Abstract
Purpose
We investigated whether lifelong football training affects the expression of healthy longevity-related muscle molecular markers.
Methods
Biopsies were collected from the vastus lateralis muscle of 10 lifelong football-trained men (68.2 ± 3.0 years) and of 10 active untrained healthy men (66.7 ± 1.3 years). Gene and protein expression was measured by RTqPCR on RNA and by western blotting on protein extracts from muscle biopsies, respectively.
Results
The expression of AMPKα1/α2, NAMPT, TFAM and PGC1α, which are markers of oxidative metabolism, and MyHC β isoform expression was higher in the muscle of football-trained men vs untrained men. Also citrate synthase activity was higher in trained than in untrained men (109.3 ± 9.2 vs 75.1 ± 9.2 mU/mg). These findings were associated with a healthier body composition in trained than in untrained men [body weight: 78.2 ± 6.5 vs 91.2 ± 11.2 kg; body mass index BMI: 24.4 ± 1.6 vs 28.8 ± 4.0 kg m−2; fat%: 22.6 ± 8.0 vs 31.4 ± 5.0%)] and with a higher maximal oxygen uptake (VO2max: 34.7 ± 3.8 vs 27.3 ± 4.0 ml/min/kg). Also the expression of proteins involved in DNA repair and in senescence suppression (Erk1/2, Akt and FoxM1) was higher in trained than in untrained men. At BMI- and age-adjusted multiple linear regression analysis, fat percentage was independently associated with Akt protein expression, and VO2max was independently associated with TFAM mRNA and with Erk1/2 protein expression.
Conclusions
Lifelong football training increases the expression of key markers involved in muscle oxidative metabolism, and in the DNA repair and senescence suppression pathways, thus providing the molecular basis for healthy longevity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aging is associated with the reduction of all health-related fitness components, in particular with such cardio-respiratory components as VO2max, and with changes in body composition (Mazzeo et al. 1998). Moreover, an increase in fat mass and a decrease in fat-free mass are associated with impaired glucose and lipid metabolism which is a condition characterised by an elevated risk of cardiovascular and metabolic diseases. Physical inactivity, on the other hand, is an independent risk factor for chronic diseases and a strong mortality predictor (Pedersen and Saltin 2006).
An active lifestyle and exercise training reduce the risk of cardiovascular and metabolic diseases (Kim et al. 2007; Ostergard et al. 2006). The beneficial effects of structured exercise sport on cardiovascular and health-related fitness are reviewed in Krustrup et al. (2010a, b). Football is an intermittent exercise that involves multiple anaerobic actions of high-intensity interspersed with periods of low-intensity recovery. It is also the most popular team sport in the world (Krustrup et al. 2010a, b). Football and recreational soccer training improves cardio-respiratory fitness and the oxidative component of muscle fibres in healthy and unhealthy individuals (Helge et al. 2014; Krustrup et al. 2010a, b; Randers et al. 2012; Schmidt et al. 2013). Despite growing evidence that football training exerts a broad spectrum of beneficial effects on cardiovascular, metabolic and musculoskeletal health variables, only one study has analysed the molecular effects of long-term (1 year) football training on muscle metabolism in healthy subjects (Alfieri et al. 2015). A recent study of the effects of lifelong football training on cardiovascular fitness and health-related body composition parameters showed that elderly subjects (veteran football players; VPG) had better cardiovascular fitness and a healthier body composition than untrained age-matched controls (control group, CG) (Schmidt et al. 2015).
Starting from these experimental findings, we assessed the effects of lifelong football training in VPG vs CG on the muscle expression of key markers involved in (1) mitochondrial biogenesis and oxidative metabolism; (2) DNA repair promotion and senescence suppression, which are two pathways involved in healthy longevity.
Methods
Subjects and sample collections
Twenty healthy male volunteers aged 65–71 years were enrolled at the University of Copenhagen and divided into two groups: 10 veteran football players (VPG; 68.2 ± 3.0 years) who in the last 10 years had trained one session per week (1.5 ± 0.6 h/session) and played in 26 ± 12 football matches (2 × 35 min) per year in local football clubs in Copenhagen; 19 active healthy untrained men (CG; 66.7 ± 1.3 years).
The inclusion criterion for study entry was the ability of volunteers to perform the battery of tests. The exclusion criterion was a history or symptoms of cardiovascular disease or cancer, type 2 diabetes, hypertension, nephropathy or musculoskeletal complaints that were considered to preclude testing. Clinical, biochemical and fitness parameters were evaluated as reported previously (Schmidt et al. 2015). Blood samples were withdrawn from a cubital vein, between 7 and 9 a.m., under standardised conditions after an overnight fast. Total cholesterol, fasting blood glucose, HDL cholesterol, LDL cholesterol and triglycerides were determined with an automated analysers (Cobas Fara, Roche, Neuilly sur Seine, France). All subjects were informed about any potential discomforts or risks related to the experimental study protocol and gave their written informed consent to the study. The study was conducted according to the Declaration of Helsinki and was approved by the local ethics committee of the University of Copenhagen; H-1-2011-013. ClinicalTrials.gov Identifier: NCT01530035.
