fatigued muscle: a combined electromyographic, mechanomyographic, and force approach. J Electromyogr Kinesiol 33:34–38. https://doi.org/10.1016/j.jelekin.2017.01.008
Søgaard K, Blangsted AK, Jørgensen LV, Madeleine P, Sjøgaard G (2003) Evidence of long term muscle fatigue following prolonged intermittent contractions based on mechano- and electromyograms. J Electromyogr Kinesiol 13:441–450. https://doi.org/10.1016/S1050-6411(03)00075-0
Stegeman DF, Zwarts MJ, Anders C, Hashimoto T (2000) Multi-channel surface EMG in clinical neurophysiology. Suppl Clin Neurophysiol 53:155–162
Stock MS, Beck TW, DeFreitas JM, Ye X (2013) Mechanomyographic responses for the biceps brachii are unable to track the declines in peak torque during 25, 50, 75, and 100 fatiguing isokinetic muscle actions. J Appl Biomech 29:769–778
Taylor DJ, Styles P, Matthews PM, Arnold DA, Gadian D, Bore P, Radda GK (1986) Energetics of human muscle: exercise-induced ATP depletion. Magn Reson Imaging 3:44–54
Tonon C, Gramegna LL, Lodi R (2012) Magnetic resonance imaging and spectroscopy in the evaluation of neuromuscular disorders and fatigue. Neuromuscul Disord 22:S187–S191. https://doi.org/10.1016/j.nmd.2012.10.008
Tous-Fajardo J, Moras G, Rodríguez-Jiménez S, Usach R, Doutres DM, Maffiuletti NA (2010) Inter-rater reliability of muscle contractile property measurements using non-invasive tensiomyography. J Electromyogr Kinesiol 20:761–766. https://doi.org/10.1016/j.jelekin.2010.02.008
Troiano A, Naddeo F, Sosso E, Camarota G, Merletti R, Mesin L (2008) Assessment of force and fatigue in isometric contractions of the upper trapezius muscle by surface EMG signal and perceived exertion scale. Gait Posture 28:179–186. https://doi.org/10.1016/j.gaitpost.2008.04.002
Tucker K, Falla D, Graven-Nielsen T, Farina D (2009) Electromyographic mapping of the erector spinae muscle with varying load and during sustained contraction. J Electromyogr Kinesiol 19:373–379. https://doi.org/10.1016/j.jelekin.2007.10.003
Udaka J, Terui T, Ohtsuki I, Marumo K, Ishiwata S, Kurihara S, Fukuda N (2011) Depressed contractile performance and reduced fatigue resistance in single skinned fibers of soleus muscle after long-term disuse in rats. J Appl Physiol 111:1080–1087. https://doi.org/10.1152/japplphysiol.00330.2011
Valenčič V, Knez N (1997) Measuring of skeletal muscles’ dynamic properties. Artif Organs 21:240–242. https://doi.org/10.1111/j.1525-1594.1997.tb04658.x
Van Deun B, Hobbelen JSM, Cagnie B, Van Eetvelde B, Van Den Noortgate N, Cambier D (2018) Reproducible measurements of muscle characteristics using the MyotonPRO device: comparison between individuals with and without paratonia. J Geriatr Phys Ther 41:194–203. https://doi.org/10.1519/JPT.0000000000000119
Vedsted P, Blangsted AK, Søgaard K, Orizio C, Sjøgaard G (2006) Muscle tissue oxygenation, pressure, electrical, and mechanical responses during dynamic and static voluntary contractions. Eur J Appl Physiol 96:165–177. https://doi.org/10.1007/s00421-004-1216-0
Viir R, Virkus A, Laiho K, Rajaleid K, Selart A, Mikkelson M (2007) Trapezius muscle tone and viscoelastic properties in sitting and supine positions. Scand J Work Environ Heal Suppl 33:76–80
Viitasalo JT, Komi PV (1981) Interrelationships between electromyographic, mechanical, muscle structure and reflex time measurements in man. Acta Physiol Scand 111:97–103
Wang D, De Vito G, Ditroilo M, Delahunt E (2017a) Effect of sex and fatigue on muscle stiffness and musculoarticular stiffness of the knee joint in a young active population. J Sports Sci 35:1582–1591. https://doi.org/10.1080/02640414.2016.1225973
Wang D, De Vito G, Ditroilo M, Delahunt E (2017b) Different effect of local and general fatigue on knee joint stiffness. Med Sci Sport Exerc 49:173–182. https://doi.org/10.1249/MSS.0000000000001086
Watanabe K, Kouzaki M, Moritani T (2013) Region-specific myoelectric manifestations of fatigue in human rectus femoris muscle. Muscle Nerve 48:226–234. https://doi.org/10.1002/mus.23739
