Abstract
Purpose
The aim of the study was to determine whether higher fibrosis markers in skeletal muscle of older adults are accompanied by increased expression of components of the canonical TGF-β signal transduction pathway.
Methods
Fourteen healthy young (21–35 years; 9 males and 5 females) and seventeen older (55–75 years; 9 males and 8 females) participants underwent vastus lateralis biopsies to determine intramuscular mRNA and protein expression of fibrogenic markers and TGF-β signaling molecules related to TGF-β1 and myostatin.
Results
Expression of mRNA encoding the pro-fibrotic factors; axin 2, collagen III, β-catenin and fibronectin, were all significantly higher (all p < 0.05) in the older participants (350, 170, 298, and 641%, respectively). Furthermore, axin 2 and β-catenin mRNA were significantly higher in older females than older males (p < 0.05). Gene expression of ActRIIB, myostatin, and TGF-β1 were higher in older adults compared to younger adults (all p < 0.05). There was, however, no difference in the total protein content of myostatin, myoD or myogenin (all p > 0.05), whereas Smad3 protein phosphorylation was 48% lower (p < 0.05) in muscle from older adults.
Conclusions
Increased abundance of mRNA of fibrotic markers was observed in muscle from older adults and was partly accompanied by altered abundance of pro-fibrotic ligands in a sex specific manner.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sarcopenia is defined as a progressive decline in skeletal muscle mass and strength with advancing age resulting in reduced capacity to perform activities of daily living in the elderly (Janssen and Ross 2005; Rosenberg 1997). The mechanism of sarcopenia is complex and involves the interplay of a surfeit of alterations with advancing age. One suggested mechanism is the loss of regenerative capacity, particularly the programing in favor of fibrosis at the expense of myogenesis (Musaro et al. 1995; Verdijk et al. 2014).
Skeletal muscle of older rodents and humans is characterized by an impaired capacity for regeneration when exposed to the same injurious stimulus as younger adults (Marsh et al. 1997; McKay et al. 2012), however, this does not always appear to be the case (Buford et al. 2014). Skeletal muscle repair in response to moderate damage is reliant upon rapid fibroblast migration, and subsequent proliferation to the site of injury (Marsh et al. 1997; Moyer and Wagner 2011). Fibroblasts produce and secrete high levels of extracellular matrix (ECM) components such as collagen, hyaluronic acid and fibronectin, which are later degraded as regeneration and growth of new myofibers proceeds. High fibrosis levels with advanced age increases the accumulation of ECM components, leading to impaired regeneration (Serrano and Munoz-Canoves 2010) and abnormal muscle fiber arrangement (Peterson and Guttridge 2008).
Given the debilitating nature of sarcopenia, establishing markers whose expression are differentially regulated in skeletal muscle with advancing age will assist in future development of therapeutic approaches to the age-related decline in skeletal muscle mass and function. Among the factors reported to be altered with age is the overproduction of the major transforming growth factor beta (TGF-β) superfamily ligand, myostatin and growth and differentiation factor 11 (Sandri et al. 2013; Schafer et al. 2016). Myostatin, as well as TGF-β1 are reported to inhibit myogenesis through several mechanisms, including repression of the transcription of the muscle regulatory factors (MRFs); MyoD and myogenin (Durieux et al. 2007; Liu et al. 2001; Murakami et al. 2008). Canonically, ligand binding to the activin type IIB receptor (ActRIIB) activates Smad2 and/or 3 to initiate signal transduction through the traditional cascade (Ge et al. 2011). Subsequent complex formation with Smad4 triggers translocation to the nucleus, where phosphorylated Smad3 binds directly to MyoD leading to its post-transcriptional repression (Liu et al. 2001; Murakami et al. 2008).
Although initially identified as a potent negative regulator of skeletal muscle mass (McPherron et al. 1997), myostatin has also been implicated in fibrosis (Morissette et al. 2009). Myostatin deletion leads to a reduction in fibrosis of the heart in aged mice (Morissette et al. 2009). Evolutionarily conserved, age-specific increases in systemic TGF-β have also been observed in mice and humans (Carlson et al. 2009). Furthermore, attenuation of TGF-β signaling has been reported to improve the regenerative response in older muscle stem cells (Carlson et al. 2009). Despite such advances in the understanding of components of the TGF-β superfamily in aging, conflicting evidence for the upregulation of this pathway exist. Particularly, reports of increased circulating (Yarasheski et al. 2002) and intramuscular myostatin levels (Leger et al. 2008; McKay et al. 2012) are in contrast with results which have demonstrated no change (Welle et al. 2002) with advancing age.