Body composition was assessed between 7 and 9 a.m. under standardised conditions after an overnight fast using a whole-body dual-energy X-ray absorptiometry scan (Prodigy Advance, Lunar Corporation, Madison, Wisconsin, USA); the effective radiation dose was 0.6 mSv/scan. Height, total body weight (kg), total lean body mass (kg), total fat percentage (fat%) and bone mass (kg) were recorded and calculated using Encore 2006 software (Lunar Corporation).
A biopsy was obtained from the vastus lateralis muscle under local anaesthesia using the Bergstrom technique as previously described (Schmidt et al. 2015) and immediately frozen in liquid nitrogen. Maximal oxygen uptake—VO2max—(Oxycon Pro, VIASYS Healthcare, Hoechberg, Germany) and resting heart rate (RHR; bpm) (Polar Team System, Polar electro Oy, Kempele, Finland) were measured during a standardised cycling protocol performed on an electronically braked ergometer bike (Monark E839, Varberg, Sweden). The subjects started exercising at a cadence of 80 rpm with a workload of 40 W, and the workload was increased by 20 W every 2 min until volitional fatigue. Oxygen uptake was measured continuously and VO2max determined as the highest value achieved in 30 s. Diastolic and systolic blood pressure (BP, mmHg) were also measured.
RNA extraction and RTqPCR
Total RNA was extracted from the muscle biopsies using an miRNeasy® kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. The RNA integrity number (RIN) of samples was assessed using a Bio-Rad Experion automated electrophoresis station (Hercules, CA, USA) before cDNA synthesis. For reverse transcription-PCR (RT-PCR) analysis, 0.5 µg of total RNA was used in the reaction with SuperScript reverse transcriptase (Life Technologies). The resulting cDNA was analysed by real-time quantitative PCR (RTqPCR) performed with the iQ5-iCycler Optical System (Bio-Rad, Hercules, CA, USA). IQ SYBR Bio-Rad protocol (100 mM KCl, 40 mM Tris–HCl pH 8.4, 0.4 mM dNTPs, iTaq DNA polymerase 50 U/ml, 6 mM MgCl2, SYBR Green I, 20 nM fluorescein, stabiliser) was applied according to the manufacturer’s instructions. Reaction mixtures were incubated at 95 °C for 30 s, followed by 2 cycles at 95 °C for 30 s and 95 °C for 3 min and by 40 cycles at 95 °C for 15 s and 60 °C for 1 min. Finally, 80 cycles were run starting at 55 °C and increasing the temperature by 5 °C every 10 s up to 95 °C. Fluorescence signals were measured during the elongation step. All measurements were performed in duplicate. The target mRNA expression levels were normalised to the levels of the peptidylprolyl isomerase A (cyclophilin A) (PPIA) and polymerase (RNA) II (DNA directed) polypeptide A (Pol2A) gene using the 2− ΔΔCT method (Liss 2002). The oligonucleotide primer sequences used in RTqPCR are reported in Supporting Information (Table 1S).
Quantification of the mitochondrial DNA to nuclear DNA (mitDNA/nucDNA) ratio
Approximately 10 mg of muscle biopsies was freeze-dried. Genomic DNA was extracted using DNeasy Blood & Tissue columns (Qiagen, Valencia, CA, USA) according to the manufacturer’s instructions. Total DNA concentration was quantified spectrophotometrically (NanoDrop). The mitDNA/nucDNA ratio was determined by quantifying the expression of mitochondrial DNA and nuclear DNA by real-time PCR (Bio-Rad,) as previously described (Nishioka et al. 2004). Purified total DNA (1 ng for mitochondrial DNA amplification and 10 ng for nuclear DNA amplification) was amplified in a 25-µL PCR containing SYBR Green Master Mix (Bio-Rad) and 100 nM of each primer. RTqPCR for the detection of mitDNA and nucDNA was performed as two separate reactions, but within the same plate run for each sample. All samples were run in duplicate for each DNA target. The primers were human specific and designed to target mitDNA (forward: CTCGCACGGACTACAACC; reverse: TGGGCGATTGATGAAAAG) or melanocortin (forward: CGCCGTGGACCGCTACATCT; reverse: GCACGGCCATGAGCACCAG). Serial dilutions of pooled DNA were run for each DNA target to generate a standard curve allowing assessment of RTqPCR amplification efficiency and quantification of mitDNA and nucDNA in relative copy number based on respective threshold cycle (Ct) values.
