Abstract
Purpose
Under electrically evoked contractions, the time interval between the onset of the stimulation pulse (Stim) and the beginning of force (F) development can be partitioned (DelayTOT), by an electromyographic (EMG), mechanomyographic (MMG) and F combined approach, into three components each containing different parts of the electrochemical and mechanical processes underlying neuromuscular activation and contraction. The aim of the study was to evaluate inter- and intra-operator reliability of the measurements and to assess the effects of fatigue on the different DelayTOT components.
Methods
Sixteen participants underwent two sets of tetanic stimulations of the gastrocnemius medialis muscle, with 10 min of rest in between. After a fatiguing protocol of 120 s, tetanic stimulations were replicated. The same protocol was repeated on a different day. Stim, EMG, MMG and F signals were recorded during contraction. DelayTOT and its three components (between Stim and EMG, Δt Stim-EMG; between EMG and MMG, Δt EMG-MMG and between MMG and F, Δt MMG-F) were calculated.
Results
Before fatigue, DelayTOT, Δt Stim-EMG, Δt EMG-MMG and Δt MMG-F lasted 27.5 ± 0.9, 1.4 ± 0.1, 9.2 ± 0.5 and 16.8 ± 0.7 ms, respectively. Fatigue lengthened DelayTOT, Δt Stim-EMG, Δt EMG-MMG and Δt MMG-F by 18, 7, 16 and 22 %, respectively. Δt Stim-EMG, Δt EMG-MMG and Δt MMG-F contributed to DelayTOT lengthening by 2, 27 and 71 %, respectively. Reliability was always from high to very high.
Conclusions
The combined approach allowed a reliable calculation of the three contributors to DelayTOT. The effects of fatigue on each DelayTOT component could be precisely assessed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The electromechanical delay (EMD) is traditionally defined as the time lag between the onset of muscle electrical activation and the onset of force production (Cavanagh and Komi 1979). During this time frame, a sequence of several physiological events transduces the electrical processes of muscle activation into a mechanical phenomenon: (1) the propagation of the action potential along the sarcolemma and the T-tubule system; (2) the coupling between the dihydropyridine and ryanodine receptors and the following release of Ca2+ from the sarcoplasmic reticulum; (3) the interaction among Ca2+, troponin and actin; (4) the cross-bridges formation; and (5) the myosin heads rotation with subsequent force transmission to the tendon insertion point through the elongation of the series elastic components (SEC). While the first four mechanisms can be considered as electrochemical in nature, the last events are mainly mechanical.
Because of the several approaches with different experimental setup and protocols, EMD values from different human skeletal muscles have been found to vary between 8 and 127 ms (Grosset et al. 2009; Hopkins et al. 2007; Nordez et al. 2009; Yavuz et al. 2010). Changes in any of the previously listed events could potentially induce EMD alterations (Cé et al. 2013; Esposito et al. 2011; Granata et al. 2000; Lacourpaille et al. 2013a; Muraoka et al. 2004; Viitasalo and Komi 1981; Yavuz et al. 2010).
To provide more insights into the sequence of events underlying muscle activation and contraction, a technique based on very high frame rate ultrasounds was utilized to determine in vivo the delay between muscle electrical activation and the onset of muscle fascicles and tendon motion (Hug et al. 2011a; Lacourpaille et al. 2013b; Nordez et al. 2009). However, this methodology requires the latest generation of echographic devices to obtain accurate measurements.
Also a combined electromyographic (EMG), mechanomyographic (MMG) and force (F) signals analysis (Cé et al. 2013; Esposito et al. 2011; Petitjean et al. 1998; Sasaki et al. 2011) can provide useful information about muscle electrical and mechanical behaviour from the same muscle area (Barry 1992; Esposito et al. 2005; Orizio et al. 1999). MMG, indeed, can be considered during contraction as the mechanical counterpart of motor unit electrical activity detected by surface EMG (Gordon and Holbourn 1948). Moreover, MMG and F characteristics during isometric contraction can be explained by a recent muscle–tendon unit mechanical model, which defines two distinct elastic elements (K1 and K2) with different compliance (Orizio et al. 1999) and can help to provide physiological explanation of the mechanical events included in EMD. Due to the highly compliant K2, indeed, the shortening of the contractile elements results first in a muscle geometry change (with dimensional changes of the transverse diameter of the muscle fibres that generates pressure waves, detectable as MMG at the skin level) with low force output. Thereafter, when the slack of the elastic-connective tissue has been taken up, the tension is efficiently transmitted to the tendon with a quick raise in F and a reduction in MMG amplitude (Orizio et al. 1999, 2003).
With this approach, EMD can be, therefore, partitioned in: (1) a component that is mainly electrochemical (time lag between the onset of EMG and the onset of MMG, Δt EMG-MMG), in which the excitation–contraction (E–C) coupling, from the propagation of the motor unit action potential at the sarcolemmal level to myosin head rotation, and pressure wave transmission to the skin surface (detected by MMG) are included (Hufschmidt 1985; Petitjean et al. 1998); and (2) a mechanical component (time lag between the onset of MMG and the onset of F, Δt MMG-F), a potential index of the time required for taking up the muscle–tendon unit slack, before force transmission becomes efficient at the tendon insertion point (Cé et al. 2013; Esposito et al. 2011; Hufschmidt 1985; Sasaki et al. 2011).
Fatigue, which is one of the factors affecting the contraction process, alters the mechanisms involved in neuromuscular activation and muscle contraction, causing an EMD elongation (Cé et al. 2013; Yavuz et al. 2010; Zhou et al. 1996, 1998). In fatigued muscle, indeed, among other phenomena, a slowing of the motor unit action potential propagation along the sarcolemma, a decrement of Ca2+ efflux from sarcoplasmic reticulum and a reduction in cross-bridge cycling rate take place (Ament and Verkerke 2009; Fitts 1994, 2008). When partitioning EMD in Δt EMG-MMG and Δt MMG-F component, fatigue has been shown to affect both of them to a similar extent (Cé et al. 2013). However, although with some controversy related to voluntary contraction (Bigland-Ritchie et al. 1982), fatigue may alter not only the processes included in EMD, but also the mechanisms at the neuromuscular junction (Kirkendall 1990; Wieser 1915).
