Abstract
Purpose
To analyze if live high–train low (LHTL) effectiveness is improved when daily training is guided by heart rate variability (HRV).
Methods
Twenty-four elite Nordic skiers took part in a 15-day LHTL study and were randomized into a HRV-guided training hypoxic group (H-HRV, n = 9, sleeping in normobaric hypoxia, FiO2 = 15.0%) and two predefined training groups sleeping either in hypoxia (H, n = 9, FiO2 = 15.0%) or normoxia (N, n = 6). HRV and training loads (TL) were recorded daily. Prior (Pre), one (Post-1), and 21 days (Post-21) following LHTL, athletes performed a 10-km roller-ski test, and a treadmill test for determination of \(\dot {V}{{\text{O}}_{{\text{2max}}}}\) was performed at Pre and Post-1.
Results
Some HRV parameters measured in supine position were different between H-HRV and H: low and high (HF) frequency power in absolute (ms2) (16.0 ± 35.1 vs. 137.0 ± 54.9%, p = 0.05) and normalized units (− 3.8 ± 10.1 vs. 53.0 ± 19.5%, p = 0.02), HF(nu) (6.3 ± 6.8 vs. − 13.7 ± 8.0%, p = 0.03) as well as heart rate (3.7 ± 6.3 vs. 12.3 ± 4.1%, p = 0.008). At Post-1, \(\dot {V}{{\text{O}}_{{\text{2max}}}}\) was improved in H-HRV and H (3.8 ± 3.1%; p = 0.02 vs. 3.0 ± 4.4%; p = 0.08) but not in N (0.9 ± 5.1%; p = 0.7). Only H-HRV improved the roller-ski performance at Post-21 (− 2.7 ± 3.6%, p = 0.05).
Conclusion
The daily individualization of TL reduced the decrease in autonomic nervous system parasympathetic activity commonly associated with LHTL. The improved performance and oxygen consumption in the two LHTL groups confirm the effectiveness of LHTL even in elite endurance athletes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It is known that in a group of athletes, the same standardized training program could induce a great diversity of responses in terms of performance and physiological adaptations (Bouchard and Rankinen 2001; Hautala et al. 2003, 2006). Age, sex, ethnicity, and genetic characteristics are among different factors that influence adaptations following endurance training (Bouchard et al. 1999; Bouchard and Rankinen 2001). In addition, the initial state of autonomic nervous activity is important for the physiological response to exercise. Since a large component of the inter-individual variability of the physiological responses to a standardized training plan is related to the balance between parasympathetic and sympathetic activities (Aubert et al. 2003; Perini et al. 1990), one may assume that an individually heart rate variability-guided (HRV-guided) training could lead to improved benefits. Despite the large number of studies showing a relationship between endurance training and HRV (Hedelin et al. 2001; Iellamo et al. 2002; Kiviniemi et al. 2006), to date only Kiviniemi et al. (2007, 2010) have reported HRV-based individual adjustment of the training. Specifically, nine young male recreational athletes (HRV-guided) performed 4 weeks of training adjusted with the HF values measured daily in the standing position, resulting in a change of \(\dot {V}{{\text{O}}_{{\text{2max}}}}\) similar as compared with those of a control group of athletes who performed a standardized training program. The change in maximal running speed was significantly greater in the HRV-guided compared to the standardized group.
The responses to altitude training are complex since hypoxia and training stress are combined (Schmitt et al. 2006a). In hypoxia, the reduced PiO2 (partial pressure of inspired oxygen) represents an additional stress, which has been shown to induce specific HRV responses (Schmitt et al. 2006a) with a combination of increased sympathetic and decreased parasympathetic nervous activities (Perini and Veicsteinas 2003; Schmitt et al. 2006a; Sevre et al. 2001; Zupet et al. 2009). Schmitt et al. (2006a, b, 2008) have shown that HRV parameters are modified differently depending on the intensity of training performed in altitude. Conversely, high-intensity exercise is not recommended during the first 7–10 days of an altitude training camp (Millet et al. 2010). In the different altitude training methods, live high–train low (LHTL) is widely recognized as the “gold standard”. Based on more than 15 years of research, LHTL is known for inducing a 1–3% improvement in specific endurance performance when compared to similar normoxic training (Bonetti and Hopkins 2009). It has also recently been demonstrated that normobaric and hypobaric LHTL stimulation produce a similar improvement in aerobic performance (Saugy et al. 2016) and increased hemoglobin mass (Hauser et al. 2016). However, the debate is not complete, as some authors have recently suggested that the beneficial effect of LHTL could be very limited in elite athletes assuming that these athletes have a reduced ability to further increase their aerobic performance following altitude training (McLean et al. 2013; Robach and Lundby 2012).
To our knowledge, the effectiveness of LTHL with daily adjustment of training load/content based on daily HRV analysis has never been investigated. The fact that the present study was performed with elite endurance athletes is of highest importance since it is known that elite athletes have smaller windows for performance improvement than their lower level counterparts and that the elite athlete’s responses to training are very specific (Burtscher et al. 1996). Therefore, the first aim of the present study was to test the hypothesis that HRV-guided LHTL training would blunt both the decrease in parasympathetic and the increase in sympathetic activities classically reported during LHTL and would enhance aerobic performance when compared with a standardized (predefined training) LHTL program. In addition, the secondary aim was to confirm that LHTL is effective even in elite endurance athletes.