Citrate synthase activity
Citrate synthase activity, which is a widely used marker of mitochondrial mass and cellular oxidative capacity, was determined as reported elsewhere (Faloona and Srere 1969; Srere and Brooks 1969) with minor modifications. Activity assays were conducted in a final volume of 1 mL containing 0.1 M tris–HCl pH 8, 1 mM DTNB, 0.3 mM acetyl CoA and 0.5 mM oxaloacetate. Mitochondria were prepared from muscle biopsies using a Qproteome Mitochondria Isolation kit (Qiagen) in accordance with the manufacturer’s instructions. Mitochondrial samples ranged between 5 and 10 µg. Assays were performed at room temperature in triplicate using a 50BIO UV–Visible spectrophotometer (Varian). Change in absorbance at 412 nmol/L was measured every 30 s for 3 min. The data were analysed using Student’s t test. The level of significance was set at p < 0.05.
Western blotting
The muscle biopsies were mechanically pulverised, solved in RIPA buffer (150 mM NaCl, 1% NP40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulphate, 50 mM Tris pH 8.0) and centrifuged at 14,000 rpm for 20 min. Supernatants were withdrawn and aliquots (1 µL) used for protein determination using a Bio-Rad Protein Assay kit (Bio-Rad). Protein extracts (each 40 µg) were resolved with 8–12% polyacrylamide gels. High molecular weight myosin α and β heavy chain protein isoforms (MyHC-α and MyHC-β) were separated on 4–20% precast gradient polyacrylamide gels (Bio-Rad), transferred to the Hybond ECL nitrocellulose membrane (GE Healthcare) and checked by Ponceau S staining to verify equal loading. The membranes were immunoblotted using antibodies against adenosine monophosphate-activated protein kinase (AMPKα), AKT serine/threonine kinase 1 (Akt), p42-44 mitogen-activated protein kinase (MAPK or Erk1/2), Forkhead Box M1 (FoxM1)polyclonal antibodies (1:1000; Cell Signaling, Technology), monoclonal mouse antibodies anti-MyHC-α and anti-MyHC-β (Abcam, 1:500), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (1:1000; Santa-Cruz Biotechnology Inc.). Blots were incubated with appropriate horseradish peroxidase-conjugated secondary antibody and target proteins were visualised by ECL detection (GE Healthcare). Densitometric measurements were carried out using Quantity One software (Bio-Rad) as reported elsewhere (Spaziani et al. 2014). GAPDH protein was used to estimate the total amount of loaded proteins. Results were normalised as a percentage of the mean of controls in each membrane.
Statistical analysis
Data were reported as means ± SEM. Relative mRNA expression was reported as relative quantitation (RQ) values, calculated as 2−ΔΔCt, where ΔCt is calculated as Cttarget gene − Cthousekeeping genes (PPIA and PolR2A mRNA expression) and analysed with Student’s t test. Relative protein abundance of Erk 1/2, FoxM1 and Akt was calculated with respect to GAPDH protein abundance and analysed with Student’s t test. All variables were tested for normal distribution by the Kolmogorov–Smirnov test. Pearson’s correlation was used to determine the correlation coefficient between molecular biomarkers and clinical variables. Multiple linear regression analysis was performed to determine the association between variables after correction for BMI and age. Differences were considered statistically significant at p < 0.05.
Results
Effect of lifelong football training on body composition, VO2max, and on the glucose and lipid profile
The clinical–biochemical characteristics of subjects enrolled in this study are reported in Table 1. The VPG had a healthier body composition than the CG. In fact, they had a lower body weight (78.2 ± 6.5 vs 91.2 ± 11.2 kg; p < 0.01), less body fat% (22.6 ± 8.0 vs 31.4 ± 5.0; p < 0.01), a lower BMI (24.4 ± 1.6 vs 28.8 ± 4.0 kg m−2; p < 0.01), and a higher VO2max by body weight (34.7 ± 3.8 vs 27.3 ± 4.0 ml/min/kg; p < 0.001). Moreover, as shown in Table 1, the glucose and lipid profile was healthier in the VPG than in the CG. In fact, we found lower levels of fasting blood glucose, total cholesterol, LDL cholesterol and triglycerides, higher HDL cholesterol values, and a lower resting heart rate in the VPG than in the CG.
Lifelong football training induces upregulation of key markers involved in muscle mitochondrial biogenesis and oxidative metabolism
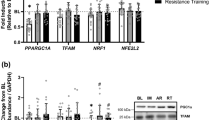
We measured mRNA and protein expression levels of the two catalytic isoforms of AMPK (AMPKα1 and α2) that regulate the muscle intermediate metabolism. As shown in Fig. 1a, b, compared with the CG, the VPG expressed significantly higher amounts of α1 and α2 messengers (p < 0.05 and p < 0.001, respectively) and of proteins (p < 0.05). The analysis of the expression of key messengers and proteins involved in muscle mitochondrial biogenesis and oxidative metabolism revealed increased expression of PGC1α (p < 0.01), NAMPT (p < 0.01) and TFAM (p < 0.01) messengers in VPG muscle compared to CG (Fig. 1c). We also found a significant increase in citrate synthase activity (p < 0.05), overexpression of the MyHC-β isoform (p < 0.05) and a positive expression trend of the MyHC-α isoform in VPG compared to CG muscle (Fig. 1d, e). Notably the levels of FNDC5 and mTOR messengers were higher in VPG than in CG, although the difference was not significant (Fig. 1f). Similarly, the mitDNA/nucDNA ratio in muscle did not differ significantly between VPG and CG (Fig. 1g), which suggests that increased oxidative metabolism was not due to a greater number of mitochondria in VPG muscle. The apparent discrepancy between the similar number of mitochondria in VPG and CG muscles (Fig. 1g) and the increased oxidative activity in VPG muscle is probably due to the higher number of type 1 fibres (as witnessed by the higher MyHC-β expression) rather than to the increase of number of mitochondria per cell. To investigate the effects of lifelong football training on angiogenesis and muscle capillarisation, we measured the expression of the key messengers eNOS and VEGF in VPG and CG muscle. The expression of both mRNAs was higher in VPG than in CG, although the difference was not significant (Fig. 1h).