Sex differences in the prevalence and degree of sarcopenia have been reported and appear to be related to aging in both human and animal models (Kirchengast and Huber 2009; Kob et al. 2015; Mitchell et al. 2012). For example, aging male rats exhibit a greater decline in muscle mass compared to female rats (Kob et al. 2015), with a similar pattern of decline reported in humans (Mitchell et al. 2012). In contrast, previous research has reported a potential crossover effect of age, with younger women (<70 years old) experiencing a higher frequency of sarcopenia compared to men, whereas this pattern is reversed in older adults (>80 years old) (Kirchengast and Huber 2009). The canonical skeletal muscle myogenesis signaling pathways are reported to be downregulated in male rodents compared to females (McMahon et al. 2003), with the discrepancy appearing to be exacerbated overtime with aging (Oldham et al. 2009). In humans, older woman exhibit an upregulation in gene expression of myostatin, Myf5, MyoD and myogenin compared to younger women, however, protein content was not measured (Raue et al. 2006). Few studies have explored the interaction of both age and sex on skeletal muscle myogenesis and fibrosis gene expression and protein signaling pathways in humans.
As such, the aim of the current study was to test the hypothesis that skeletal muscle of older adults is characterized by higher fibrosis markers and increased expression of components of the canonical TGF-β signal transduction pathway. Subsequently, we aimed to explore the potential interaction of sex and age on skeletal muscle myogenesis and fibrosis protein signaling.
Materials and methods
Participants
Fourteen young adults (9 males and 5 females; age 24.7 ± 1.0 years; height 173.1 ± 2.7 cm; weight 72.2 ± 4.1 kg; body mass index (BMI) 23.9 ± 0.9 kg/m2; mean ± SE) and seventeen older adults (9 males and 8 females; age 67.5 ± 1.7 years; height 167.9 ± 1.8 cm; weight 84.0 ± 4.1 kg; BMI 29.6 ± 1.1 kg/m2) participated in the study. This study was approved by the Victoria University Human Research Ethics Committee and carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans.
Muscle biopsy
Participants presented to the laboratory in a fasted state. Following 30 min of supine resting, a muscle sample was collected from the vastus lateralis under local anesthesia (xylocaine 1%) by percutaneous needle biopsy technique (Bergstrom 1962), modified to include suction (Evans et al. 1982). Excised muscle tissue from each biopsy was immediately frozen and stored in liquid nitrogen for later analysis.
Gene expression analysis
RNA extraction, reverse transcription and qPCR were performed as previously described (Watts et al. 2013; Caldow et al. 2013) and gene expression was calculated from quantification cycle (C q) values. The efficacy of cyclophilin A as an endogenous control was examined using the equation \(2^{{ - \Delta C_{\text{q}} }}\). No differences were observed between groups (all p > 0.05; data not shown); therefore, cyclophilin A was deemed as a suitable control. Primer sequences were designed using Primer Express (Applied Biosystems, Foster City, CA, USA) and validated by BLAST sequence homology and melt-curve analysis. See Table 1 for primer sequence details.
Protein extraction and Western blotting
Western blotting was performed as previously described (Watts et al. 2013). Smad3 (#9520) and phospho-Smad3 (#9513; Ser423/425) antibodies were purchased from Cell Signaling Technology (Danvers, MA, USA). TGF-β1 (#sc-146), myostatin (#AB3239) and actin (#A2066) antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA), Chemicon (Billerica, MA, USA) and Sigma (Sydney, Australia), respectively. In brief, muscle biopsy samples (~30 mg) were homogenized in cold lysis buffer (20 mM Tris–HCl, 5 mM EDTA, 10 mM Na4P2O7, 100 mM NaF, 2 mM Na3VO4, 1% Igepal, 10 μg/ml aprotinin, 10 μg/ml leupeptin, 3 mM benzamidine, 1 mM PMSF). Denatured protein from whole skeletal muscle lysates were separated by SDS-PAGE and transferred onto a nitrocellulose membrane. Nitrocellulose membranes were then blocked in 5% BSA/TBST (p-Smad3, Smad3, actin) and 5% cold fish gelatin (myostatin, TGF-β1), and then incubated overnight at 4 °C with primary antibody, followed by 1 h incubation with horseradish peroxidase-conjugated secondary antibody (in respective blocking buffer). Immunoreactive bands were detected by enhanced chemiluminescence (Western Lighting Chemiluminescent Reagent Plus, Perkin-Elmer, Boston, MA, USA) and densitometric analysis was performed (Kodak Imaging software, Kodak ID 3.5, Perkin-Elmer). Phospho-Smad3 membranes were stripped using Restore Western Blot Stripping Buffer (Thermo Fisher, Australia) before being re-probed with the total Smad3 antibody. Actin antibody was used as the loading control due to its stable expression between skeletal muscle of young and older adults.