Methods
Subjects
Subjects were 24 elite Nordic-skiers, members of the cross-country ski and Nordic-combined French national teams with 19 men (age 23.3 ± 3.6 years; \(\dot {V}{{\text{O}}_{{\text{2max}}}}\) 67.8 ± 3.6 mL kg−1 min−1) and 5 women (age 22.8 ± 4.1 years; \(\dot {V}{{\text{O}}_{{\text{2max}}}}\) 58.9 ± 2.5 mL kg−1 min−1). All these athletes compete in European cup or World cups and some are medalists in World championships. The subjects were assigned into a HRV-guided training normobaric hypoxic group (H-HRV, n = 9, simulated altitude of 2700 m, FiO2 = 15.0%), and two predefined training groups sleeping either in normobaric hypoxia (H, n = 9, simulated altitude of 2700 m, FiO2 = 15.0%) or in normoxia (N, n = 6). The three groups were randomly matched for Nordic disciplines (cross-country ski vs. Nordic combined) and gender (male vs. female). No athletes were under any medical treatment which might influence HRV during the study. The characteristics of subjects by groups in gender (men, women), age (years), mass (kg), height (cm), and fat mass (%) were:
For H-HRV: 7 men (22.9 ± 4.3 year, 68.1 ± 5.5 kg, 177.3 ± 5.8 cm, 9.5 ± 0.9%) and 2 women (20.5 ± 0.7 year, 56.5 ± 5.0 kg, 163.5 ± 6.4 cm, 19.0 ± 1.5%).
For H: 6 men (21.8 ± 1.3 year, 72.5 ± 6.3 kg, 180.2 ± 3.4 cm, 9.8 ± 0.8%) and 3 women (24.3 ± 4.9 year, 55.0 ± 5.0 kg, 163.7 ± 5.8 cm, 18.0 ± 1.4%).
For N: 5 men (25.7 ± 3.5 year, 69.7 ± 5.2 kg, 177.7 ± 6.3 cm, 10.0 ± 1.1%).
Study design
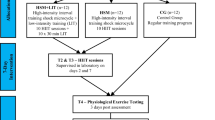
The study design is shown in Fig. 1. The study was approved by the local ethical committee (French National Conference of Research Ethics Committee; N°CPP EST I: 2014/33; Dijon, France). All experimental procedures conformed to the standards set by the Declaration of Helsinki. All subjects provided written informed consent to participate in the study after having been informed in detail about the experimental procedure. Exclusion criteria for participation included any history of altitude-related sickness and health risks that could compromise the subject’s safety during training and/or hypoxic exposure. The study was performed at the French National Ski-Nordic Centre located at an altitude of 1200 m which contains 11 hypoxic rooms. These hypoxic chambers were of medium size (15 ± 1 m2) and equipped with conventional beds. Two subjects were in each room and primarily spent time sleeping or resting between training sessions.
Experimental design. Heart rate variability (HRV), hypoxic HRV-guided group (H-HRV, n = 9), hypoxic predefined group (H, n = 9), normoxic predefined group (N, n = 6), maximal oxygen uptake (\(\dot {V}{{\text{O}}_{{\text{2max}}}}\)), night peripheral oxygen saturation (SpO2), and heart rate (HR). Vertical arrows indicate the day of the tests and horizontal arrows define the day-tests period
Measurements
Daily measurements
Each morning of the 15-day LHTL camp, the following parameters were recorded: duration of hypoxic exposure in hypoxic chambers, night peripheral oxygen saturation (SpO2) and heart rate (HR), morning heart rate variability (HRV), and a questionnaire validated by the consensus group on overtraining of the French Society of Sports Medicine (QSFMS) (Maso et al. 2004) was administrated and training load (TL) calculated.
Night peripheral oxygen saturation (SpO2) and heart rate (HR)
SpO2 and HR were recorded continuously each night from Pre to Post-1 at 0.25 Hz with a wrist oximeter connected to a finger sensor (Wristox 3150® with 8000SM-WO Sensor, Nonin, Plymouth, MN, USA). Data were obtained and the average value was computed for further analysis.