Effects of lifelong football training on the expression of markers involved in the muscle metabolism pathway. a Skeletal muscle mRNA and b protein expression of AMPKα1 and α2; c mRNA expression of PGC1α, NAMPT and TFAM; d citrate synthase activity; e MyHC-α and MyHC-β protein expression; f mRNA expression of FNDC5 and mTOR; g mitDNA/nucDNA ratio; h mRNA expression of eNOS and VEGF. Messenger and protein expression, citrate synthase activity and mitDNA/nucDNA ratio were determined in muscle biopsies from 10 CG subjects (black bars) and 10 VPG subjects (grey bars). An arbitrary value of 1 was assigned to the level of expression of each messenger in CG. GAPDH served as loading control. Representative blots are reported for each protein analysed. Data were expressed as relative percentage of protein expression vs CG. a–h Data represent the means (±SEM) of 3 different experiments and were analysed using Student’s t test. Differences were considered significant at *p < 0.05, **p < 0.01 and ***p < 0.001, respectively, compared to CG
Lifelong football training affects the muscle expression of key markers involved in DNA repair and senescence suppression
To assess the effects of lifelong football training on healthy longevity, we measured the expression of key proteins involved in the muscle DNA repair promotion and senescence suppression pathways, namely Erk1/2, Akt and FoxM1 in muscle biopsies of the VPG and the CG. All four proteins were significantly higher (p < 0.05) in the VPG than in the CG (Fig. 2a–c). This suggests that lifelong football training has positive effects on these pathways and so contribute to healthy longevity.
Effects of lifelong football training on the expression of key markers involved in DNA repair promotion and senescence suppression pathways. a–c Protein expression levels of Erk1/2, Akt and FoxM1 in muscle biopsies from 10 controls (black bars) and 10 VPG subjects (grey bars) analysed by western blotting. GAPDH served as loading control. Representative blots are reported for each protein analysed. Data were expressed as percentage of CG. Data were compared using Student’s t test and represent the means (±SEM) of 3 different experiments. Differences were considered significant at *p < 0.05 vs CG
Correlation of the study variables
Pearson’s correlation analysis showed correlations between most of the molecular markers and clinical and biochemical variables examined (Table 2). In particular, fat% was negatively associated with Erk1/2 (r −0.52; p = 0.04) and Akt (r −0.58; p = 0.02) protein expression and with VO2max (r −0.71; p < 0.001). Conversely, VO2max was positively associated with citrate synthase activity (r 0.49; p = 0.03), TFAM (r 0.55; p = 0.01), AMPKα2 (r 0.59; p = 0.01) and PGC1α (r 0.56; p = 0.01) mRNA expression and with Erk1/2 (r 0.66; p = 0.01) protein expression. At BMI- and age-adjusted multiple regression analysis, fat% was independently associated with Akt protein expression (Table 3; p = 0.01) and VO2max was independently associated with TFAM (p = 0.001) mRNA and with Erk1/2 (p < 0.001) protein expression (Table 4).
Discussion
To our knowledge, this is the first study to investigate the effects of lifelong football training on the muscle expression profile of molecular markers (genes and proteins) involved in healthy longevity. Skeletal muscle is a plastic tissue, and regular exercise is the most effective tool with which to counteract muscle atrophy pathways (Arany 2008). Exercise induces an increase in several molecular stress signals in skeletal muscle, a process that activates AMPK isoforms. AMPK regulates cellular energy status during exercise: AMPK activation induces downstream upregulation of molecular markers correlated to mitochondrial biogenesis and oxidative metabolism in muscle (PGC1α, NAMPT and TFAM), and to the molecular targets controlling cellular respiration, mitochondrial fusion and transcription (Mounier et al. 2015). AMPK expression levels were lower in muscle from elderly vs young rats, and was associated with decreased mitochondrial content (Reznick et al. 2007) and with a lower mitochondrial oxidative capacity (Lantier et al. 2014), which collectively contribute to an age-related decline in metabolic active muscle mass.