Statistical analysis
Data were checked for normality and analyzed using Predictive Analytics Software (PASW v21, SPSS Inc., Chicago, WI, USA). Comparisons of multiple means were examined using a two-way analysis of variance (age × sex). Significant interaction and main effects were further probed using Fisher’s LSD test. Results are presented as mean ± SE. Statistical comparisons were considered significant at p ≤ 0.05.
Data availability statement
The datasets generated during and/or analyzed during the current study are not publicly available due to ethical considerations. The study was approved by, and conducted in accordance with the Victoria University Human Research Ethics Committee (VUHREC) which stipulates that raw data collected from participants is confidential (as per storage of medical records) and only accessible to researchers named on the ethics approval by the VUHREC. Raw de-identified data can be accessed upon request to the corresponding author pending approval by the VUHREC.
Results
Significant age × sex interaction effects were detected for axin 2 (p = 0.02) and beta-catenin (p = 0.01). No other significant interaction effects were detected (all p > 0.05). Main effects for age were detected for gene expression of collagen III (p = 0.01), fibronectin (p < 0.01), ActRIIB (p < 0.01), myostatin (p = 0.04), TGF-β1 (p < 0.05), Myf5 (p = 0.03), and Smad3 protein phosphorylation (p = 0.04). No other main effects for age or sex were detected (all p > 0.05).
Higher fibrogenic markers in skeletal muscle from older adults
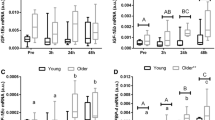
Older adults had significantly higher axin 2 (350%, Fig. 1a), beta-catenin (298%, Fig. 1b), collagen III (170%, Fig. 1c) and fibronectin (641%, Fig. 1d) gene expression, compared to young individuals. An age-related increase in axin 2 mRNA was mainly attributable to significantly higher expression in muscle from female participants (p < 0.05, Fig. 1a). β-Catenin mRNA was significantly increased for both older males (167%) and females (478%), however, was significantly higher for female participants than males (p < 0.05, Fig. 1b). No sex differences were observed for gene expression of collagen III and fibronectin (both p > 0.05, Fig. 1c, d).
Transcript levels of axin 2 (a), beta-catenin (b), collagen III (c), and fibronectin (d) relative to cyclophilin A, were determined by RT-PCR in skeletal muscle from young and older adults (black columns) and sex comparisons between males (white columns) and females (checkered columns). Statistically significant difference (*P < 0.05; **P < 0.01; ***P < 0.001) between young and older adults and between male and female († P < 0.05). Data are presented as mean ± SE and expressed relative (fold difference) to the combined young group for the combined analysis and the young male group for the between sex analysis
Altered Smad3 signaling in skeletal muscle from older adults
Older adults had significantly higher ActRIIB (365%), myostatin (120%), and TGF-β1 (72%) gene expression compared to young individuals (p < 0.05, Fig. 2a–c). No sex differences were detected for ActRIIB, myostatin, or TGF-β1 mRNA gene expression (all p > 0.05, Fig. 2a–c). Older adults demonstrated 48% lower (p < 0.05) Smad3 phosphorylation compared to the young group (Fig. 3a), whereas no difference in total protein content of myostatin or TGF-β1 was detected between young and older participants (p > 0.05; Fig. 3b, c).
Transcript levels of ActRIIB (a), myostatin (b) and TGF-β1 (c) relative to cyclophilin A, were determined by RT-PCR in skeletal muscle from young and older adults (black columns) and sex comparisons between males (white columns) and females (checkered columns). Statistically significant difference (*P < 0.05; **P < 0.01; ***P < 0.001) between young and older adults and between male and female († P < 0.05). Data are presented as mean ± SE and expressed relative (fold difference) to the combined young group for the combined analysis and the young male group for the between sex analysis
Western blot analysis of total protein content and/or phosphorylation of Smad3 (a), myostatin (b) and TGF- β1 (c), in skeletal muscle from young and older adults (black columns) and sex comparisons between males (white columns) and females (checkered columns). Statistically significant difference (*P < 0.05) between the young and older adults. Representative blots are also displayed (d). Data are presented as mean ± SE normalized to total SMAD3 or actin where specified; and expressed relative (fold difference) to the combined young group for the combined analysis and the young male group for the between sex analysis
Higher Myf5 expression in skeletal muscle from older adults
Myf5 expression was higher in the older adult group compared to the young group (74%, Fig. 4a; p < 0.05) with no difference in the gene expression of MyoD or myogenin (Fig. 4b, c, respectively).