Heart rate variability tests
The protocol of HRV tests is presented in detail in previous articles (Schmitt et al. 2013, 2016). Briefly, the HRV test consisted of a 15-min RR interval recording at rest with 8 min supine (SU) followed by 7 min standing (ST). The HRV recording was performed daily in the morning immediately after waking and voiding the urinary bladder. HRV analyses were performed on RR intervals between the 3rd and 8th min supine, and between the 9th and 14th min standing. Measurement of the interval duration between two R waves of the cardiac electrical activity was performed with a HR monitor (Ambit3 Peak, Suunto®, Vantaa, Finland). The spectral power was calculated with the fast Fourier transform (FFT) using a software (Nevrokard® HRV, Medistar, Ljubljana, Slovenia). The power of spectral density was measured by frequency bands in ms2 Hz−1 and the spectral power was expressed in ms2 (Task-Force 1996). The high-frequency (HF) power band (0.15–0.40 Hz) reflects alteration of the parasympathetic influence on the heart and is related to the respiratory sinus arrhythmia (Pomeranz et al. 1985). While the low-frequency (LF) power band (0.04–0.15 Hz) is also driven by parasympathetic tone, and presently considered responsible for carrying vagal resonances to either changes in vasomotor tone (often sympathetic) or in the central modulation of sympathetic tone (Reyes Del Paso et al. 2013). Spectral power in the LF power band has also been shown to be related to fluctuations of arterial blood pressure (Akselrod et al. 1981; Pomeranz et al. 1985) and to activity of the baroreflex (Goldstein et al. 2011). Both in supine (SU) and in standing (ST) positions, LF and HF were calculated in absolute spectral power units (ms2) and in normalized units (nu) with LF(nu) = LF/(LF + HF) × 100 and HF(nu) = HF/(HF + LF) × 100. The total spectral power (TP) was calculated from the sum of LF and HF.
Performance measurements at Pre, Post-1, and Post-21
A maximal roller-ski test was performed on an official FIS-accredited roller-ski asphalt track of the National French Ski-Nordic Centre, with an alternation of uphill, downhill, and flat terrain. Determined by the coaches, the women performed 2 laps in classical technique and the men 3 laps of 3.3 km in skating technique. Measurements were performed on each subjects own roller-skis and poles while maintaining the same equipment use for each test.
Incremental maximal treadmill test at Pre 1, Post-1 and Post-21
The incremental running treadmill \(\dot {V}{{\text{O}}_{{\text{2max}}}}\) protocol used in the present study is routinely used for the medical follow-up of the French Nordic-skiers in the same laboratory. The test started with 3-min stages at 8 km h−1 and 2% grade, followed by 9 km h−1 and 2%, 9 km h−1 and 4%, 9 km h−1 and 6%, and 10 km h−1 and 6% grade. Every 3 min, the stage increased first by speed, then by grade until the second ventilatory threshold (VT2) was passed. After VT2, the protocol changed to 1-min stages increasing grade to a maximum of 16% until exhaustion. During all tests, heart rate (HR) was continuously monitored using a telemetry-based HR monitor (Ambit3 Peak, Suunto®, Vantaa, Finland) and the maximum was obtained for further analysis. Oxygen (O2) and carbon dioxide (CO2) levels were continuously measured and monitored as breath-by-breath values in expired gas (Ultima Cardio 2® gas exchange analysis system, MGC Diagnostics with Breezesuite software, Saint Paul, MN, USA). The flow meter and the gas analyser were calibrated prior to each test with a 3-L volume calibration syringe (Hans Rudolph®, Medgraphics) and with a 5% CO2 and 12% O2 and 21% O2 gas mixtures (Medgraphics). The \(\dot {V}{{\text{O}}_{{\text{2max}}}}\) was determined by the highest 30 s average value with a “plateau” or levelling off in \(\dot {V}{{\text{O}}_2}\), RER > 1.1, and HR > 95% of theoretical age predicted HRmax. VT1 corresponded to the first non-linear increases in \(\dot {V}{\text{C}}{{\text{O}}_2}\), and VT2 was determined as the first rise in the ventilatory equivalent of oxygen (\({\dot {V}_{\text{E}}}\)/\(\dot {V}{{\text{O}}_2}\)) without a concurrent rise in the ventilatory equivalent of carbon dioxide (\({\dot {V}_{\text{E}}}\)/\(\dot {V}{\text{C}}{{\text{O}}_2}\)) (Reinhard et al. 1979).
Training loads
The training load (TL) was organized into four training zones depending on the intensity, which was based on the ventilatory levels of the first ventilatory threshold (VT1) and the second ventilatory threshold (VT2). These levels were identified as: intensity I for endurance training at an intensity below VT1; intensity II for endurance training at an intensity between VT1 and VT2; intensity III for training at an intensity at VT2, interval training at intensity above VT2, and competitions; and intensity IV for strength and speed training sessions.
Training loads were quantified as in Mujika et al. (1996), but slightly adapted to Nordic skiing and expressed in arbitrary units (a.u.) by multiplying the training duration (in min) spent in each intensity zone by a coefficient (i.e., 1, 2, 4, and 8 for the zones I, II, III, and IV, respectively).
Daily adjustment of training by HRV analysis
To ensure that the subjects were in a good condition at the beginning of the study period, the LHTL camp was organized at the end of the “base training” period (in July) of the yearly season, in which no training sessions had been completed that year at an intensity above VT2. In addition, the training volume was progressively increased and a recovery week was placed prior to the pre-tests in order to ensure subjects were healthy and well prepared. During the 2 months preceding the LHTL intervention, no athletes used altitude training and all lived and trained between 800 and 1200 m of altitude; i.e., typical residence. The training content was similar for each group (i.e., male cross-country skiers, female cross-country skiers, and male Nordic-combined athletes) and prescribed by the national coaches.