We previously demonstrated that long-term football training (64 weeks) increased the muscle expression of AMPKα1 and α2 messengers in trained vs untrained healthy adults (Alfieri et al. 2015). Here, we provide evidence that lifelong football training counteracts the reduction in muscle mitochondrial oxidative capacity, which is a hallmark of the aging process. In fact, the expression of the AMPKα1 and α2 isoforms was higher in VPG muscle than in controls. Also the downstream markers of the muscle oxidative metabolism pathway, i.e. isoforms PGC1α, TFAM, NAMPT and MyHC-β, were significantly higher in muscle from veterans vs untrained healthy elderly subjects. In addition, mitochondrial citrate synthase enzyme activity, a key marker of muscle oxidative capacity (Gerhart-Hines et al. 2007; Short et al. 2003) was higher in VPG muscle than in control muscle (see Fig. 3). The sustained increase in PGC1α and TFAM expression was closely correlated with reduction of fat mass percentage and to a healthier body composition and metabolic profile (lipid and glucose) in VPG but not in controls. This suggests that lifelong football training is a tool with which to induce and sustain muscle oxidative capacity in the elderly. Several studies reported that concurrent endurance and resistance exercise contributes to the regulation of the expression of two molecular markers (AMPK and PGC1α) and that this increases muscle oxidative capacity (Egan et al. 2010; Skovgaard et al. 2016). Furthermore, increased PGC1α expression in muscle is the initial event in the regulation of oxidative metabolism and exercise intensity influences its expression (Nordsborg et al. 2010). Most of the above-cited studies focused on the acute effects of training in young or middle-aged subjects. Here we demonstrate that lifelong football training, which has been proven to be a combination of endurance training and strength training (Krustrup et al. 2010a, b), induces similar adaptations in oxidative metabolism in elderly muscle also in a chronic condition. However, we cannot rule out the possibility that similar adaptation can be obtained in elderly subjects trained at the same metabolic intensity for a minimum of 6 months.
Schematic representation of the effects of lifelong football training on molecular pathways related to longevity. Long-term football training induces upregulation of key markers involved in the mitochondrial biogenesis, oxidative metabolism pathways (AMPK, PGC1α, NAMPT and TFAM) and in DNA repair promotion and senescence suppression (Akt, ERK1/2 and FoxM1) in muscle from VPG. Blue increased expression. Grey non-analysed molecules. Pink mildly increased expression. Green arrows positive input. Red arrows negative input
Our results confirm the previously reported improved cardio-respiratory fitness in the VPG vs the CG (Schmidt et al. 2015). In particular, the expression of protein MyHC-β, the peculiar isoform of slow, oxidative skeletal muscle fibres (Paoli et al. 2013), was higher in the VPG than in controls; this finding is correlated to the increased VO2max observed in VPG.
Nitric oxide reduction bioavailability is an hallmark of endothelial dysfunction, which in turn is an independent risk factor for adverse cardiac events in the elderly (Nyberg et al. 2012). Lifelong endurance training counteracts the age-related decline in nitric oxide bioavailability (Rubinshtein et al. 2010). Interestingly, we found increased levels of the angiogenesis and capillarisation markers eNOS and VEGF messengers in VPG muscle.
We also investigated the effects of lifelong football training on skeletal muscle aging. Erk1/2 and Akt are two of the most widely studied longevity markers. Erk controls muscle cell proliferation and differentiation thereby playing an important role in the myogenic response to exercise (Carlson et al. 2001; Parkington et al. 2004). Thus far, the effect of Akt on suppression of muscle senescence has been controversial. Indeed, increased Akt expression in adult mouse muscle models was correlated with growth and hypertrophy (Bodine et al. 2001; Pallafacchina et al. 2002), whereas Akt expression did not differ between muscle from elderly active and muscle from sedentary subjects (Sandri et al. 2013). In the present study, Erk1/2 and Akt expression was higher in VPG muscle than in muscle from active, untrained elderly subjects. This finding suggests that there is an adaptive response of skeletal muscle to long-term exercise training that counteracts the consequences of senescence (see Fig. 3). In line with our findings, Frosig et al. (2007) reported that Akt expression was higher in muscle from trained elderly subjects that in untrained elderly subjects. Moreover, AMPK and Akt, respectively, negatively and positively regulated mTOR expression (Gwinn et al. 2008; Manning et al. 2002), which is a master regulator of cell growth and longevity (Harrison et al. 2009; Katewa and Kapahi 2011). In fact, mTOR forms two different protein complexes, mTORC1 and mTORC2. Inhibition of mTORC1 has been reported to extend lifespan and delay aging in various organisms (Sharples et al. 2015), whereas inhibition of mTORC2 negatively affects mammalian health and longevity (Arriola Apelo and Lamming 2016). In mouse models, mTOR expression was higher in the skeletal muscle of aging mice than in young (Lamming et al. 2012; Reznick et al. 2007). The role of mTOR in human muscle aging and sarcopenia is not completely understood. In fact, no differences were identified in mTOR expression in muscle among young, active and sedentary old subjects due to interindividual variability (Sandri et al. 2013). Accordingly, the muscle expression of mTOR messenger did not significantly differ between VPG subjects and our active untrained controls.
