Abstract
Stroke induces bilateral neurological impairment and muscle weakness yielding neurologically more (MA; paretic) and less affected (LA; non-paretic) sides. “Cross-education” refers to training one side of the body to increase strength in the same muscles on the untrained side. Past work showed dorsiflexion training of the LA side produced bilateral strength increases after stroke. The current study explored the presence and extent of cross-education after arm strength training in chronic stroke. Twenty-four chronic stroke participants completed 5 weeks of maximal wrist extension training using their LA arm. Maximal voluntary contraction force, arm motor impairment and functional performance were measured before and after training. Both spinal cord plasticity (n = 12: reciprocal inhibition and cutaneous reflexes, University of Victoria) and cortical plasticity (n = 12: cortical silent period, short-interval intracortical inhibition, intracortical facilitation and transcallosal inhibition, University of British Columbia) were assessed. Five weeks after training, 20 participants completed a follow-up maximal wrist extension retention test. LA wrist extension force increased 42% and MA by 35%. Strength gains were maintained in the follow-up test. Clinically meaningful increases in Fugl-Meyer scores were noted in four participants. Muscle activation was correlated with cutaneous reflex amplitudes after training in the MA arm. LA cortical silent period and transcallosal inhibition from both hemispheres significantly decreased after training. This study shows that high-intensity training with the neurologically less affected “non-paretic” arm can improve strength bilaterally and alter both spinal and cortical plasticity. The extent to which this plasticity can be enhanced or functionally exploited remains to be examined.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Stroke-induced neural damage leads to loss of inputs to motor neurons on the contralesional side as well as altered intra-cortical communication. Strength and sensorimotor functions are impaired bilaterally and asymmetrically which present as paretic, neurologically more affected (MA) side and non-paretic, less affected (LA) side (Zehr 2011). The benefits of post-stroke strength training have been well recognized (Ada et al. 2006). Patten et al. completed a systematic review emphasizing strength training after stroke is useful and does not exacerbate spasticity, or reduce joint range of motion (Patten et al. 2004). However, directly training the MA side is often extremely difficult for those with severe muscle weakness or limited joint range of motion.
Training one side of the body to increase strength in the same muscles on the untrained sides (“cross-education”) was first reported in (Scripture et al. 1894) and can occur in both arm and leg muscles of neurologically intact participants (Yue and Cole 1992; Dragert and Zehr 2011; Hortobagy et al. 1997). According to the “restoring symmetry hypothesis”, Farthing and Zehr proposed that cross-education training, an asymmetrical intervention, should be applied to offset asymmetrical neuromuscular deficits after stroke (Farthing and Zehr 2014).
After stroke, cross-education training with the LA leg can facilitate dorsiflexion strength gains on the MA side. Significantly improved voluntary strength (~ 30%) and tibialis anterior muscle activation in the MA ankle with improved walking ability after 6 weeks of dorsiflexion training using the LA side (Dragert and Zehr 2013). In addition, Urbin et al. found 16 sessions of wrist extension training on the LA side increased active wrist range of motion on the MA side and altered corticospinal plasticity (Urbin et al. 2015).
Studies clearly indicate that unilateral training affects neural pathways bilaterally at both spinal and cortical level (Dragert and Zehr 2011, 2013; Hortobagyi et al. 2011; Latella et al. 2012; Lee and Carroll 2007). Altered excitability in spinal pathways that project to the contralateral side has been assessed by changes in H-reflex amplitudes and extent of reciprocal inhibition (Dragert and Zehr 2011, 2013). Dragert and Zehr (2011) reported that dorsiflexion training altered soleus H-reflex amplitudes in neurologically intact participants and enhanced reciprocal inhibition from soleus to tibialis anterior muscle on the untrained sides in individuals with stroke (Dragert and Zehr 2013). Reduced inhibition in the cortical and corticospinal pathways have also been recorded following unilateral training (Hortobagyi et al. 2011; Latella et al. 2012). Strong correlation between strength transfer and decreased interhemispheric inhibition were seen following unilateral strength training in dorsal interosseous muscle suggesting cross education may affect by the adaptations in interhemispheric inhibition from the trained to the non-trained primary motor cortex (Hortobagyi et al. 2011). Although training-induced neural adaption has been found in both spinal and cortical pathways in neurologically intact participants, less is known about neural adaption following upper limb cross-education training in stroke.