With electrically evoked contractions, the simultaneous detection of the stimulation current (Stim) together with the surface EMG may provide additional information on the processes at the pre-synaptic and synaptic level (Δt Stim-EMG). Indeed, the time delay between Stim, applied either on the nerve or on the muscle motor point, and the generated M-wave reflects the latency between the origin of the action potential at the axonal level and the action potential along the sarcolemma, thus including also the synaptic latency. Consequently, in addition to the traditionally defined EMD, the latency between the beginning of Stim and force development can be calculated (DelayTOT).
Methodological concerns about EMD measurement, though, have been recently raised by Hug et al. (2011b). In particular, EMG electrodes positioning may greatly influence onset detection of electrical muscle activation (up to 20 ms), thus affecting EMD measurement reliability. A detailed and reliable characterization of DelayTOT and its components may represent an important aid for the evaluation of neuromuscular activation and muscle contraction in clinical, rehabilitative and physical training fields.
Therefore, the aim of the present study was twofold: (1) to evaluate the intra- and inter-operator reliability of the measurement of each single DelayTOT component, both before and after fatigue; and (2) to assess the effects of fatigue on DelayTOT and its components, and the time course of recovery. To this purpose, participants underwent, on two different days, electrically evoked tetanic stimulations before and 1, 3 and 7 minutes after a fatiguing protocol.
Methods
Participants
After receiving a full explanation of the aim of the study and of the experimental procedures, 16 physically active males (age: 25.0 ± 3.9 years, body mass: 77.5 ± 13.8 kg; stature: 1.79 ± 0.08 m; mean ± standard deviation, SD) gave their written informed consent to volunteer in the study. Participants were all clinically healthy with no history of previous lower limb injuries. They were asked to abstain from caffeine or similar beverages in the 24 h preceding tests and to report to the laboratory without any form of physical exercise of heavy intensity of the lower limbs in the previous 48 h. The study was approved by the local University Ethical Committee and had been performed in accordance with the principles of the 1975 Declaration of Helsinki.
Experimental design
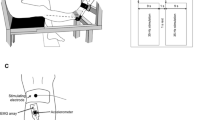
After a first visit for familiarization purposes, participants reported to the laboratory on two different days (D1 and D2), with at least 48 h in between. In both occasions, they were tested at about the same time of the day, to minimize possible differences induced by circadian effects, and with the same protocol to assess inter-day reliability. A schematic representation of the testing procedures is given in Fig. 1.
Experimental design. During D1, two sessions (SA and SB) of three tetanic stimulations (50 Hz for 3 s, with 5 min of rest in between) were applied on the motor point of the gastrocnemius medialis muscle. Between SA and SB, 10 min of rest was allowed. Thereafter, a fatiguing stimulation protocol (35 Hz for 120 s) was administered. To monitor the time course of the investigated parameters during recovery, the same tetanic stimulations (50 Hz for 3 s) before fatigue were delivered at minute 1, 2 and 7 from the end of the fatiguing protocol (POST1, POST2 and POST7, respectively). Identical procedures were followed during D2. Pooled data from SA and SB during D1 and D2 of both operators were considered as the PRE condition
Each test consisted of two sessions (SA and SB) of three tetanic stimulations of the gastrocnemius medialis muscle (GM), for the assessment of intra-day (inter-session) reliability. SA and SB were divided by 10 min of rest and were considered as the PRE condition. Thereafter, a fatiguing stimulation of 120 s began, after which tetanic stimulations at minute 1, 2 and 7 (POST1, POST2 and POST7, respectively) were administered to assess the effects of fatigue and to monitor the time course of the investigated parameters during recovery.
Experimental procedures and measurements
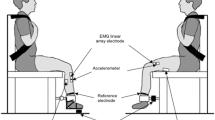
All experiments were performed in a laboratory at constant temperature (22 ± 1 °C) and relative humidity (50 ± 5 %). During tests, all participants sat on a custom-built ergometer with a resonant frequency >200 Hz. As shown in Fig. 2 (panel A), the knee of the dominant limb was fully extended and, to minimize the elongation of the triceps surae muscle at the distal level and the pre-tensioning of the fibres [which could per se alter EMD duration (Muraoka et al. 2004)], the ankle joint was fixed at 20° in plantarflexion, the reference position (0°) being perpendicularity of the tibia relative to the sole. The foot was attached by Velcro® straps (Velcro Industries, Willemstad, Netherlands Antilles) to a metal plate provided with a heel support and connected to a load cell.
Schematic representation of the experimental setup. Panel A Positioning of the participant on the ergometer. Panel B Positioning of the stimulation and EMG electrodes and of the accelerometer for MMG detection on the gastrocnemius medialis muscle. For stimulation, the cathode was positioned on the motor point, whereas the anode was positioned posteriorly at the third distal of the leg. The ankle joint was fixed at 20° in plantarflexion, with a total angle of 110° between the foot and the leg
Neuromuscular transcutaneous electrical stimulations under isometric condition were delivered to GM in monopolar technique by an electrical stimulator (St-Pro Multichannel Programmable Neuromuscular Stimulator, LiSin, Turin, Italy). Electrical stimulation was used to activate only a target muscle (Muraoka et al. 2004), to by-pass central nervous system inhibitory mechanisms and to focus mainly on the peripheral mechanisms of fatigue-induced muscle alterations (Bigland-Ritchie et al. 1982; Fitts 2008). The cathode (90 × 40 mm) was placed over the most proximal motor point of GM (Gobbo et al. 2011), whereas the anode (130 × 100 mm) was positioned posteriorly at the third distal of the leg. A set of brief 2-Hz stimulations of increasing amplitude was administered to determine the maximum compound motor unit stimulus (M-wave). Once detected the stimulus that elicited the maximal peak-to-peak M-wave, participants rested for 5 min. Hence, a session of three tetanic stimulations (SA), consisting of a train of pulses (wave shape: biphasic; pulse duration: 304 μs; stimulation frequency: 50 Hz; current amplitude: 110 % of the maximum M-wave; duration: 3 s), was delivered with 5 min of rest in between. After SA, participants rested for 10 min while sitting on the ergometer. Then, the same set of stimulations was repeated (S B ). Thereafter, the fatiguing stimulation protocol (120 s at 35 Hz) previously utilized by Esposito et al. (2009) was administered, after which GM was stimulated tetanically at minute 1, 2 and 7 with the same train of pulses as before fatigue.