During the experimental period, the training adjustment was performed every morning using HRV analysis by the same highly experienced researcher. We have reported in a previous study (Schmitt et al. 2015) that the main type of fatigue can be diagnosed by a 50% minimum decrease in HF and LF spectral powers in both supine and standing positions. On the basis of these previous values, the threshold for training adjustment was chosen as 30% of the mean of the previous day in the present study.
The daily HRV-guided modulation of training load was adjusted based on the following situations:
-
Maintained or increased HF induced a higher TL than the previous day.
-
Decreased HF equal to or more than 30% led to lower TL.
-
Two consecutive days of decreased HF prompted a resting day.
-
After a rest day, a low-intensity and medium-volume session was prescribed.
The TL of the first day of the study was at medium volume and low intensity (intensity II, between VT1 and VT2). This medium volume was calculated for each subject as the mean volume of the three training weeks preceding the study.
Overall, the HRV-guided modulation of training can lead to either increased or decreased TL than in the predefined training program. In the first week of LHTL, the change in TL was only due to change in training volume since no “intense” session (i.e., at or above VT2) was scheduled to respect the acclimatization phase (Millet et al. 2010), with a 20% increase in TL when HF was either maintained or increased. In the second week of LHTL, the increase in TL was incorporated with additional training sessions lasting 45 min in duration at VT2.
Hematological parameters
Analysis of venous blood was performed from a 4.9-mL sample (4.9 mL EDTA tube, Sarstedt, Nümbrecht, Germany) to measure hematological parameters of erythrocyte concentration (T L−1), hemoglobin (Hb, g dL−1), hematocrit (Htc, %), mean cell volume (MCV, fL), reticulocytes (g dL−1), and ferritin (ng mL−1) using a blood flow cytometric analysis (Sysmex XN-2000, Sysmex, Kobe, Japan).
Data analysis and statistics
Data are reported as mean and standard deviation (SD). Data were tested for equality of variance (Fisher–Snedecor F test) and for normality (Shapiro–Wilk test). When both conditions were met, a two-way repeated measures ANOVA [condition (H-HRV, H and N) vs. measurement (pre, post-1, and post-21)] were performed with pairwise multiple comparison procedures (post hoc, Tukey method). Differences in percentage changes between the conditions were tested with a Wilcoxon signed rank sum test. When either equality of variance or normality were not satisfied, variables were analyzed for each condition using a Friedman test for repeated measures to determine time effects using pairwise multiple comparison procedures (Bonferroni test). In this case, differences between the H-HRV, H, and N condition at baseline (pre-) were tested using a Mann–Whitney rank sum test. Null hypotheses were rejected at p < 0.05. All analyses were completed using SigmaStat 3.5 software (Systat Software, San Jose, CA, USA).
Results
Hypoxic dose and nocturnal measurements
Daily hypoxic dose was similar between H-HRV and H (14.3 ± 1.6 vs. 14.1 ± 1.8 h). Total hypoxic dose in LHTL intervention was similar between H-HRV and H (215.4 ± 6.0 vs. 211.5 ± 9.7 h). Night SpO2 was similar between H-HRV and H, but lower than in N (90.4 ± 1.3 vs. 91.1 ± 1.6 vs. 94.2 ± 0.8%, p < 0.001).
Training loads
Despite the potential individual daily adjustment of the training content in H-HRV, overall training loads were similar between H-HRV and H, but lower than in N (3365 ± 425 vs. 3481 ± 179 vs. 3847 ± 433 a.u., p < 0.05). During the 15-day LHTL period, the daily training was adjusted on 3.3 ± 2.3 days in the H-HRV subjects.
No significant differences between groups were observed in the distribution of time spent training in the different intensity zones.
Questionnaire of overtraining
No significant differences in the overtraining QSFMS scores were found at any moment of the LHTL period between H-HRV, H, and N. The values in QSFMS scores were Pre (1.4 ± 1.7, 1.2 ± 1.9, and 2.2 ± 1.7) and Post-1 (1.0 ± 1.1, 1.9 ± 2.4, and 1.8 ± 2.6) in H-HRV, H, and N, respectively.
Heart rate variability
Table 1 shows the Pre test absolute values in heart rate and HRV parameters in supine and standing positions. No differences were observed between H-HRV, H, and N in HR, LF, HF, LF + HF, LFnu, or HFnu.
Change (%) in HR and HRV parameters during LHTL and post-LHTL periods between H-HRV, H, and N are shown in Table 2 and Fig. 2.
Incremental maximal treadmill test
Maximal oxygen uptake (\(\dot {V}{{\text{O}}_{{\text{2max}}}}\)) and oxygen consumption at the second ventilatory threshold (\(\dot {V}{{\text{O}}_{{\text{2VT2}}}}\)) obtained at Pre and Post-1 are shown in Table 3. At Post-1, \(\dot {V}{{\text{O}}_{{\text{2max}}}}\) (mL min−1 kg−1) and \(\dot {V}{{\text{O}}_{{\text{2VT2}}}}\) (mL min−1 kg−1) were improved in H-HRV (+ 3.8 ± 3.1%, p < 0.05 and + 6.7 ± 6.1%, p < 0.01) and to a (non-significant) lesser extent in H (+ 3.0 ± 4.4%, p = 0.08 and + 5.0 ± 5.7%, p = 0.06) but not in N (+ 0.9 ± 5.1%, ns and − 2.5 ± 5.1%, ns).