Resistance training-induced improvements in balance and gait performance (Flansbjer et al. 2012, 2008; Yang et al. 2006), and reduced arm motor impairment (Winstein et al. 2004) are noted when the MA side is trained. Unilateral strength training in the ankle can improve strength and these changes may have the potential to transfer to improve function in chronic stroke participants (Dragert and Zehr 2013). However, whether MA arm strength training-induced functional changes could transfer to the untrained side in individuals with chronic stroke has not been tested.
To explore whether unilateral wrist extension could induce cross-education in strength, spinal and cortical plasticity, and motor function after stroke, 24 chronic stroke participants completed a 5-week maximal wrist extension intervention using the LA arm. We hypothesized that unilateral resistance training with the less-affected wrist would improve strength, produce neural adaptation at spinal and cortical levels and induce clinically meaningful changes bilaterally after stroke.
Methods
Participants
Twenty-four participants with chronic (> 6 months post lesion) stroke and associated arm weakness were recruited, detailed participants’ information was provided in Table 1. Twelve participants trained at the University of Victoria (UVIC) and 12 at University of British Columbia (UBC). The protocol was approved by the University of Victoria Human Research Ethics Board (Protocol Number: 07-480-04d) and University of British Columbia Clinical Research Ethics Board (Protocol Number: H15-00055) in accordance with Declaration of Helsinki. Written informed consent was obtained before data collection.
Control procedures
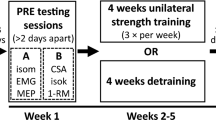
The current study utilized a within-subject multiple baseline design (Butefisch et al. 1995). Three baseline tests (PRE1, PRE2 and PRE3; separated by 4–7 days) and one post-test (POST, within 1 week after training) were performed. Maximal wrist extension strength, spinal and cortical plasticity, and clinical assessments were performed at PRE1-3 and POST. Retention of strength gains was assessed in follow-up tests with wrist maximal extension force and Wolf Motor Function Test (WMFT) measured 5 weeks after the last training session.
Although this multiple baseline design requires more time and labor, it has been validated as a replacement of the control group (Butefisch et al. 1995; Dragert and Zehr 2011, 2013; Klarner et al. 2014, 2016a, 2016b; Kaupp et al. 2018), allows participants to create a reliable baseline and act as their own control, and ensures all receive treatment. To evaluate individual subject data, a 95% confidence interval (95%CI) of the wrist extension force was calculated from the 3 baselines and those whose POST value was outside this range were defined as a responder (Klarner et al. 2016a).
Training protocol
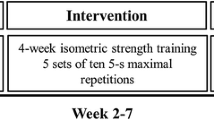
Five weeks of training were completed with 3 sessions (one in lab, two at home) per week consisting of 5 sets × 5 reps × 5 s maximal wrist extension contractions in the LA arm (3 s breaks between contractions and 2 min breaks between sets) (Dragert and Zehr 2011, 2013; Barss et al. 2018). Before training, a warm-up session with 3 sets × 5 rep × 5 s 50% maximal wrist extension contraction were completed. Training was performed with the participant seated in a comfortable position with LA arm strapped to the customized training device to ensure the wrist angle was constant during contraction (Fig. 1A). When training at home, standardized audio instructions were provided with cueing of when to contract and relax during warm-up and training, as well as verbal encouragement to ensure the instruction and timing were consistent between sessions. To ensure participants followed protocol when training at home, each training device included a load cell to record contractions and a micro SD card to save the data. Data from the training device were recorded and analyzed for those training at UVIC. Training devices were piloted with two neurologically intact volunteers prior to data collection. The full training protocol was completed to ensure the device was comfortable and easy to use through the training. To test the reliability of the training devices, load cell readings were recorded by adding and removing standard weights across 5 different days. High reliability was suggested based on significant intraclass correlation for all the devices (Pearson correlation > 0.98, p = 0.000).