Signal acquisition
The stimulation current, the surface EMG and MMG and F signals were recorded during contraction. MMG probe and electrode positioning on the investigated muscle are shown in Fig. 2 (panel B). The stimulation current and the surface EMG and MMG signals from the GM were acquired by a multichannel amplifier (mod. EMG-USB, OtBioelettronica, Turin, Italy; input impedance: >90 MΩ; CMRR: >96 dB; EMG and MMG bandwidth: 10–750 and 0.7–100 Hz, respectively; gain: *1, *1,000 and *2 for stimulation current, EMG and MMG, respectively) with a sampling rate of 10,240 Hz. F signal was recorded by a calibrated load cell (mod. SM-1000 N, Interface, UK) operating linearly between 0 and 1,000 N, amplified (gain: *200) by a 16 bits A/D converter (mod. UM150, Biopac System, CA, USA) and driven to the auxiliary input of the EMG amplifier. EMG signal was detected in single differential modality by a linear array of four electrodes (mod. ELSCH004, OtBioelettronica, Turin, Italy; linear array 45 × 20 mm; electrode 2 × 1 mm; inter-electrode distance 10 mm) fixed to the skin by dual-adhesive foam (mod. AD004, OTBioelettronica, Turin, Italy) filled with conductive gel (Cogel, Comedical, Trento, Italy). The EMG array was oriented with the major axis parallel to the muscle fibres direction and with the EMG electrodes positioned perpendicularly to the major axes of muscle fibres, in accordance with the European recommendations about surface EMG (Hermens et al. 1999). The skin area under the electrodes was cleaned carefully with ethyl alcohol and gently abraded with a special abrasive and conductive cream (Nuprep, Weaver and Co., Aurora, USA) to achieve an inter-electrode impedance below 2,000 Ω. The third electrode of the EMG array was removed and replaced by a mono-directional accelerometer (mod. ADXL103, Analog Devices, Norwood, MA, USA; device weight: <1.0 g; sensitivity: 1,000 mV/g; measure range: ± 1.7 g) placed directly on the skin over the muscle belly for MMG detection. Consequently, the inter-electrode distance from which surface EMG was detected (between the second and the fourth EMG electrodes displaced across the accelerometer) became 20 mm. To minimize site-to-site variability in EMG and MMG signals during repeated measurements on different days, a map with the EMG–MMG probe and the stimulation electrodes position, together with some skin repere points (moles, angiomas and scars), was drawn on a transparency. EMG electrodes were also positioned over the belly of the tibialis anterior muscle to exclude the presence of simultaneous contractions of the GM antagonist muscle.
To evaluate any possible temperature effect on the investigated variables, skin temperature was measured in proximity of the EMG and MMG probes by an infrared thermometer with a laser beam pointer (mod. 826-T2, Testo, Lenzkierch, Germany) throughout the entire testing sessions.
Data analysis
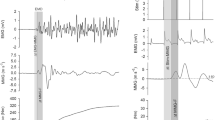
Data analysis was performed off-line by a custom-built routine using a commercially available software (Labview 7.1, National Instruments, Austin, TX, USA). In Fig. 3, the four raw signals from a representative participant are given.
Stimulation current (Stim), EMG, MMG and F signals in a representative participant. Continuous, dashed, dashed-and-dotted, and dotted lines indicate the onset of the stimulation current (Stim), EMG, MMG, and force (F) signals, respectively. First grey area represents the synaptic component, light grey area shows the E–C coupling component, dark grey area indicates the mechanical component, top dark grey area represents the traditionally computed EMD and the very dark grey area indicates the DelayTOT
We considered the first positive peak of the recorded signal to determine Stim onset. The EMG onset corresponded to the first negative peak of the stimulation artefact (Yavuz et al. 2010). These determinations were based on automatic procedures. According to previous studies (Cé et al. 2013; Esposito et al. 2011), MMG and F signals onsets required to exceed, for three consecutive points, the three SD of the mean value obtained in a 100-ms interval of the resting condition immediately preceding the contraction. The three-SD method is a good compromise between operator visual recognition and objective thresholds assessments, even though the risk of an overestimation cannot be ruled out. Signals were visually inspected and the interval was moved when necessary, e.g. when this time frame contained spurious oscillations that would contaminate mean and SD values and, in turn, MMG and F signals onset. This procedure could potentially induce an operator-dependent variation in delays measurement. Therefore, delays calculation was conducted by two independent and expert operators (Op. 1 and Op. 2) to assess inter-operator reliability.
The overall time delay between the onset of the stimulation current and the onset of F development, DelayTOT, and its partitioning in the time delay between Stim and EMG (Δt Stim-EMG, which includes the processes between the action potential at the axonal level and the action potential along the sarcolemma, thus containing also the synaptic latency), between EMG and MMG (Δt EMG-MMG, which includes the E–C coupling processes, cross-bridge formation and the time for pressure waves transmission to the skin), and between MMG and F (Δt MMG-F, the mechanical component from pressure wave propagation to the skin to force transmission at the bone insertion point) were then calculated. Therefore, by adding Δt EMG-MMG to Δt MMG-F, the time delay corresponding to the classical EMD, as defined by Cavanagh and Komi (1979), can be obtained. Consequently, the addition of Δt Stim-EMG to EMD (Δt EMG-MMG + Δt MMG-F) leads to DelayTOT.