Roller-ski performance
At Pre, Post-1, and Post-21, temperature and humidity were similar with no wind.
The roller-ski performance was not improved in any of the groups at Post-1. At Post-21, H-HRV and H groups improved roller-ski performance (− 2.7 ± 3.6%, p < 0.05 vs. − 2.5 ± 3.5%, p = 0.07) while there was no improvement for the N group (− 1.1 ± 2.3%, ns). When pooled together, the two LHTL hypoxic groups (H-HRV + H) improved roller-ski performance at Post-21 (− 2.6 ± 3.4%, p < 0.01) significantly more than the normoxic group.
Hematological parameters
The hematological parameters measured at Pre and Post-1 in the three groups are reported in Table 4.
Discussion
This study, demonstrated that the daily individualized adjustment of the training loads based on morning HRV measurement blunted both the decrease in parasympathetic and the increase in sympathetic activities in the HRV-guided hypoxic (H-HRV) group in comparison to the predefined training hypoxic (H) group in elite male and female Nordic skiers. This study also confirmed the effectiveness of the living high–training low training method.
An important finding is that the daily individualized adjustment of training loads based on morning HRV measurement blunted both the decrease in parasympathetic and the increase in sympathetic activities in the HRV-guided hypoxic (H-HRV) group in comparison to the predefined training hypoxic (H) group in elite male and female Nordic skiers. It is known that the high-frequency power (HF, ms2) in the HRV spectral analysis represents the parasympathetic influence on the sinus node of the heart (Akselrod et al. 1981), and that the low frequency (LF, ms2) is also driven by parasympathetic tone in addition to carrying vagal resonances to either changes in vasomotor tone (often sympathetic) or central modulation of sympathetic tone (Reyes Del Paso et al. 2013). We have relayed in a preceding study (Schmitt et al. 2013) that in case of fatigue, the changes in HRV parameters were mainly due to a decrease in both HF and LF spectral power accompanied by an improvement in the relative influence of the LF band (LFnu) on the total spectral activity, observed only in the supine position. A further consideration for the present results is the influence of the menstrual cycle on the autonomic activity and HR responses in female subjects. In the present study for an adequate control of the experimental factors (training content, training and testing environmental conditions, motivational aspects), it was paramount that all athletes trained together and therefore impossible to adjust the period on the basis of the individual phase of the menstrual cycle for any female athlete. We cannot rule out that this methodological flaw might have slightly modified the HRV changes in some female athletes. In order to individually modulate the daily training adaptations in H-HRV, this study followed a previously reported protocol (Kiviniemi et al. 2007). The main difference was that the present HRV measurements were in the supine position whereas HRV records were standing in the Kiviniemi’s study. However in both studies, TL adjustments were based on the change in HF spectral energy. In the present study, several HRV parameters followed a different pattern of change throughout the 15-day LHTL period between H-HRV and H, showing globally a better adaptation in the H-HRV group, likely due to improved regulation of the combination between the hypoxic and the training stimuli as possible through daily HRV analysis. These results demonstrated an increase in LFnu (53.0 ± 19.5%) and HR (12.3 ± 4.1%) associated to a decrease in HFnu (− 13.7 ± 8.0%) in H, whereas these parameters remained unchanged in H-HRV with LFnu (− 3.8 ± 10.1%), HR (3.7 ± 6.3%), and HFnu (6.3 ± 6.8%) during the LHTL period. These latter results indicated a stronger sympathetic predominance and a decreased parasympathetic activity in H as is classically observed in hypoxic conditions (Passino et al. 1996; Perini et al. 1996). Conversely, in H-HRV, a better response to the hypoxic stimulus was possible, due to the daily TL adaptation as follows: the inclusion of moderate aerobic training when HF (ms2) decreased by more than 30% or the increase in TL when HRV was improved. Similarly, the increase in HR as well as the increase in LFnu and LF (ms2) was observed in H but not in H-HRV, which provides clear evidence of the moderation of the hypoxia-induced sympathetic predominance in the latter group. Finally, the subjective feeling of fatigue was not statistically different between the groups. In our view, this point highlights the need for both objective (i.e., HRV) and subjective assessment of fatigue in athletes. This is especially needed during the acclimatization period (i.e., first week) in altitude.