Transcript levels of Myf5 (a), MyoD (b) and myogenin (c) relative to cyclophilin A, were determined by RT-PCR in skeletal muscle from young and older adults (black columns) and sex comparisons between males (white columns) and females (checkered columns). Statistically significant difference (*P < 0.05) between the young and older adults. Data are presented as mean ± SE and expressed relative (fold difference) to the combined young group for the combined analysis and the young male group for the between sex analysis
Discussion
We report higher gene expression of fibrosis markers axin 2, beta-catenin, collagen III and fibronectin, in skeletal muscle of older adults compared to young individuals. In addition, gene expression of the pro-fibrotic ligands ActRIIB, myostatin and TGF-β1, were higher in older adults compared to young individuals. The data suggest that elevated fibrotic activation observed in the muscle from older adults is partly accompanied by altered gene expression of pro-fibrotic ligands in both males and females. Furthermore, increased gene expression of axin 2 and beta-catenin in older adults were largely dependent on greater gene expression in females compared to males, supporting a potential interaction of age and sex with skeletal muscle fibrosis and myogenesis molecular signaling.
Aging is associated with morphological and functional changes in skeletal muscle. However, the cellular and molecular determinants of skeletal muscle fibrosis are not clear. Studying the processes involved in fibrotic tissue accrual within the musculature is pivotal to understanding the clinical complications of aging (Rosenberg 1997). Increased fibrosis of the ECM contiguous with the diminishing skeletal muscle of the elderly is associated with muscle stiffness, reduced whole muscle function and contributes to the impairment of satellite cell maturation (Kragstrup et al. 2011). The concentrations of dominant ECM components, including collagen III, are frequently used to measure the degree of skeletal muscle fibrosis (Haus et al. 2007). The higher mRNA expression of collagen III and other associated fibrotic markers, axin 2, beta-catenin and fibronectin, we observed in the older participants is consistent with the established literature (Brack and Rando 2007; Goldspink et al. 1994). We also observed higher levels of axin 2 and beta-catenin mRNA expression in older females compared to older males. Axin 2 and beta-catenin also play a role in skeletal muscle myogenesis (Suzuki et al. 2015), and thus may reflect increased myogenic gene expression reported in older women when compared to younger women (Raue et al. 2006). As such, care should be taken when combining data from male and females. Changes in the levels of hormones such as estrogen and its effects on skeletal muscle physiology after menopause may account for such sex-related differences with aging.
It has previously been reported that the TGF-β family pathway, the expression of the major ligands TGF-β1 and myostatin, as well as their canonical signaling components, are altered in skeletal muscle of older adults compared to their young counterparts (Carlson et al. 2009; Leger et al. 2008). In the current study, ActRIIB and TGF-β1 mRNAs were significantly higher in older adults compared to young individuals. Sub-analysis revealed no sex differences in the effect of aging on ActRIIB and TGF-β1 mRNA, suggesting increased expression of canonical TGF-β signal transduction pathway may be involved in the development of muscle fibrosis during the aging process in both sexes. Future studies should explore the cellular and clinical implications of inhibiting TGF-β signal transduction pathway in older adults.
Myostatin is known as a potent negative regulator of skeletal muscle mass in adulthood (Durieux et al. 2007; McPherron et al. 1997). In the current study, myostatin mRNA was higher in the older adult group compared to the young group. Older myostatin-null mice are characterized by the absence of skeletal muscle atrophy and an increased propensity to undergo muscle regeneration in response to injury (Siriett et al. 2006). It is important to note that much of the evidence for the possible role of TGF-β signal transduction pathway arises from in vitro studies comparing either age-associated alterations to gene expression in muscle precursor cells isolated from young and elderly adult rodents (Beggs et al. 2004) or the regenerative response following TGF-β1 neutralization in isolated human cells (Carlson et al. 2009). In contradiction to previous reports of attenuated myostatin mRNA expression and increased protein content in older aged skeletal muscle (Sandri et al. 2013), or the reported lack of difference in myostatin mRNA (Welle et al. 2002), we report elevated myostatin gene expression in the absence of differences in myostatin protein content in older aged skeletal muscle. The discrepancy in findings are unclear, but may stem from differences in population demographics (e.g. age) and the inherent inter-individual variability of humans which often impair the transferability of mouse and cell culture findings to human studies (Sandri et al. 2013). Furthermore, others have also reported discrepancies between myostatin RNA expression and myostatin protein content in human and rodent skeletal muscle (McKay et al. 2012; Oldham et al. 2009). The discrepancy between gene expression and protein content may arise through variations in protocol/methodology and antibody specificity, or more likely through post-transcriptional, translational and protein degradation regulation processes (Vogel and Marcotte 2012).