After identifying the peak F (pF) as the highest level of F achieved, only the transient phases corresponding to muscle contraction (on-phase) between 5 and 95 % of the pF were considered for calculations (Esposito et al. 2009). Subsequently, the rate of F development (RFD, i.e. the slope of F raise during the on-phase), was determined together with MMG peak-to-peak (MMG p-p).
Force and MMG variables were computed off-line by a custom-built routine of a commercially available software (Labview 7.1, National Instruments, Austin, TX, USA).
Statistical analysis
Raw data were analysed using a statistical software package (IBM SPSS Statistics v. 19, Armonk, NY, USA). To check the normal distribution of the sampling, a Kolgomorov–Smirnov test was applied. A sample size of 16 participants was selected to ensure a statistical power higher than 0.80. A one-way analysis of variance (ANOVA) for repeated measures and an intraclass-coefficient correlation (ICC) analysis were performed to determine the repeatability of the three tetanic stimulations within each session (SA and SB of both D1 and D2) before fatigue. A three-way ANOVA for repeated measures was applied on each variable before fatigue to assess the effects of session, day and operator, and the interaction among the three factors. The effect of fatigue and the time course during recovery were determined by a one-way ANOVA for repeated measures. The location of possible differences was assessed by a Holm–Sidak post hoc test. The magnitude of the changes was assessed using effect size (ES) statistics with the lower and upper 95 % confidence interval (CI). ES was classified as trivial for ES values <0.2, small between 0.2 and 0.6, moderate between 0.6 and 1.2, large between 1.2 and 2.0, and very large when >2.0. Intra- and inter-operator reliability among sessions and days was assessed using a two-way, mixed ICC model and the standard error of measurements (SEM). ICC values were consider as very high if >0.90, high if between 0.70 and 0.89 and moderate if between 0.50 and 0.69. The sensitivity of the different delays was checked by calculating the minimum detectable change at 95 % confidence as a percentage (MDC95 %). The level of significance was set at α < 0.05. Unless otherwise stated, the results are expressed as mean ± standard error (SE).
Results
PRE and POST (POST1, POST2 and POST7) values were assessed according to the following results and considerations.
Before fatigue, the repeatability analysis among the values of the parameters was calculated from the three tetanic stimulations within each session for the two operators. ICC values ranged from high to very high for all the variables (0.874–0.996) and SEM was comprised between 0.78 and 6.61 %. Moreover, ANOVA did not disclose significant differences among the stimulations. Thus, the three values obtained for each session were averaged (SA and SB for the first and the second session within the same day, respectively) and used for further statistical analysis. Moreover, no significant differences were detected by the three-way ANOVA (session, day and operator) among results of each variable. All the data were then pooled together and used as PRE value for further comparisons.
After fatigue, two-way ANOVA (day and operator) disclosed no significant differences in each variable. Thus, for further comparisons, all the data were pooled together and considered as POST1, POST2 and POST7 for the values after 1, 2 and 7 min from the end of the fatiguing stimulation, respectively.
Reliability
MDC95 % values were 11.0, 3.7, 21.5 and 11.1 % for DelayTOT, Δt Stim-EMG, Δt EMG-MMG and Δt MMG-F, respectively.
In Table 1, the mean values (n = 16) of DelayTOT and its components calculated by both operators (Op. 1 and Op. 2) for each session (SA and SB) in both days (D1 and D2) before fatigue are given. Inter-operator analysis for all the considered variables showed ICC values between 0.901 and 0.999, indicating a very high reliability. Intra-operator analysis revealed a very high intra-day reliability between sessions (SA and SB, from 0.919 to 0.989), while reliability was from high to very high for between-day analysis (Inter-day reliability, D1 and D2, from 0.781 to 0.981). SEM values ranged from 1.79 to 8.71 % of the relative mean value.
Reliability analysis after fatigue is provided in Table 2. Inter-operator reliability results were between 0.847 and 0.999, indicating a high to very high reliability for all variables. Intra-operator analysis showed values between 0.863 and 0.991 for all the variables during recovery (POST1, POST2 and POST7), evidencing a high to very high reliability of the measurement. SEM values after fatigue ranged from 1.77 to 11.29 % of the relative mean value.
Effects of fatigue
The effects of fatigue on F and MMG signals are presented in Fig. 4. After fatigue, ANOVA disclosed a pF reduction from 687 ± 51 to 639 ± 51 N (P < 0.05, ES = 0.9, CI 0.19–1.65). pF remained depressed during the entire recovery period (POST2: 671 ± 60 N and POST7: 655 ± 60 N; P < 0.05, ES = 0.3, CI 0.42–0.98 and ES = 0.6, CI 0.15–1.27 for POST2 and POST7, respectively). Fatigue reduced significantly also RFD from 860 ± 111 to 555 ± 57 N/s (P < 0.05, ES = 3.4, CI 2.29–4.45), which returned to baseline within 2 min from the end of the fatiguing stimulation. Lastly, MMG p-p decreased significantly after the fatiguing stimulation from 19.6 ± 1.9 to 15.9 ± 1.6 m/s2 (P < 0.05, ES = 2.0, CI 1.20–2.91), then remaining significantly lower for the entire recovery period (17.4 ± 1.7 m/s2 at minute 7 of recovery; ES = 1.2, CI 0.44–1.94, P < 0.05).
Main force and MMG parameters. Peak force (pF), rate of force development (RFD) and MMG peak-to-peak (MMG p-p) values before (PRE) and 1, 2 and 7 min after the fatiguing protocol (POST1, POST2 and POST7, respectively), normalized to the value before fatigue. PRE values were pooled from each session (SA and SB) of both days (D1 and D2). POST values were pooled from results of both days (D1 and D2). The vertical grey bar represents the fatiguing protocol. Data are expressed as mean ± SE. *P < 0.05
In Fig. 5, the effects of fatigue on DelayTOT and its components are shown.