Another finding of the present study, is the significant improvement in performance and aerobic capacity both in H-HRV and H, without significant differences of change between these groups. Many potential reasons could be suggested. Firstly, the training was prescribed by a highly qualified national coach with therefore very little “room” for improvement, even if the study was performed in the “base training” phase. This is attested by the large improvement in \(\dot {V}{{\text{O}}_{{\text{2max}}}}\) in the H group, following the current dose–response consensus of 1.1% increase of Hbmass for 100 h of adequate altitude exposure (Gore et al. 2013). With around 211 h of total hypoxic dose it could be expected an improvement of 2.2% in \(\dot {V}{{\text{O}}_{{\text{2max}}}}\). As we observed an increase in \(\dot {V}{{\text{O}}_{{\text{2max}}}}\) of 3.0%, that this difference is likely due to the additional effect of the training performed. In addition, since these athletes are performing at a high level, there are smaller possibilities of physiological change (Burtscher et al. 1996). Further, the quantity (3.3 ± 2.3 a.u. per subject) of daily TL change in the H-HRV group was moderate and mainly performed during the first week of LHTL. Moreover, the sample size was relatively low as often in studies with elite athletes since by definition these athletes are scarce. Finally, one cannot rule out that the favorable HRV responses observed in the H-HRV group could translate to larger performance enhancement over time and/or at least would minimize the risks of overreaching in these athletes (Halson and Jeukendrup 2004; Hedelin et al. 2000).
The present study confirms the beneficial effects of LHTL (in the present case, in normobaric hypoxia) even in “true” (competing at European and World cup levels) elite Nordic-skiers. There was a large improvement (2.6 ± 3.4%, p < 0.01) in the hypoxic groups while the normoxic group did not improve the endurance performance measured in a sport-specific way (i.e., a roller-ski time-trial) 21 days after the LHTL period. This result is in accordance with the 1–3% of aerobic capacity or performance improvement (Bonetti and Hopkins 2009; Brocherie et al. 2015; Hauser et al. 2016) associated with LHTL and confirms that LHTL is effective also when using chambers in normobaric condition (Saugy et al. 2016). The present results contradict a recent view that elite endurance athletes with initial elevated values of hemoglobin mass (Hbmass) would not benefit from LHTL (Robach and Lundby 2012). Regarding the physiological responses to LHTL in the present study, there were several beneficial adaptations (\(\dot {V}{{\text{O}}_{{\text{2max}}}}\); \(\dot {V}{{\text{O}}_{{\text{2VT2}}}}\); roller-ski performance) in the two groups who slept in hypoxia. Further, there were no significant differences between H-HRV and H, and additionally no improvement in the control normoxic group. Specifically, in the two LHTL groups, \(\dot {V}{{\text{O}}_{{\text{2max}}}}\) and \(\dot {V}{{\text{O}}_{{\text{2VT2}}}}\) at Post-1 and roller-ski time-trial performance at Post-21 have been significantly enhanced. These changes in \(\dot {V}{{\text{O}}_{{\text{2max}}}}\) and \(\dot {V}{{\text{O}}_{{\text{2VT2}}}}\) are in line with the change in blood parameters shown in Table 4, particularly a significant increase in hemoglobin in H-HRV group. The positive changes observed in the hypoxic groups are similar to a vast body of literature that consistently report the effectiveness of LHTL (Levine and Stray-Gundersen 1997; Schmitt et al. 2006b; Stray-Gundersen et al. 2001), but also with Kiviniemi et al. (Kiviniemi et al. 2007, 2010) who have demonstrated similar improvements in \(\dot {V}{{\text{O}}_{2{\text{peak}}}}\) between HRV-guided and standardized training groups.
Conclusion
This study demonstrated that the daily individualized adjustment of the training loads based on morning HRV measurement blunted both the decrease in parasympathetic and the increase in sympathetic activities commonly associated with LHTL in H-HRV vs. H. We confirmed the first hypothesis and speculate that this result would reduce the overreaching risk. In addition, the second hypothesis was also validated in that LHTL is effective even with “true” elite endurance athletes since this study showed an improved performance and oxygen consumption in the two LHTL groups.