The FoxM1 transcription factor regulates a broad range of biological functions, including cell proliferation, cell cycle progression, renewal, differentiation, cell migration, and angiogenesis. FoxM1 overexpression has been reported in liver, prostate, brain, breast and colon cancers (Zona et al. 2014). It is also involved in senescence suppression and in the DNA damage repair process, being particularly active in case of age-dependent accumulation of DNA damage (Behrens et al. 2014). Reduced FoxM1 expression has been associated with an age-related decline in cell proliferation, whereas increased FoxM1 expression has been implicated in the promotion of stem cell renewal, DNA repair and senescence suppression (Bella et al. 2014).
This is the first study to investigate the effects of lifelong training on the expression of molecular markers involved in DNA repair and senescence suppression in skeletal muscle. Here we demonstrate that lifelong football training positively affects the expression of checkpoint markers involved in the DNA repair and senescence suppression pathways, including FoxM1 (see Fig. 3). A limit of this study is the small number of participants and muscle biopsies analysed.
Conclusions
Lifelong football training is a tool with which to modulate expression levels of key messengers involved in muscle oxidative metabolism, DNA repair and senescence suppression. Our results confirm the improvements in body composition and cardiovascular or microvascular function and the healthier glucose and lipid profile found in VPG subjects (Schmidt et al. 2015). All these factors counteract the risk of metabolic diseases and negative cardiovascular events and promote healthy longevity. The current study provides further evidence that lifelong football training can be useful to counteract metabolic decline in the elderly.
Abbreviations
- AMPKα1/α2:
-
Adenosine monophosphate-activated protein kinase α1/ α2
- BMI:
-
Body mass index
- eNOS:
-
Endothelial nitric oxide synthase
- Erk1/2:
-
p42-44 mitogen-activated protein kinase—MAPK
- FNDC5:
-
Fibronectin type III domain-containing protein 5
- FoxM1:
-
Forkhead box M1
- MyHC-α/β:
-
Myosin α/β heavy chain
- mTOR:
-
Mammalian target of rapamycin
- NAMPT:
-
Nicotinamide phosphoribosyl transferase
- PGC1α:
-
Peroxisome proliferator-activated receptor-coactivator-1α
- TFAM:
-
Transcription factor A, mitochondrial
- VEGF:
-
Vascular endothelial growth factor
References
Alfieri A, Martone D, Randers MB, Labruna G, Mancini A, Nielsen JJ, Bangsbo J, Krustrup P, Buono P 2015 Effects of long-term football training on the expression profile of genes involved in muscle oxidative metabolism. Mol Cell Probes 29:43–47
Arany Z (2008) PGC-1 coactivators and skeletal muscle adaptations in health and disease. Curr Opin Genet Dev 18:426–434
Arriola Apelo SI, Lamming DW (2016) Rapamycin: an InhibiTOR of aging emerges from the soil of easter Island. J Gerontol A Biol Sci Med Sci 71(7):841–849
Behrens A, van Deursen JM, Rudolph KL, Schumacher B 2014 Impact of genomic damage and ageing on stem cell function. Nat Cell Biol 16:201–207
Bella L, Zona S, de Moraes GN, Lam EW 2014 FOXM1: a key oncofoetal transcription factor in health and disease. Semin Cancer Biol 29:32–39
Bodine SC, Stitt TN, Gonzalez M, Kline WO, Stover GL, Bauerlein R, Zlotchenko E, Scrimgeour A, Lawrence JC, Glass DJ, Yancopoulos GD (2001) Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol 3:1014–1019
Carlson CJ, Fan Z, Gordon SE, Booth FW (2001) Time course of the MAPK and PI3-kinase response within 24 h of skeletal muscle overload. J Appl Physiol (1985) 91:2079–2087
Egan B, Carson BP, Garcia-Roves PM, Chibalin AV, Sarsfield FM, Barron N, McCaffrey N, Moyna NM, Zierath JR, O’Gorman DJ (2010). Exercise intensity-dependent regulation of peroxisome proliferator-activated receptor coactivator-1 mRNA abundance is associated with differential activation of upstream signalling kinases in human skeletal muscle. J Physiol doi:10.1113/jphysiol.2010.188011
Faloona GR, Srere PA (1969) Escherichia coli citrate synthase. Purification and the effect of potassium on some properties. BioChemistry 8:4497–4503
Frosig C, Sajan MP, Maarbjerg SJ, Brandt N, Roepstorff C, Wojtaszewski JF, Kiens B, Farese RV, Richter EA (2007) Exercise improves phosphatidylinositol-3,4,5-trisphosphate responsiveness of atypical protein kinase C and interacts with insulin signalling to peptide elongation in human skeletal muscle. J Physiol 582:1289–1301