DelayTOT and its subcomponents. DelayTOT, ∆t Stim-EMG, ∆t EMG-MMG and ∆t MMG-F values before (PRE) and 1, 2 and 7 min after the fatiguing protocol (POST1, POST2 and POST7, respectively), normalized to the value before fatigue. PRE values were pooled from the data measured by the two operators in each session (SA and SB) of both days (D1 and D2). POST values were pooled from results of both operators in the 2 days (D1 and D2). The vertical grey bar represents the fatiguing protocol. Data are expressed as mean ± SE. *P < 0.05
After the fatiguing stimulation, ANOVA revealed a significant increase in DelayTOT by 4.7 ± 0.7 ms (P < 0.05, ES = 1.5 and CI 0.73–2.31). All DelayTOT components were significantly affected by fatigue, with an increase in ∆t Stim-EMG, in ∆t EMG-MMG and in ∆t MMG-F by 0.106 ± 0.028, 1.30 ± 0.31 and 3.35 ± 0.54 ms, respectively (P < 0.05, ES = 0.8 and CI 0.06–1.50, ES = 0.6 and CI 0.06–1.36, ES = 1.4 and CI 0.60–2.13 for ∆t Stim-EMG, ∆t EMG-MMG and ∆t MMG-F, respectively). When partitioning each contribution to the overall fatigue-induced increase in DelayTOT, ∆t Stim-EMG, ∆t EMG-MMG and ∆t MMG-F contributed by about 2.2, 27.3 and 70.5 %, respectively. During recovery, ∆t Stim-EMG and ∆t EMG-MMG recovered within 2 min from the fatiguing stimulation, while DelayTOT returned to baseline within 7 min. On the contrary, ∆t MMG-F was still elongated after 7 min of recovery.
Discussion
The novel finding of the present study was that intra- and inter-operator reliability of the measurement of each single DelayTOT component was from high to very high, When assessing reliability after the effects of fatigue, reliability was still from high to very high. Collectively, these data indicate that when delays are assessed under a controlled and standardized condition, very reliable and sensible data can be obtained. Fatigue lengthened DelayTOT by about 18 %, which recovered within 7 min. DelayTOT components were all altered by fatigue, with a larger effect on the mechanical aspect. Moreover, Δt Stim-EMG and Δt EMG-MMG recovered within 2 min, while mechanical events took longer to restore.
Reliability
The electromechanical latency reliability has been already evaluated only for EMD (Almosnino et al. 2009; Cé et al. 2013; Hopkins et al. 2007; Howatson et al. 2009; Lacourpaille et al. 2013b; Sasaki et al. 2011), while reliability assessment for DelayTOT and its components is still missing in the literature. Moreover, only few studies tested EMD reliability over different days (Almosnino et al. 2009; Cé et al. 2013; Lacourpaille et al. 2013b). Therefore, the present study is the first to assess the consistency of the measurement of each DelayTOT component when data refer to different sessions, different days and also different operators. As previously mentioned (see “Data analysis”), indeed, an operator-dependent variation in MMG and F signal onset detection could exist. Intra-operator reliability analysis compared the data of the different sessions and the different days within each operator, and showed values from high to very high for all DelayTOT components, both between different sessions within the same day (inter-session reliability, SA vs. SB) and between different days (inter-day reliability, D1 vs. D2). Δt Stim-EMG and Δt EMG-MMG showed the highest ICC values for inter-session and inter-day reliability. Inter-operator reliability assessment (Op. 1 vs. Op. 2) showed a high consistency of the measurement for the delays calculated by the operators, both between sessions and days. Therefore, this approach can provide repeatable measurements, even when performed by different operators on different days.
Lastly, the significant changes of the different components above the MDC95 found after fatigue indicate that this approach can afford adequate levels of sensitivity under this experimental condition.
Electrochemical and mechanical contribution to DelayTOT
∆t Stim-EMG in the present study was about 1.44 ms, a period of time compatible with the duration of the mechanisms included (see “Introduction”). Indeed, the neurotransmitter release by the motor nerve impulse starts after 210 μs (Hubbard and Schmidt 1963) and lasts 200 μs (Martyn et al. 2009). The time to depolarize the end-plate region takes less than 150 μs (Katz and Miledi 1965) and the delay between the excitatory post-synaptic potential and the firing of the postsynaptic cell have been shown to range between 210 and 650 μs, respectively (Sabatini and Regehr 1999).
Although slightly longer than in previous reports (Esposito et al. 2011; Hufschmidt 1985; Nordez et al. 2009; Petitjean et al. 1998; Sasaki et al. 2011), also ∆t EMG-MMG (about 9 ms) lasted for a period of time that can reasonably include all the processes involved in this latency. Indeed, after sarcolemmal depolarization, dihydropyridine and ryanodine receptors interaction occurs within 2 ms (Fill and Copello 2002), Ca2+ release by sarcoplasmic reticulum has a time to peak between 1.6 and 4.0 ms (Baylor and Hollingworth 2007), and the time for troponin activation of about 2.7 ms (Solzin et al. 2007). Lastly, the time for the pressure wave generated by myosin heads rotation to propagate toward the skin should also be included, even though its duration has not been measured yet.
The slight discrepancy between ∆t EMG-MMG duration in the present study and that previously reported (Esposito et al. 2011; Hufschmidt 1985; Petitjean et al. 1998; Sasaki et al. 2011) could be possibly explained by differences in the reference point adopted for EMG onset detection, in the criteria adopted for signal onset detection, in the transducers utilized for monitoring muscle mechanical activity, in the transducer position with respect to EMG electrodes location, in fibre typing of the investigated muscles, in the intensities and durations of stimulation and in the joint angles investigated (Hug et al. 2011b; Lacourpaille et al. 2013a; Muraoka et al. 2004; Nilsson et al. 1977; Viitasalo and Komi 1981; Yavuz et al. 2010).
Lastly, the duration of ∆t MMG-F (about 17 ms) is within the range of previous studies reporting values between 8 and 42 ms (Cé et al. 2013; Esposito et al. 2011; Nordez et al. 2009; Petitjean et al. 1998; Sasaki et al. 2011). Differences in muscle fibre type composition, muscle temperature, subject positioning on the ergometer, joint angle, site of stimulation, frequency and amplitude of the current delivered to the muscle, and criteria for detecting signals onset could explain also in this case the difference.