Surprisingly, Smad3 phosphorylation in skeletal muscle from older adults was lower compared to younger individuals despite the detection of elevated TGF-β1 and myostatin mRNA expression. Smad signaling is reported to be involved in higher risk for insulin resistance-related muscle atrophy through attenuation of muscle regulatory factors independent of changes in myostatin or TGF-β1 protein content (Watts et al. 2013). Furthermore, others have also reported Smad3 signaling to be involved in skeletal muscle hypertrophy signaling pathways, such as the Akt/mammalian target of rapamycin (mTOR) pathway, independent of myostatin driven mechanisms (Winbanks et al. 2012). The physiological relevance of attenuated Smad3 phosphorylation in skeletal muscle of older populations remains unclear, and warrants further exploration.
The loss of type II muscle fibers accompanied by a fiber-specific decline in satellite cell content is a contributor to disrupted skeletal muscle regeneration with advancing age (Verdijk et al. 2014). Disruption to the timely expression by satellite cells of any of the MRFs may compromise postnatal growth, repair and maintenance of mature skeletal muscle and augment collagen deposition and fibrosis (Charge and Rudnicki 2004). Given that complete ablation of Smad3 in mice is attributed to impaired satellite cell renewal (Ge et al. 2011), we sought to determine the possibility that diminished Smad3 in the muscle of older adults may be accompanied by dysregulated expression of the aforementioned transcription. The gene expression of MyoD and myogenin revealed no significant differences between whole muscle lysate of young and older adults. The increase in Myf5 mRNA in the older adults appears paradoxical considering the propensity for reduced skeletal muscle regeneration with aging, as well as previous reports of its down regulation in response to Smad3 ablation (Ge et al. 2011). However, increased MRF transcription in older sarcopenic mice following a continuous decline through the postnatal period has been described (Musaro et al. 1995).
In contrast to axin 2 and beta-catenin gene expression, gene expression and protein signaling of other MRFs did not appear to be effected by sex. These findings contradict previous work in rodents which have reported upregulation of myogenic signaling in skeletal muscle of female rodents compared to male rodents (McMahon et al. 2003), with this discrepancy appearing to be more pronounced overtime with aging (Oldham et al. 2009). It has also been reported that older woman (aged 80–89 years old) exhibit upregulated gene expression of myostatin, Myf5, MyoD and myogenin compared to younger women (aged 18–30 years) (Raue et al. 2006), however, protein content was not measured. The discrepancy to the current findings may be due to the large variation in investigated age groups. Certainly, the prevalence of sarcopenia is reported to be greater in females under the age of 70 compared to males, whereas this pattern is reversed with adults over the age of 80 (Kirchengast and Huber 2009). Nevertheless, we provide new data suggesting similar skeletal muscle gene expression and protein content of canonical MRFs between male and females in both the young and older aged groups investigated.
This study was reliant upon human muscle biopsy samples, an invasive protocol which generates small quantities of muscular tissue. Thus, limited muscle availability precluded both morphological and immunohistochemical analysis. Although sample size may be a limiting factor of the study, previous invasive human studies have used similar sample sizes to investigate age- and sex-related differences in skeletal muscle myogenesis gene expression and protein signaling pathways (Raue et al. 2006; Bamman et al. 2003). Other potential study limitations include the omission of muscle fiber type and direct measures of muscle fibrosis between the young and older participants. The protein concentration of some signal transduction proteins varies between fiber isoforms, presumably reflecting concentration differences related to myofiber functioning (Atherton et al. 2004). While the bulk of RNA isolated from muscle tissue is derived from myonuclei, age differences in the abundance of other contributing cell types could also be an important factor that was not accounted for in this current study. Finally, future studies would benefit by stratifying age even further, such as investigating skeletal muscle of young, middle-aged, and older adults.