Abbreviations
- ANOVA:
-
Analysis of variance
- FFT:
-
Fast Fourier transform
- FiO2 :
-
Inspired fraction of oxygen
- H:
-
Hypoxic
- HF:
-
High-frequency power
- HFnu:
-
High-frequency power in normalized units
- Hbmass :
-
Hemoglobin mass
- HR:
-
Heart rate
- HRV:
-
Heart rate variability
- LF:
-
Low-frequency power
- LFnu:
-
Low-frequency power in normalized units
- LHTL:
-
Live high–train low
- N:
-
Normoxic
- PiO2 :
-
Partial pressure of inspired oxygen
- QSFMS:
-
Questionnaire of the French Society of Sports Medicine
- SpO2 :
-
Pulse oxygen saturation
- TL:
-
Training load
- \({\dot {V}_{\text{E}}}\)/\(\dot {V}{\text{C}}{{\text{O}}_2}\) :
-
Ventilatory equivalent of carbon dioxide
- \({\dot {V}_{\text{E}}}\)/\(\dot {V}{{\text{O}}_2}\) :
-
Ventilatory equivalent of oxygen
- \(\dot {V}{{\text{O}}_{{\text{2max}}}}\) :
-
Maximal oxygen consumption
- VT1:
-
First ventilatory threshold
- VT2:
-
Second ventilatory threshold
References
Akselrod S, Gordon D, Ubel FA, Shannon DC, Berger AC, Cohen RJ (1981) Power spectrum analysis of heart rate fluctuation: a quantitative probe of beat-to-beat cardiovascular control. Science 213:220–222
Aubert AE, Seps B, Beckers F (2003) Heart rate variability in athletes. Sports Med 33:889–919
Bonetti DL, Hopkins WG (2009) Sea-level exercise performance following adaptation to hypoxia: a meta-analysis. Sports Med 39:107–127
Bouchard C, Rankinen T (2001) Individual differences in response to regular physical activity. Med Sci Sports Exerc 33:S446–S451 (discussion S452–443)
Bouchard C, An P, Rice T, Skinner JS, Wilmore JH, Gagnon J, Perusse L, Leon AS, Rao DC (1999) Familial aggregation of VO(2max) response to exercise training: results from the HERITAGE Family Study. J Appl Physiol (1985) 87:1003–1008
Brocherie F, Millet GP, Hauser A, Steiner T, Rysman J, Wehrlin JP, Girard O (2015) “Live high-train low and high” hypoxic training improves team-sport performance. Med Sci Sports Exerc 47:2140–2149
Burtscher M, Nachbauer W, Baumgartl P, Philadelphy M (1996) Benefits of training at moderate altitude versus sea level training in amateur runners. Eur J Appl Physiol Occup Physiol 74:558–563
Goldstein DS, Bentho O, Park MY, Sharabi Y (2011) Low-frequency power of heart rate variability is not a measure of cardiac sympathetic tone but may be a measure of modulation of cardiac autonomic outflows by baroreflexes. Exp Physiol 96:1255–1261
Gore CJ, Sharpe K, Garvican-Lewis LA, Saunders PU, Humberstone CE, Robertson EY, Wachsmuth NB, Clark SA, McLean BD, Friedmann-Bette B, Neya M, Pottgiesser T, Schumacher YO, Schmidt WF (2013) Altitude training and haemoglobin mass from the optimised carbon monoxide rebreathing method determined by a meta-analysis. Br J Sports Med 47(Suppl 1):i31–i39
Halson SL, Jeukendrup AE (2004) Does overtraining exist? An analysis of overreaching and overtraining research. Sports Med 34:967–981
Hauser A, Schmitt L, Troesch S, Saugy JJ, Cejuela-Anta R, Faiss R, Robinson N, Wehrlin JP, Millet GP (2016) Similar hemoglobin mass response in hypobaric and normobaric hypoxia in athletes. Med Sci Sports Exerc 48:734–741
Hautala AJ, Makikallio TH, Kiviniemi A, Laukkanen RT, Nissila S, Huikuri HV, Tulppo MP (2003) Cardiovascular autonomic function correlates with the response to aerobic training in healthy sedentary subjects. Am J Physiol Heart Circ Physiol 285:H1747–H1752
Hautala AJ, Kiviniemi AM, Makikallio TH, Kinnunen H, Nissila S, Huikuri HV, Tulppo MP (2006) Individual differences in the responses to endurance and resistance training. Eur J Appl Physiol 96:535–542
Hedelin R, Kentta G, Wiklund U, Bjerle P, Henriksson-Larsen K (2000) Short-term overtraining: effects on performance, circulatory responses, and heart rate variability. Med Sci Sports Exerc 32:1480–1484
Hedelin R, Bjerle P, Henriksson-Larsen K (2001) Heart rate variability in athletes: relationship with central and peripheral performance. Med Sci Sports Exerc 33:1394–1398
Iellamo F, Legramante JM, Pigozzi F, Spataro A, Norbiato G, Lucini D, Pagani M (2002) Conversion from vagal to sympathetic predominance with strenuous training in high-performance world class athletes. Circulation 105:2719–2724
Kiviniemi AM, Hautala AJ, Makikallio TH, Seppanen T, Huikuri HV, Tulppo MP (2006) Cardiac vagal outflow after aerobic training by analysis of high-frequency oscillation of the R–R interval. Eur J Appl Physiol 96:686–692
Kiviniemi AM, Hautala AJ, Kinnunen H, Tulppo MP (2007) Endurance training guided individually by daily heart rate variability measurements. Eur J Appl Physiol 101:743–751
Kiviniemi AM, Hautala AJ, Kinnunen H, Nissila J, Virtanen P, Karjalainen J, Tulppo MP (2010) Daily exercise prescription on the basis of HR variability among men and women. Med Sci Sports Exerc 42:1355–1363
Levine BD, Stray-Gundersen J (1997) “Living high-training low”: effect of moderate-altitude acclimatization with low-altitude training on performance. J Appl Physiol 83:102–112