Overall, DelayTOT was about 27.5 ms. When partitioning DelayTOT in its three sub-components, the relative contribution of Δt Stim-EMG, ∆t EMG-MMG and ∆t MMG-F was about 5, 34 and 61 %, respectively. When extracting EMD (Δt EMG-MMG + Δt MMG-F) from DelayTOT, this latency was about 26 ms, which is within the range of the previously reported values between 11.6 and 45 ms (Esposito et al. 2011; Hufschmidt 1985; Nordez et al. 2009; Petitjean et al. 1998; Sasaki et al. 2011). The relative contribution of ∆t EMG-MMG and ∆t MMG-F to EMD was about 35 and 65 %, respectively, indicating that the relative weight of the mechanical component was predominant. This observation is qualitatively in line with previous findings, in which the electrochemical contribution to EMD ranged from 17 to 40 % (Hufschmidt 1985; Petitjean et al. 1998; Sasaki et al. 2011).
Noticeably, Nordez et al. (2009) found by a high frame rate ultrasound technique that the delay between the electrical stimulation and the onset of fascicle motion and between the onset of fascicle motion and the onset of F production contributed to EMD by 52 and 48 %, respectively. The first delay had an absolute duration relatively similar to that found in the present study (about 6.0 and 9.2 ms for Nordez et al. and the present study, respectively). The slightly shorter duration in Nordez et al. can be reasonably ascribed to the ultrasound technique, which permits to detect the first mechanical event, fascicle motion, before pressure wave transmission to the skin, where the accelerometer can detect it. On the contrary, the absolute time required for the processes included in the second delay differs markedly between these two studies (about 5.5 and 16.8 ms for Nordez et al. and the present study, respectively). This discrepancy may be attributed to the different criteria utilized for force onset detection (first point with a negative derivative in the reverse direction of time and 3 SD above baseline, for Nordez et al. and the present study, respectively) and the ankle joint angle utilized during isometric contraction. Indeed, the 10° angle in plantarflexion adopted by Nordez et al. compared to the 20° in the present study likely induced a higher level of passive pre-tensioning of the muscle–tendon unit, thus shortening force transmission to the tendon insertion point. Moreover, inter-subject variability of the mechanical properties of the passive elements involved in tension transmission along the muscle and the tendon should not be disregarded (Nordez et al. 2009).
Acute effects of fatigue on delays
In the present study, DelayTOT and its three subcomponents all lengthened after fatigue.
The elongation of Δt Stim-EMG suggests a fatigue-induced effect on several mechanisms, among which a propagation failure at axonal level (Sieck and Prakash 1995), an alteration of synapsis functionality (Sieck and Prakash 1995; Wieser 1915), a desensitization of the post-synaptic end plate (Sieck and Prakash 1995) and an impairment of the propagation of the action potential at the sarcolemmal level (Juel 1988). Given some controversial findings, whether this lengthening occurs also during voluntary contraction is still a matter of debate (Kirkendall 1990) and requires further investigation.
Similarly, also ∆t EMG-MMG elongated after fatigue. Such an increase depends on the well-known alterations induced by fatigue on the several factors included in this latency, such as a decrease in sarcolemmal propagation velocity (Edwards et al. 2012), an impairment in Ca2+ release by sarcoplasmic reticulum (Ament and Verkerke 2009; Fitts 2008), an alteration of the ryanodine receptor functionality (Debold 2012), and of the actin–myosin cross-bridges cycle rate (Ament and Verkerke 2009; Fitts 2008).
Lastly, a significant and large ∆t MMG-F elongation occurred after fatigue, suggesting modifications of the MTU mechanical properties, such as a decrease in stiffness (Jaskolski et al. 2007; Taylor et al. 1997; Zhang and Rymer 2001). A reduced MTU stiffness, indeed, causes an elongation of the time necessary to tension the SEC and, consequently, to transfer F to the insertion point (Yavuz et al. 2010).
Overall, the distribution of the effects of fatigue appeared to affect mainly the mechanical factors. Indeed, DelayTOT elongation after the fatiguing stimulation was distributed among the three sub-components by 2, 27 and 71 % for Δt Stim-EMG, Δt EMG-MMG and Δt MMG-F, respectively.
Taking into account that before fatigue Δt Stim-EMG represents only 5 % of DelayTOT, its variations may have paltry effects on the overall delay.
The present study is the first that stepped forward to evaluate the time course of recovery of DelayTOT and its sub-components, showing that during recovery, DelayTOT returned to baseline within 7 min from the end of the fatiguing stimulation. This was the case also for ∆t MMG-F, but not for Δt Stim-EMG and Δt EMG-MMG, which recovered earlier (within 2 min), indicating that the mechanical events occurring after myosin head rotation took longer to restore than those involved in synaptic transmission, action potential propagation, and E–C coupling processes. Only one previous study evaluated the time course of recovery of the electromechanical latency (EMD, not DelayTOT) changes after a fatiguing protocol and found that the fatigue-induced elongation recovered within 5 min (Zhou 1996). However, the mechanisms responsible for this behaviour could not be identified, as EMD partitioning was not applied.
Lastly, a final consideration should be taken into account. EMG onset assessment may influence markedly the exact duration of the first two components of DelayTOT (Δt Stim-EMG and Δt EMG-MMG). In the present study, we decided not to remove the stimulation artefact to avoid partial EMG signal cancellation due to artefact suppression (O’Keeffe et al. 2001). Consequently, the beginning of M-wave was fused with the stimulation artefact. Therefore, the choice of the stimulation artefact as the beginning of muscle electrical activation, which was adopted also in many other investigations (Lacourpaille et al. 2013a, b; Muraoka et al. 2004; Nordez et al. 2009), may slightly anticipate the EMG onset and, therefore, introduce the risk of a bias in Δt Stim-EMG (underestimation) and Δt EMG-MMG (overestimation) measurement.