A Customized strength training device. Participants aligned the wrist crease to the middle of the training device at the hinge. A load cell was installed underneath. Blue circle indicates the compartment with data acquisition circuit, battery and micro SD. B, C MVC force at wrist vertical (B) and horizontal (C) positions. Black arrow indicates force sensor
Measures of strength (n = 24, participants from UVIC and UBC)
During PRE, POST and follow-up tests, participants were seated comfortably with forearm and wrist supported in a customized device (Fig. 1B, C). Maximal voluntary contraction (MVC) wrist extension force was measured with the wrist at horizontal (pronated) and vertical (mid-supinated) positions bilaterally using a 6-axis force sensor (ATI, Industrial Automation Gamma DAQ F/T Transducer, Apex, NC, USA). MVC force was calculated from a 10 ms window around the peak with custom written MATLAB programs (Version R2013b, The Mathworks, Natick, MA, USA).
During training, wrist extension force was measured by a load cell and recorded on a Micro SD card on the training device (Fig. 1A). Data were analyzed with a customized LabVIEW program for those training at UVIC (n = 12). If the load cell reading showed a quick increase with a clear plateau > 3 s, that trial was considered as a “qualified” MVC. According to the training protocol described in the previous section, a total of 375 MVC (5 reps × 5 sets × 3 sessions × 5 weeks) were intended to be completed by each participant. The average number of “qualified” MVC for the 12 participants at UVIC was 288 ± 65.
Measures of spinal plasticity (n = 12, participants from UVIC)
Electromyography (EMG) of extensor (ECR) and flexor carpi radialis (FCR) muscles was recorded using disposable surface electrodes (Thought Technology Ltd., Quebec, Canada). EMG was amplified (× 5000), bandpass filtered from 100 to 300 Hz (GRASS P511, Astromed-Grass Inc.) and sampled at 2000 Hz through a customized LabVIEW program (National Instruments, Austin, TX). Maximal EMG in ECR muscle (EMGMAX) during wrist extension was determined on both sides, reciprocal inhibition and cutaneous reflexes were examined at four contraction levels (10, 15, 25 and 50% EMGMAX of the same arm).
Reciprocal inhibition from wrist flexors to extensors and cutaneous reflexes evoked by median (MED) and superficial radial (SR) nerve stimulation were assessed bilaterally with similar procedures as found in previous studies (Thompson et al. 2008; Zehr and Kido 2001; Kido et al. 2004). Reciprocal inhibition was evoked by a single 1.0 ms pulse applied over the median nerve just above the elbow under the curve of the biceps brachii. Stimulation intensity was set at 1.2 times the threshold that evoked a direct muscle response (M-wave motor threshold) in FCR. For cutaneous reflexes, trains of 5 × 1.0 ms pulses at 300 Hz were applied to the superficial SR or MED nerves at the wrist. Intensity was set as 2 times radiating threshold (RT), the lowest intensity at which a sensation of radiating paresthesia could be evoked in the innervation territory of the nerve, while not considered noxious by study participants.
Twenty data sweeps were collected and sampled by triggering pseudo-randomly every 1.5–3 s (reciprocal inhibition) or 2–3 s (cutaneous reflexes). Target EMG was presented on a computer screen during each trial so participants could match targets between stimulations. Since most participants could not generate four distinct levels of wrist extension contraction on their MA sides, two to three trials were performed with different ECR background EMG.
Typical muscle responses from reciprocal inhibition and cutaneous reflexes trials are presented in Fig. 2. Reciprocal inhibition was calculated as the difference between the mean background EMG and the mean of a 10 ms window around the post-stimulus minima with a latency to the peak of the response of ~ 30 to 40 ms. Early latency cutaneous reflexes were analyzed as the difference between the mean background EMG and the mean of a 10 ms window around post-stimulation minima at ~ 50 to 75 ms latency.