Conclusions
Elevated activation of fibrotic markers observed in muscle from older adults is partly accompanied by altered abundance of pro-fibrotic ligands in males and females. The onset of sarcopenia of the elderly is likely to be triggered by a complex regulatory network of signaling pathways. Future studies exploring how these signaling components interact to produce sarcopenia with advancing age will help contribute towards the development of strategies to counteract the age-related declines in morphology and function.
Abbreviations
- ActRIIB:
-
Activin type IIB receptor
- ECM:
-
Extracellular matrix
- MRFs:
-
Muscle regulatory factors
- TGF-β:
-
Transforming growth factor beta
References
Atherton PJ, Higginson JM, Singh J, Wackerhage H (2004) Concentrations of signal transduction proteins exercise and insulin responses in rat extensor digitorum longus and soleus muscles. Mol Cell Biochem 261(1–2):111–116
Bamman MM, Hill VJ, Adams GR, Haddad F, Wetzstein CJ, Gower BA, Ahmed A, Hunter GR (2003) Gender differences in resistance-training-induced myofiber hypertrophy among older adults. J Gerontol A Biol Sci Med Sci 58(2):108–116
Beggs ML, Nagarajan R, Taylor-Jones JM, Nolen G, Macnicol M, Peterson CA (2004) Alterations in the TGFbeta signaling pathway in myogenic progenitors with age. Aging Cell 3(6):353–361. doi:10.1111/j.1474-9728.2004.00135.x
Bergstrom J (1962) Muscle electrolytes in man determined by neutron activation analysis on needle biopsy specimens. Scand J Clin Lab Invest 14(Suppl 68):1–110
Brack AS, Rando TA (2007) Intrinsic changes and extrinsic influences of myogenic stem cell function during aging. Stem Cell Rev 3(3):226–237
Buford TW, MacNeil RG, Clough LG, Dirain M, Sandesara B, Pahor M, Manini TM, Leeuwenburgh C (2014) Active muscle regeneration following eccentric contraction-induced injury is similar between healthy young and older adults. J Appl Physiol 116(11):1481–1490. doi:10.1152/japplphysiol.01350.2012
Caldow MK, Cameron-Smith D, Levinger P, McKenna MJ, Levinger I (2013) Inflammatory markers in skeletal muscle of older adults. Eur J Appl Physiol 113(2):509–517. doi:10.1007/s00421-012-2458-x
Carlson ME, Conboy MJ, Hsu M, Barchas L, Jeong J, Agrawal A, Mikels AJ, Agrawal S, Schaffer DV, Conboy IM (2009) Relative roles of TGF-beta1 and Wnt in the systemic regulation and aging of satellite cell responses. Aging Cell 8(6):676–689. doi:10.1111/j.1474-9726.2009.00517.x
Charge SB, Rudnicki MA (2004) Cellular and molecular regulation of muscle regeneration. Physiol Rev 84(1):209–238. doi:10.1152/physrev.00019.2003
Durieux AC, Amirouche A, Banzet S, Koulmann N, Bonnefoy R, Pasdeloup M, Mouret C, Bigard X, Peinnequin A, Freyssenet D (2007) Ectopic expression of myostatin induces atrophy of adult skeletal muscle by decreasing muscle gene expression. Endocrinology 148(7):3140–3147. doi:10.1210/en.2006-1500
Evans WJ, Phinney SD, Young VR (1982) Suction applied to a muscle biopsy maximizes sample size. Med Sci Sports Exerc 14(1):101–102
Ge X, McFarlane C, Vajjala A, Lokireddy S, Ng ZH, Tan CK, Tan NS, Wahli W, Sharma M, Kambadur R (2011) Smad3 signaling is required for satellite cell function and myogenic differentiation of myoblasts. Cell Res 21(11):1591–1604. doi:10.1038/cr.2011.72
Goldspink G, Fernandes K, Williams PE, Wells DJ (1994) Age-related changes in collagen gene expression in the muscles of mdx dystrophic and normal mice. Neuromuscul Disord 4(3):183–191
Haus JM, Carrithers JA, Trappe SW, Trappe TA (2007) Collagen, cross-linking, and advanced glycation end products in aging human skeletal muscle. J Appl Physiol 103(6):2068–2076. doi:10.1152/japplphysiol.00670.2007
Janssen I, Ross R (2005) Linking age-related changes in skeletal muscle mass and composition with metabolism and disease. J Nutr Health Aging 9(6):408–419