Conclusion
The combined EMG, MMG and F approach allowed the partitioning of the time interval between neuromuscular activation and force production (DelayTOT). The three components, (Δt Stim-EMG, Δt EMG-MMG and ∆t MMG-F) contributed to the total delay by 5, 34 and 61 %, respectively. After the administration of a fatiguing protocol, our approach was sufficiently sensitive to detect fatigue-induced elongations of each sub-component. In spite of methodological issues raised on previous investigations, the present study indicates that the measurement of DelayTOT and its components presents a very high intra- and inter-operator reliability within the same session and between different days, both before and after fatigue. Collectively, these findings indicate that, when the measurement is performed under controlled and standardized conditions, sensitive and reliable data on the investigated latencies can be obtained. Consequently, this combined approach could be a valid means to detect changes induced by physiological and/or pathological processes, such as the effects of training, fatigue, muscle temperature as well as myotonic phenomena, even when the evolution of the changes has to be monitored in different laboratories over a long period of time.
Abbreviations
- D1 and D2 :
-
Day 1 and day 2
- DelayTOT :
-
Latency between the onset of stimulation current and force development
- ∆t Stim-EMG:
-
Time interval between the onset of stimulation current and the onset of muscle electrical activation. Synaptic component
- ∆t EMG-MMG:
-
Time interval between the onset of muscle electrical activation and the onset of muscle contraction. E–C coupling component
- ∆t MMG-F:
-
Time interval between the onset of muscle contraction and the force development. Mechanical component
- EMD:
-
Electromechanical delay. Time interval between the onset of muscle electrical activation and force development
- EMG:
-
Electromyography
- F:
-
Force
- GM:
-
Gastrocnemius medialis muscle
- MMG:
-
Mechanomyography
- MMG p-p:
-
MMG peak-to-peak
- MTU:
-
Muscle–tendon unit
- Op. 1 and Op. 2:
-
Operator 1 and operator 2
- pF:
-
Peak force
- RFD:
-
Rate of force development
- SA and SB :
-
Session A and session B
- Stim:
-
Stimulation current
References
Almosnino S, Pelland L, Pedlow SV, Stevenson JM (2009) Between-day reliability of electromechanical delay of selected neck muscles during performance of maximal isometric efforts. Sports Med Arthrosc Rehabil Ther Technol 1:22
Ament W, Verkerke GJ (2009) Exercise and fatigue. Sports Med 39:389–422
Barry DT (1992) Vibrations and sounds from evoked muscle twitches. Electromyogr Clin Neurophysiol 32:35–40
Baylor SM, Hollingworth S (2007) Simulation of Ca2+ movements within the sarcomere of fast-twitch mouse fibers stimulated by action potentials. J Gen Physiol 130:283–302
Bigland-Ritchie B, Kukulka CG, Lippold OC, Woods JJ (1982) The absence of neuromuscular transmission failure in sustained maximal voluntary contractions. J Physiol 330:265–278
Cavanagh PR, Komi PV (1979) Electromechanical delay in human skeletal muscle under concentric and eccentric contractions. Eur J Appl Physiol Occup Physiol 42:159–163
Cé E, Rampichini S, Agnello L, Limonta E, Veicsteinas A, Esposito F (2013) Effects of temperature and fatigue on the electromechanical delay components. Muscle Nerve 47:566–576
Debold EP (2012) Recent insights into the molecular basis of muscular fatigue. Med Sci Sport Exer 44:1440–1452
Edwards JN, Cully TR, Shannon TR, Stephenson DG, Launikonis BS (2012) Longitudinal and transversal propagation of excitation along the tubular system of rat fast-twitch muscle fibres studied by high speed confocal microscopy. J Physiol 590:475–492
Esposito F, Cè E, Gobbo M, Veicsteinas A, Orizio C (2005) Surface EMG and mechanomyogram disclose isokinetic training effects on quadriceps muscle in elderly people. Eur J Appl Physiol 94:549–557
Esposito F, Ce E, Rampichini S, Veicsteinas A (2009) Acute passive stretching in a previously fatigued muscle: electrical and mechanical response during tetanic stimulation. J Sports Sci 27:1347–1357
Esposito F, Limonta E, Ce E (2011) Passive stretching effects on electromechanical delay and time course of recovery in human skeletal muscle: new insights from an electromyographic and mechanomyographic combined approach. Eur J Appl Physiol 111:485–495
Fill M, Copello JA (2002) Ryanodine receptor calcium release channels. Physiol Rev 82:893–922
Fitts RH (1994) Cellular mechanisms of muscle fatigue. Physiol Rev 74:49–94
Fitts RH (2008) The cross-bridge cycle and skeletal muscle fatigue. J Appl Physiol 104:551–558
Gobbo M, Gaffurini P, Bissolotti L, Esposito F, Orizio C (2011) Transcutaneous neuromuscular electrical stimulation: influence of electrode positioning and stimulus amplitude settings on muscle response. Eur J Appl Physiol 111:2451–2459
Gordon G, Holbourn AH (1948) The sounds from single motor units in a contracting muscle. J Physiol 107:456–464
Granata KP, Ikeda AJ, Abel MF (2000) Electromechanical delay and reflex response in spastic cerebral palsy. Arch Phys Med Rehabil 81:888–894
Grosset JF, Piscione J, Lambertz D, Perot C (2009) Paired changes in electromechanical delay and musculo-tendinous stiffness after endurance or plyometric training. Eur J Appl Physiol 105:131–139
Hermens H, Freiks B, Merletti R, Stegeman D, Blok J, Rau G, Disselhorst-Klug C, Hagg G (1999) Eurpoean recommendations for surface electromyography. RRD, The Netherlands
Hopkins JT, Feland JB, Hunter I (2007) A comparison of voluntary and involuntary measures of electromechanical delay. Int J Neurosci 117:597–604