Typical muscle responses for reciprocal inhibition (A) and cutaneous reflex (B) trials with stimulation artifact removed (blank area in figures). A Shaded area indicates the reciprocal inhibition response measured at latency to largest effect, a 10 ms window around the lowest value that was used for data analysis at the latency around 30–40 ms. There was a 20 ms window of background EMG data recorded before stimulation onset. The average value of the pre-stimulation background EMG was presented as a long dash line. B Dark grey area indicates the early latency cutaneous reflex, a 10 ms window around the lowest value that was used for data analysis at the latency around 50–75 ms. There was a 100 ms window of data recorded before stimulation onset. The average value of the pre-stimulation background EMG was presented as a long dash line
Measures of cortical plasticity (n = 12, participants from UBC)
Cortical silent period (CSP), transcallosal inhibition (TCI), short-interval intracortical inhibition (SICI) and intracortical facilitation (ICF) were measured during PRE, POST and follow-up test.
As described previously (Mang et al. 2015), CSP and TCI were elicited by single-pulse TMS with a Magstim 2002 stimulator unit and a figure-of-eight coil (70 mm, P/N 9790, Magstim Co. Ltd., Whitland, Carmarthenshire, UK) at a frequency of 0.25 Hz. CSP was measured as the prolonged decrease in ECR EMG following a motor evoked potential (MEP). During TCI trails, participants produced 50% maximum grip contraction ipsilateral to the stimulation. Ten TMS stimulations at 150% resting motor threshold were delivered over the ECR motor cortex representation to elicit ipsilateral silent period (iSP). Mean and minimum EMG amplitude during the iSP (iSP-mean, iSP-max) from both contralesional (CL) and ipsilesional (IL) side were measured. The normalized iSP-mean was calculated as iSP-mean/pre-stimulus.
SICI and ICF were evoked by paired-pulse TMS (Chen et al. 1998; Kujirai et al. 1993). SICI was defined as the suppression of the MEP evoked by a subthreshold conditioning stimulus and a suprathreshold test stimulus with a 2 ms interval. ICF is a period of increased intracortical excitability in response to conditioning stimulus and test stimulus with a 12 ms interval. The amplitude of conditioning stimulation was set at 80% active motor threshold and the test stimulus was set at the necessary stimulus intensity to consistently evoke an MEP with an amplitude of 0.3–0.5 mV in ECR. Ten test stimuli, 10 SICI, and 10 ICF stimulations were delivered in a pseudo-randomized order. The percentages of SICI and ICF to unconditioned test stimuli MEP were calculated. All TMS data analyzed offline with custom MATLAB program. SICI and ICF were evoked on the ipsilesional sides of four participants during one baseline test. Therefore, only these four PRE and POST datasets were used for statistical analysis.
Clinical measurements
The Fugl-Myer Upper Extremity (FM-UE) assessment indexed arm motor impairment in the MA arm for all participants (n = 24) before and after training. FM-UE assessment has been commonly used in measuring motor impairment during stroke recovery (Gladstone et al. 2002). Here, FM-UE assessed joint movement from four sections: upper extremity (36 points), wrist (10 points), hand (14 points) and coordination/speed (6 points) using a 3-point scale with higher scores indicating less motor impairment (max score 66).
To evaluate arm motor function, the Wolf Motor Function Test (WMFT) (Wolf et al. 2001) were performed by the 12 participants at UBC. Due to time constraints, an abbreviated version of the Wolf Motor Function Test (abb-WMFT) were performed by the 12 participants at UIVC. The abb-WMFT included three tasks: pick up a can (gross motor), pick up a paper clip (fine motor) and fold a towel (functional task); both arms are tested during the WMFT. Performance time of each task was converted to rate of performance (Hodics et al. 2012):
The rates of performance were averaged among the tasks and compared statistically between PRE and POST. Participant information and clinical baseline measurements are in Table 1.