Gerhart-Hines Z, Rodgers JT, Bare O, Lerin C, Kim SH, Mostoslavsky R, Alt FW, Wu Z, Puigserver P (2007) Metabolic control of muscle mitochondrial function and fatty acid oxidation through SIRT1/PGC-1α. EMBO J 26:1913–1923
Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS, Turk BE, Shaw RJ (2008) AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell 30:214–226
Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, Nadon NL, Wilkinson JE, Frenkel K, Carter CS, Pahor M, Javors MA, Fernandez E, Miller RA (2009) Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature 460:392–395
Helge EW, Andersen TR, Schmidt JF, Jorgensen NR, Hornstrup T, Krustrup P, Bangsbo J (2014) Recreational football improves bone mineral density and bone turnover marker profile in elderly men. Scand J Med Sci Sports 24(Suppl 1):98–104
Katewa SD, Kapahi P (2011) Role of TOR signaling in aging and related biological processes in Drosophila melanogaster. Exp Gerontol 46:382–390
Kim ES, Im JA, Kim KC, Park JH, Suh SH, Kang ES, Kim SH, Jekal Y, Lee CW, Yoon YJ, Lee HC, Jeon JY (2007) Improved insulin sensitivity and adiponectin level after exercise training in obese Korean youth. Obesity (Silver Spring) 15:3023–3030
Krustrup P, Aagaard P, Nybo L, Petersen J, Mohr M, Bangsbo J (2010a) Recreational football as a health promoting activity: a topical review. Scand J Med Sci Sports 20 Suppl 1:1–13
Krustrup P, Christensen JF, Randers MB, Pedersen H, Sundstrup E, Jakobsen MD, Krustrup BR, Nielsen JJ, Suetta C, Nybo L, Bangsbo J (2010b) Muscle adaptations and performance enhancements of soccer training for untrained men. Eur J Appl Physiol 108:1247–1258
Lamming DW, Ye L, Katajisto P, Goncalves MD, Saitoh M, Stevens DM, Davis JG, Salmon AB, Richardson A, Ahima RS, Guertin DA, Sabatini DM, Baur JA (2012) Rapamycin-induced insulin resistance is mediated by mTORC2 loss and uncoupled from longevity. Science 335:1638–1643
Lantier L, Fentz J, Mounier R, Leclerc J, Treebak JT, Pehmoller C, Sanz N, Sakakibara I, Saint-Amand E, Rimbaud S, Maire P, Marette A, Ventura-Clapier R, Ferry A, Wojtaszewski JF, Foretz M, Viollet B (2014) AMPK controls exercise endurance, mitochondrial oxidative capacity, and skeletal muscle integrity. FASEB J 28:3211–3224
Liss B (2002) Improved quantitative real-time RT-PCR for expression profiling of individual cells. Nucleic Acids Res 30:e89
Manning BD, Tee AR, Logsdon MN, Blenis J, Cantley LC (2002) Identification of the tuberous sclerosis complex-2 tumor suppressor gene product tuberin as a target of the phosphoinositide 3-kinase/akt pathway. Mol Cell 10:151–162
Mazzeo RS, Cavanagh P, Evans WJ, Fiatarone M, Hagberg J, McAuley E, Startzell J (1998) Exercise and physical activity for older adults. Med Sci Sports Exerc 30:992–1008
Mounier R, Theret M, Lantier L, Foretz M, Viollet B (2015) Expanding roles for AMPK in skeletal muscle plasticity. Trends Endocrinol Metab 26:275–286
Nishioka T, Soemantri A, Ishida T (2004) mtDNA/nDNA ratio in 14484 LHON mitochondrial mutation carriers. J Hum Genet 49:701–705
Nordsborg NB, Lundby C, Leick L, Pilegaard H (2010). Relative workload determines exercise-induced increases in PGC-1alpha mRNA. Med Sci Sports Exerc 1477–1484. doi:10.1249/MSS.0b013e3181d2d21c
Nyberg M, Jensen LG, Thaning P, Hellsten Y, Mortensen SP (2012) Role of nitric oxide and prostanoids in the regulation of leg blood flow and blood pressure in humans with essential hypertension: effect of high-intensity aerobic training. J Physiol 590:1481–1494
Ostergard T, Nyholm B, Hansen TK, Rasmussen LM, Ingerslev J, Sorensen KE, Botker HE, Saltin B, Schmitz O (2006) Endothelial function and biochemical vascular markers in first-degree relatives of type 2 diabetic patients: the effect of exercise training. Metabolism 55:1508–1515
Pallafacchina G, Calabria E, Serrano AL, Kalhovde JM, Schiaffino S (2002) A protein kinase B-dependent and rapamycin-sensitive pathway controls skeletal muscle growth but not fiber type specification. Proc Natl Acad Sci USA 99:9213–9218
Paoli A, Pacelli QF, Cancellara P, Toniolo L, Moro T, Canato M, Miotti D, Reggiani C (2013). Myosin isoforms and contractile properties of single fibers of human Latissimus Dorsi muscle. Biomed Res Int 2013:249398. doi:10.1155/2013/249398.