Howatson G, Glaister M, Brouner J, van Someren KA (2009) The reliability of electromechanical delay and torque during isometric and concentric isokinetic contractions. J Electromyogr Kinesiol 19:975–979
Hubbard JI, Schmidt RF (1963) An electrophysiological investigation of mammalian motor nerve terminals. J Physiol 166:145–167
Hufschmidt A (1985) Acoustic phenomena in the latent period of skeletal muscle: a simple method for in vivo measurement of the electro-mechanic latency (EML). Pflugers Arch 404:162–165
Hug F, Gallot T, Catheline S, Nordez A (2011a) Electromechanical delay in biceps brachii assessed by ultrafast ultrasonography. Muscle Nerve 43:441–443
Hug F, Lacourpaille L, Nordez A (2011b) Electromechanical delay measured during a voluntary contraction should be interpreted with caution. Muscle Nerve 44:838–839
Jaskolski A, Andrzejewska R, Marusiak J, Kisiel-Sajewicz K, Jaskolska A (2007) Similar response of agonist and antagonist muscles after eccentric exercise revealed by electromyography and mechanomyography. J Electromyogr Kinesiol 17:568–577
Juel C (1988) Muscle action-potential propagation velocity changes during activity. Muscle Nerve 11:714–719
Katz B, Miledi R (1965) Effect of temperature on synaptic delay at neuromuscular junction. J Physiol 181:656–670
Kirkendall DT (1990) Mechanisms of peripheral fatigue. Med Sci Sports Exerc 22:444–449
Lacourpaille L, Hug F, Nordez A (2013a) Influence of passive muscle tension on electromechanical delay in humans. PLoS One 8:e53159
Lacourpaille L, Nordez A, Hug F (2013b) Influence of stimulus intensity on electromechanical delay and its mechanisms. J Electromyogr Kinesiol 23:51–55
Martyn JAJ, Fagerlund MJ, Eriksson LI (2009) Basic principles of neuromuscular transmission. Anaesthesia 64:1–9
Muraoka T, Muramatsu T, Fukunaga T, Kanehisa H (2004) Influence of tendon slack on electromechanical delay in the human medial gastrocnemius in vivo. J Appl Physiol 96:540–544
Nilsson J, Tesch P, Thorstensson A (1977) Fatigue and EMG of repeated fast voluntary contractions in man. Acta Physiol Scand 101:194–198
Nordez A, Gallot T, Catheline S, Guevel A, Cornu C, Hug F (2009) Electromechanical delay revisited using very high frame rate ultrasound. J Appl Physiol 106:1970–1975
O’Keeffe DT, Lyons GM, Donnelly AE, Byrne CA (2001) Stimulus artifact removal using a software-based two-stage peak detection algorithm. J Neurosci Methods 109:137–145
Orizio C, Baratta RV, Zhou BH, Solomonow M, Veicsteinas A (1999) Force and surface mechanomyogram relationship in cat gastrocnemius. J Electromyogr Kinesiol 9:131–140
Orizio C, Gobbo M, Veicsteinas A, Baratta RV, Zhou BH, Solomonow M (2003) Transients of the force and surface mechanomyogram during cat gastrocnemius tetanic stimulation. Eur J Appl Physiol 88:601–606
Petitjean M, Maton B, Fourment A (1998) Summation of elementary phonomyograms during isometric twitches in humans. Eur J Appl Physiol Occup Physiol 77:527–535
Sabatini BL, Regehr WG (1999) Timing of synaptic transmission. Ann Rev Physiol 61:521–542
Sasaki K, Sasaki T, Ishii N (2011) Acceleration and force reveal different mechanisms of electromechanical delay. Med Sci Sports Exerc 43:1200–1206
Sieck GC, Prakash YS (1995) Fatigue at the neuromuscular junction. Branch point vs presynaptic vs postsynaptic mechanisms. Adv Exp Med Biol 384:83–100
Solzin J, Iorga B, Sierakowski E, Alcazar DPG, Ruess DF, Kubacki T, Zittrich S, Blaudeck N, Pfitzer G, Stehle R (2007) Kinetic mechanism of the Ca2 + -dependent switch-on and switch-off of cardiac troponin in myofibrils. Biophys J 93:3917–3931
Taylor DC, Brooks DE, Ryan JB (1997) Viscoelastic characteristics of muscle: passive stretching versus muscular contractions. Med Sci Sports Exerc 29:1619–1624
Viitasalo JT, Komi PV (1981) Interrelationships between electromyographic, mechanical, muscle structure and reflex time measurements in man. Acta Physiol Scand 111:97–103
Wieser F (1915) The elongation of the latency time of nerve-ending organs through fatigue. Z Biol Munich 65:449–467
Yavuz SU, Sendemir-Urkmez A, Turker KS (2010) Effect of gender, age, fatigue and contraction level on electromechanical delay. Clin Neurophysiol 121:1700–1706
Zhang LQ, Rymer WZ (2001) Reflex and intrinsic changes induced by fatigue of human elbow extensor muscles. J Neurophysiol 86:1086–1094
Zhou S (1996) Acute effect of repeated maximal isometric contraction on electromechanical delay of knee extensor muscle. J Electromyogr Kinesiol 6:117–127
Zhou S, McKenna MJ, Lawson DL, Morrison WE, Fairweather I (1996) Effects of fatigue and sprint training on electromechanical delay of knee extensor muscles. Eur J Appl Physiol Occup Physiol 72:410–416
Zhou S, Carey MF, Snow RJ, Lawson DL, Morrison WE (1998) Effects of muscle fatigue and temperature on electromechanical delay. Electromyogr Clin Neurophysiol 38:67–73
Acknowledgments
The authors wish to thank all the participants involved in the study, for their patience and committed involvement and Pietro Brioschi for his valuable technical assistance. The study was supported by a PUR Grant (2008) of the University of Milan assigned to Prof. Fabio Esposito.
Conflict of interest
Authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Toshio Moritani.
Rights and permissions
About this article
Cite this article
Rampichini, S., Cè, E., Limonta, E. et al. Effects of fatigue on the electromechanical delay components in gastrocnemius medialis muscle. Eur J Appl Physiol 114, 639–651 (2014). https://doi.org/10.1007/s00421-013-2790-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-013-2790-9