Kirchengast S, Huber J (2009) Gender and age differences in lean soft tissue mass and sarcopenia among healthy elderly. Anthropol Anz 67(2):139–151
Kob R, Fellner C, Bertsch T, Wittmann A, Mishura D, Sieber CC, Fischer BE, Stroszczynski C, Bollheimer CL (2015) Gender-specific differences in the development of sarcopenia in the rodent model of the Aging high-fat rat. J Cachexia Sarcopenia Muscle 6(2):181–191. doi:10.1002/jcsm.12019
Kragstrup TW, Kjaer M, Mackey AL (2011) Structural, biochemical, cellular, and functional changes in skeletal muscle extracellular matrix with aging. Scand J Med Sci Sports 21(6):749–757. doi:10.1111/j.1600-0838.2011.01377.x
Leger B, Derave W, De Bock K, Hespel P, Russell AP (2008) Human sarcopenia reveals an increase in SOCS-3 and myostatin and a reduced efficiency of Akt phosphorylation. Rejuvenation Res 11(1):163–175B. doi:10.1089/rej.2007.0588
Liu D, Black BL, Derynck R (2001) TGF-beta inhibits muscle differentiation through functional repression of myogenic transcription factors by Smad3. Genes Dev 15(22):2950–2966. doi:10.1101/gad.925901
Marsh DR, Criswell DS, Carson JA, Booth FW (1997) Myogenic regulatory factors during regeneration of skeletal muscle in young, adult, and old rats. J Appl Physiol 83(4):1270–1275
McKay BR, Ogborn DI, Bellamy LM, Tarnopolsky MA, Parise G (2012) Myostatin is associated with age-related human muscle stem cell dysfunction. FASEB J 26(6):2509–2521. doi:10.1096/fj.11-198663
McMahon CD, Popovic L, Jeanplong F, Oldham JM, Kirk SP, Osepchook CC, Wong KW, Sharma M, Kambadur R, Bass JJ (2003) Sexual dimorphism is associated with decreased expression of processed myostatin in males. Am J Physiol Endocrinol Metab 284(2):E377–E381. doi:10.1152/ajpendo.00282.2002
McPherron AC, Lawler AM, Lee SJ (1997) Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature 387(6628):83–90. doi:10.1038/387083a0
Mitchell WK, Williams J, Atherton P, Larvin M, Lund J, Narici M (2012) Sarcopenia, dynapenia, and the impact of advancing age on human skeletal muscle size and strength; a quantitative review. Front Physiol 3:260. doi:10.3389/fphys.2012.00260
Morissette MR, Stricker JC, Rosenberg MA, Buranasombati C, Levitan EB, Mittleman MA, Rosenzweig A (2009) Effects of myostatin deletion in aging mice. Aging Cell 8(5):573–583. doi:10.1111/j.1474-9726.2009.00508.x
Moyer AL, Wagner KR (2011) Regeneration versus fibrosis in skeletal muscle. Curr Opin Rheumatol 23(6):568–573. doi:10.1097/BOR.0b013e32834bac92
Murakami M, Ohkuma M, Nakamura M (2008) Molecular mechanism of transforming growth factor-beta-mediated inhibition of growth arrest and differentiation in a myoblast cell line. Dev Growth Differ 50(2):121–130. doi:10.1111/j.1440-169X.2007.00982.x
Musaro A, Cusella De Angelis MG, Germani A, Ciccarelli C, Molinaro M, Zani BM (1995) Enhanced expression of myogenic regulatory genes in aging skeletal muscle. Exp Cell Res 221(1):241–248
Oldham JM, Osepchook CC, Jeanplong F, Falconer SJ, Matthews KG, Conaglen JV, Gerrard DF, Smith HK, Wilkins RJ, Bass JJ, McMahon CD (2009) The decrease in mature myostatin protein in male skeletal muscle is developmentally regulated by growth hormone. J Physiol 587(3):669–677. doi:10.1113/jphysiol.2008.161521
Peterson JM, Guttridge DC (2008) Skeletal muscle diseases, inflammation, and NF-kappaB signaling: insights and opportunities for therapeutic intervention. Int Rev Immunol 27(5):375–387. doi:10.1080/08830180802302389
Raue U, Slivka D, Jemiolo B, Hollon C, Trappe S (2006) Myogenic gene expression at rest and after a bout of resistance exercise in young (18–30 yr) and old (80–89 yr) women. J Appl Physiol 101(1):53–59. doi:10.1152/japplphysiol.01616.2005