Westerblad H (2016) Acidosis is not a significant cause of skeletal muscle fatigue. Med Sci Sports Exerc 48:2339–2342. https://doi.org/10.1249/MSS.0000000000001044
Westerblad H, Allen DG (2003) Cellular mechanisms of skeletal muscle fatigue. Adv Exp Med Biol 538:563–570 (discussion 571)
Wiewelhove T, Raeder C, Simola RADP, Schneider C, Döweling A, Ferrauti A (2017) Tensiomyographic markers are not sensitive for monitoring muscle fatigue in elite youth athletes: a pilot study. Front Physiol 8:1–9. https://doi.org/10.3389/fphys.2017.00406
Wilkie DR (1986) Muscular fatigue: effects of hydrogen ions and inorganic phosphate. Fed Proc 45:2921–2923
Wilson JR, McCully KK, Mancini DM, Boden B, Chance B (1988) Relationship of muscular fatigue to pH and diprotonated Pi in humans: a 31P-NMR study. J Appl Physiol 64:2333–2339. https://doi.org/10.1152/jappl.1988.64.6.2333
Xie HB, Zheng YP, Jing-Yi G (2009) Detection of chaos in human fatigue mechanomyography signals. Conf Proc IEEE Eng Med Biol Soc. https://doi.org/10.1109/IEMBS.2009.5333485
Yang ZF, Kumar DK, Arjunan SP (2009) Mechanomyogram for identifying muscle activity and fatigue. Conf Proc IEEE Eng Med Biol Soc. https://doi.org/10.1109/IEMBS.2009.5333666
Yoshitake Y, Ue H, Miyazaki M, Moritani T (2001) Assessment of lower-back muscle fatigue using electromyography, mechanomyography, and near-infrared spectroscopy. Eur J Appl Physiol 84:174–179. https://doi.org/10.1007/s004210170001
Zhang LQ, Rymer WZ (2001) Reflex and intrinsic changes induced by fatigue of human elbow extensor muscles. J Neurophysiol 86:1086–1094
Zinder SM, Padua DA (2011) Reliability, validity, and precision of a handheld myometer for assessing in vivo muscle stiffness. J Sport Rehabil. https://doi.org/10.1123/jsr.2010-0051
Zuniga JM, Housh TJ, Camic CL, Hendrix CR, Schmidt RJ, Mielke M, Johnson GO (2010) A mechanomyographic fatigue threshold test for cycling. Int J Sports Med 31:636–643. https://doi.org/10.1055/s-0030-1255112
Zwarts MJ, Keidel M (1991) Relationship between electrical and vibratory output of muscle during voluntary contraction and fatigue. Muscle Nerve 14:756–761. https://doi.org/10.1002/mus.880140810
Zwarts MJ, Stegeman DF (2003) Multichannel surface EMG: basic aspects and clinical utility. Muscle Nerve 28:1–17. https://doi.org/10.1002/mus.10358
Author information
Authors and Affiliations
Contributions
All authors listed have made a substantial, either direct or intellectual, contribution to the work. All authors have approved the manuscript for publication. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed. The authors declare no conflict of interest and give their contribution to the study as follows: EC: review design; literature research and analysis; drafting the article or revising it critically for important intellectual content; SL: review design; literature research and analysis; drafting the article or revising it critically for important intellectual content; EL: review design; drafting the article or revising it critically for important intellectual content; GC: review design; drafting the article or revising it critically for important intellectual content; SR: review design; literature research and analysis; drafting the article or revising it critically for important intellectual content; FE: review design; drafting the article or revising it critically for important intellectual content. All authors approved the final version of the manuscript and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Conflict of interest
The authors report no conflict of interest. This work is known to and agreed by the co-authors identified on the manuscript’s title page.
Additional information
Communicated by Michael Lindinger.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cè, E., Longo, S., Limonta, E. et al. Peripheral fatigue: new mechanistic insights from recent technologies. Eur J Appl Physiol 120, 17–39 (2020). https://doi.org/10.1007/s00421-019-04264-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-019-04264-w