Parkington JD, LeBrasseur NK, Siebert AP, Fielding RA (2004) Contraction-mediated mTOR, p70S6k, and ERK1/2 phosphorylation in aged skeletal muscle. J Appl Physiol (1985) 97:243–248
Pedersen BK, Saltin B (2006) Evidence for prescribing exercise as therapy in chronic disease. Scand J Med Sci Sports 16(Suppl 1):3–63
Randers MB, Petersen J, Andersen LJ, Krustrup BR, Hornstrup T, Nielsen JJ, Nordentoft M, Krustrup P (2012) Short-term street soccer improves fitness and cardiovascular health status of homeless men. Eur J Appl Physiol 112:2097–2106
Reznick RM, Zong H, Li J, Morino K, Moore IK, Yu HJ, Liu ZX, Dong J, Mustard KJ, Hawley SA, Befroy D, Pypaert M, Hardie DG, Young LH, Shulman GI (2007) Aging-associated reductions in AMP-activated protein kinase activity and mitochondrial biogenesis. Cell Metab 5:151–156
Rubinshtein R, Yang EH, Rihal CS, Prasad A, Lennon RJ, Best PJ, Lerman LO, Lerman A (2010) Coronary microcirculatory vasodilator function in relation to risk factors among patients without obstructive coronary disease and low to intermediate Framingham score. Eur Heart J 31:936–942
Sandri M, Barberi L, Bijlsma AY, Blaauw B, Dyar KA, Milan G, Mammucari C, Meskers CG, Pallafacchina G, Paoli A, Pion D, Roceri M, Romanello V, Serrano AL, Toniolo L, Larsson L, Maier AB, Munoz-Canoves P, Musaro A, Pende M, Reggiani C, Rizzuto R, Schiaffino S (2013) Signalling pathways regulating muscle mass in ageing skeletal muscle: the role of the IGF1-Akt-mTOR-FoxO pathway. Biogerontology 14:303–323
Schmidt JF, Andersen TR, Horton J, Brix J, Tarnow L, Krustrup P, Andersen LJ, Bangsbo J, Hansen PR (2013) Soccer training improves cardiac function in men with type 2 diabetes. Med Sci Sports Exerc 45:2223–2233
Schmidt JF, Andersen TR, Andersen LJ, Randers MB, Hornstrup T, Hansen PR, Bangsbo J, Krustrup P (2015) Cardiovascular function is better in veteran football players than age-matched untrained elderly healthy men. Scand J Med Sci Sports 25:61–69
Sharples AP, Hughes DC, Deane CS, Saini A, Selman C, Stewart CE (2015). Longevity and skeletal muscle mass: the role of IGF signalling, the sirtuins, dietary restriction and protein intake. Aging Cell. doi:10.1111/acel.12342
Short KR, Vittone JL, Bigelow ML, Proctor DN, Rizza RA, Coenen-Schimke JM, Nair KS (2003) Impact of aerobic exercise training on age-related changes in insulin sensitivity and muscle oxidative capacity. Diabetes 52:1888–1896
Skovgaard C, Brandt N, Pilegaard H, Bangsbo J. (2016). Combined speed endurance and endurance exercise amplify the exercise-induced PGC-1α and PDK4 mRNA response in trained human muscle. Physiol Rep. doi:10.14814/phy2.12864.
Spaziani S, Imperlini E, Mancini A, Caterino M, Buono P, Orru S (2014) Insulin-like growth factor 1 receptor signaling induced by supraphysiological doses of IGF-1 in human peripheral blood lymphocytes. Proteomics 14:1623–1629
Srere PA, Brooks GC (1969) The circular dichroism of glucagon solutions. Arch Biochem Biophys 129:708–710
Zona S, Bella L, Burton MJ, de Moraes GN, Lam EWF (2014) FOXM1: an emerging master regulator of DNA damage response and genotoxic agent resistance. Biochim Biophys Acta 1839:1316–1322
Acknowledgements
We thank Jean Ann Gilder (Scientific Communication srl, Naples, Italy) for revising and editing the text. Dr Vitucci, Dr Imperlini and Dr Labruna were supported by the IRCCS SDN, Naples, Italy. The project was also supported by Nordea-fonden, the FIFA Medical Assessment and Research Centre (F-MARC), Preben og Anna Simonsens Fond; PROGETTO CREME (Campania Research in Experimental Medicine) POR Campania FSE 2007/2013, CUP B25B09000050007; the Italian Ministry of Health (GR-2010-2317596) and the University of Naples Parthenope, “Bando per la ricerca individuale, annualità 2015” to MA, PB and SO.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All the authors declare that they have no conflicts of interest.
Additional information
Communicated by Olivier Seynnes.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mancini, A., Vitucci, D., Labruna, G. et al. Effect of lifelong football training on the expression of muscle molecular markers involved in healthy longevity. Eur J Appl Physiol 117, 721–730 (2017). https://doi.org/10.1007/s00421-017-3562-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-017-3562-8