Maso F, Lac G, Filaire E, Michaux O, Robert A (2004) Salivary testosterone and cortisol in rugby players: correlation with psychological overtraining items. Br J Sports Med 38:260–263
McLean BD, Buttifant D, Gore CJ, White K, Kemp J (2013) Year-to-year variability in haemoglobin mass response to two altitude training camps. Br J Sports Med 47(Suppl 1):i51–i58
Millet GP, Roels B, Schmitt L, Woorons X, Richalet JP (2010) Combining hypoxic methods for peak performance. Sports Med 40:1–25
Mujika I, Busso T, Lacoste L, Barale F, Geyssant A, Chatard JC (1996) Modeled responses to training and taper in competitive swimmers. Med Sci Sports Exerc 28:251–258
Passino C, Bernardi L, Spadacini G, Calciati A, Robergs R, Anand I, Greene R, Martignoni E, Appenzeller O (1996) Autonomic regulation of heart rate and peripheral circulation: comparison of high altitude and sea level residents. Clin Sci (Lond) 91 Suppl:81–83
Perini R, Veicsteinas A (2003) Heart rate variability and autonomic activity at rest and during exercise in various physiological conditions. Eur J Appl Physiol 90:317–325
Perini R, Orizio C, Baselli G, Cerutti S, Veicsteinas A (1990) The influence of exercise intensity on the power spectrum of heart rate variability. Eur J Appl Physiol Occup Physiol 61:143–148
Perini R, Milesi S, Biancardi L, Veicsteinas A (1996) Effects of high altitude acclimatization on heart rate variability in resting humans. Eur J Appl Physiol Occup Physiol 73:521–528
Pomeranz B, Macaulay RJ, Caudill MA, Kutz I, Adam D, Gordon D, Kilborn KM, Barger AC, Shannon DC, Cohen RJ et al (1985) Assessment of autonomic function in humans by heart rate spectral analysis. Am J Physiol 248:H151–H153
Reinhard U, Muller PH, Schmulling RM (1979) Determination of anaerobic threshold by the ventilation equivalent in normal individuals. Respir Int Rev Thorac Dis 38:36–42
Reyes Del Paso GA, Langewitz W, Mulder LJ, van Roon A, Duschek S (2013) The utility of low frequency heart rate variability as an index of sympathetic cardiac tone: a review with emphasis on a reanalysis of previous studies. Psychophysiology 50:477–487
Robach P, Lundby C (2012) Is live high-train low altitude training relevant for elite athletes with already high total hemoglobin mass? Scand J Med Sci Sports 22:303–305
Saugy JJ, Schmitt L, Hauser A, Constantin G, Cejuela R, Faiss R, Wehrlin JP, Rosset J, Robinson N, Millet GP (2016) Same performance changes after live high-train low in normobaric vs. hypobaric hypoxia. Front Physiol 7:138
Schmitt L, Hellard P, Millet GP, Roels B, Richalet JP, Fouillot JP (2006a) Heart rate variability and performance at two different altitudes in well-trained swimmers. Int J Sports Med 27:226–231
Schmitt L, Millet G, Robach P, Nicolet G, Brugniaux JV, Fouillot JP, Richalet JP (2006b) Influence of “living high-training low” on aerobic performance and economy of work in elite athletes. Eur J Appl Physiol 97:627–636
Schmitt L, Fouillot JP, Millet GP, Robach P, Nicolet G, Brugniaux J, Richalet JP (2008) Altitude, heart rate variability and aerobic capacities. Int J Sports Med 29:300–306
Schmitt L, Regnard J, Desmarets M, Mauny F, Mourot L, Fouillot JP, Coulmy N, Millet G (2013) Fatigue shifts and scatters heart rate variability in elite endurance athletes. PLoS One 8:e71588
Schmitt L, Regnard J, Parmentier AL, Mauny F, Mourot L, Coulmy N, Millet GP (2015) Typology of “fatigue” by heart rate variability analysis in elite nordic-skiers. Int J Sports Med 36:999–1007
Schmitt L, Regnard J, Auguin D, Millet G (2016) Monitoring training and fatigue status with heart rate variability: case study in a swimming Olympic champion. J Fitness Res 5:38–45
Sevre K, Bendz B, Hanko E, Nakstad AR, Hauge A, Kasin JI, Lefrandt JD, Smit AJ, Eide I, Rostrup M (2001) Reduced autonomic activity during stepwise exposure to high altitude. Acta Physiol Scand 173:409–417
Stray-Gundersen J, Chapman RF, Levine BD (2001) “Living high-training low” altitude training improves sea level performance in male and female elite runners. J Appl Physiol 91:1113–1120
Task-Force (1996) Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Eur Heart J 17:354–381
Zupet P, Princi T, Finderle Z (2009) Effect of hypobaric hypoxia on heart rate variability during exercise: a pilot field study. Eur J Appl Physiol 107:345–350
Acknowledgements
We thank the athletes of the French national Nordic-combined and Cross-country skiing (men and women) teams and their coaches: for Nordic-combined—Jérôme Laheurte and Cyril Michaud-Fidey; for cross-country skiing—Vincent Vittoz and Thibaut Chene.
Funding
No external funding was received for this work from NIH; Welcome Trust; HHMI; others.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Communicated by Massimo Pagani.
Rights and permissions
About this article
Cite this article
Schmitt, L., Willis, S.J., Fardel, A. et al. Live high–train low guided by daily heart rate variability in elite Nordic-skiers. Eur J Appl Physiol 118, 419–428 (2018). https://doi.org/10.1007/s00421-017-3784-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-017-3784-9