Rosenberg IH (1997) Sarcopenia: origins and clinical relevance. J Nutr 127(5 Suppl):990S–991S
Sandri M, Barberi L, Bijlsma AY, Blaauw B, Dyar KA, Milan G, Mammucari C, Meskers CG, Pallafacchina G, Paoli A, Pion D, Roceri M, Romanello V, Serrano AL, Toniolo L, Larsson L, Maier AB, Munoz-Canoves P, Musaro A, Pende M, Reggiani C, Rizzuto R, Schiaffino S (2013) Signalling pathways regulating muscle mass in Aging skeletal muscle: the role of the IGF1-Akt-mTOR-FoxO pathway. Biogerontology 14(3):303–323. doi:10.1007/s10522-013-9432-9
Schafer MJ, Atkinson EJ, Vanderboom PM, Kotajarvi B, White TA, Moore MM, Bruce CJ, Greason KL, Suri RM, Khosla S, Miller JD, Bergen HR 3rd, LeBrasseur NK (2016) Quantification of GDF11 and myostatin in human aging and cardiovascular disease. Cell Metab 23(6):1207–1215. doi:10.1016/j.cmet.2016.05.023
Serrano AL, Munoz-Canoves P (2010) Regulation and dysregulation of fibrosis in skeletal muscle. Exp Cell Res 316(18):3050–3058. doi:10.1016/j.yexcr.2010.05.035
Siriett V, Platt L, Salerno MS, Ling N, Kambadur R, Sharma M (2006) Prolonged absence of myostatin reduces sarcopenia. J Cell Physiol 209(3):866–873. doi:10.1002/jcp.20778
Suzuki A, Scruggs A, Iwata J (2015) The temporal specific role of WNT/β-catenin signaling during myogenesis. J Nat Sci 1(8):e143
Verdijk LB, Snijders T, Drost M, Delhaas T, Kadi F, van Loon LJ (2014) Satellite cells in human skeletal muscle; from birth to old age. Age 36(2):545–547. doi:10.1007/s11357-013-9583-2
Vogel C, Marcotte EM (2012) Insights into the regulation of protein abundance from proteomic and transcriptomic analyses. Nat Rev Genet 13(4):227–232. doi:10.1038/nrg3185
Watts R, McAinch AJ, Dixon JB, O’Brien PE, Cameron-Smith D (2013) Increased Smad signaling and reduced MRF expression in skeletal muscle from obese subjects. Obesity (Silver Spring) 21(3):525–528. doi:10.1002/oby.20070
Welle S, Bhatt K, Shah B, Thornton C (2002) Insulin-like growth factor-1 and myostatin mRNA expression in muscle: comparison between 62–77 and 21–31 yr old men. Exp Gerontol 37(6):833–839
Winbanks CE, Weeks KL, Thomson RE, Sepulveda PV, Beyer C, Qian H, Chen JL, Allen JM, Lancaster GI, Febbraio MA, Harrison CA, McMullen JR, Chamberlain JS, Gregorevic P (2012) Follistatin-mediated skeletal muscle hypertrophy is regulated by Smad3 and mTOR independently of myostatin. J Cell Biol 197(7):997–1008. doi:10.1083/jcb.201109091
Yarasheski KE, Bhasin S, Sinha-Hikim I, Pak-Loduca J, Gonzalez-Cadavid NF (2002) Serum myostatin-immunoreactive protein is increased in 60–92 year old women and men with muscle wasting. J Nutr Health Aging 6(5):343–348
Acknowledgements
A/Prof. Levinger was supported by Future Leader Fellowship (ID 100040) from the National Heart Foundation of Australia. The authors wish to thank Dr. Andrew Garnham (Victoria University University) and Dr. Mitchell Anderson for performing the muscle biopsies.
Author information
Authors and Affiliations
Contributions
RW and MC carried out the experimental procedures; LP carried out the statistical analysis; DCS, IL and PL designed the study and collected the data. All authors assisted with drafting of the manuscript, critically reviewed the manuscript, and have read and approved the final version.
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts of interest to disclose.
Additional information
Communicated by Fabio Fischetti.
Rights and permissions
About this article
Cite this article
Parker, L., Caldow, M.K., Watts, R. et al. Age and sex differences in human skeletal muscle fibrosis markers and transforming growth factor-β signaling. Eur J Appl Physiol 117, 1463–1472 (2017). https://doi.org/10.1007/s00421-017-3639-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-017-3639-4