Statistical analysis
One-way repeated measures analyses of variance (rmANOVA) were performed (SPSS 20, Chicago, IL) to assess whether force changes over time. If there was no significant difference between PRE1, PRE2 and PRE3, baseline data were averaged to one PRE value. (Klarner et al. 2016a, b). To test whether strength improved significantly after training, the main effect of TIME was tested for PRE and POST force data (n = 24). To test whether the strength gains were retained after training, one-way rmANOVA was performed to test the main effect of TIME on PRE, POST and follow-up force data (n = 20).
To assess the strength changes for individual participants, a 95% confidence interval (95%CI) was calculated from the 3 baseline tests with maximal of 9 MVC wrist extension contractions. If the averaged post-test strength outside the range of 95% CI, the strength improvement was considered significant and that participant was defined as a responder.
For reciprocal inhibition and cutaneous reflexes (n = 12), linear regression analyses between baseline EMG and reflex amplitudes were performed and Pearson r values calculated for each pool of paired data (df = n−1). For significant linear relation, the slope and y-intercept were compared between PRE and POST data, with critical t distribution values (df = n1 + n2–4) used to establish significance (Dragert and Zehr 2013).
CSP from the contralesional side (n = 12), SICI and ICF values on each hemisphere (contralesional: n = 12 and ipsilesional n = 4) were examined across time (PRE, POST, follow-up) by one-way rmANOVA. Two-way rmANOVA with the main effect of Time and Hemisphere (CL, IL) was used for TCI measurements (n = 12). Correlation analysis was performed between the percentage change in strength gain and TMS measures for the responders.
Paired t tests were used to compare averaged rate of performance in abb-WMFT, full-WMFT and the Fugl-Meyer between PRE and POST tests. Statistical significance was set at p ≤ 0.05.
Results
Force measurements
Wrist extension force significantly increased by 42% (F(1,23) = 5.603, p = 0.027) and 35% (F(1,23) = 4.510, p = 0.045) on the LA and MA sides in the trained wrist horizontal position. Paired t test showed that the percent gain did not differ between the two arms. A significant main effect of Time was found in the 20 participants comparing PRE, POST and follow-up (F(1,23) = 4.484, p = 0.018). No significant difference between POST and follow-up suggests maintained strength. Strength improvement in the wrist horizontal position did not transfer to wrist vertical position for either hand. Figure 3 shows the averaged maximal wrist extension force during PRE, POST and follow-up tests. Force measurements for each participant are presented in Table 2.
Wrist extension MVC force at PRE, POST and follow-up at wrist horizontal (A, B) and vertical (C, D) positions. Grey, black and white bars represent force amplitude at PRE (n = 24), POST (n = 24) and follow-up (n = 20), respectively. Each bar represents mean ± one standard error of the mean. Asterisk indicates significant difference (p < 0.05). ns represent there is no significant effect
Single subject analysis showed that wrist extension force in 17 of 24 participants significantly improved in the trained arm. These 17 participants were considered LA responder. Within the17 LA responders, strength transfer occurred in 8 participants, which were determined as MA responder. Fifteen LA responders completed the follow-up tests and showed strength maintenance in 8 with only 3 MA responders.
Spinal plasticity measurements—UVIC
Significant correlation between background EMG and reciprocal inhibition was found on the LA side before and after training with no differences between linear regression slopes (Fig. 4). Early latency cutaneous reflexes from MED and SR nerve stimulation were also significantly correlated with background EMG in the LA arm (see Fig. 5).
Reciprocal inhibition evoked at different background muscle activation levels. Linear regression analyses and Pearson r values were calculated for each best-fit line. Grey and black dots represent reflex amplitudes at PRE and POST tests, respectively. *Indicates significant linear correlation. X-axis represents background ECR muscle activation. Y-axis represents reciprocal inhibition amplitudes
Cutaneous reflexes evoked from superficial radial (SR; A, B) and median (MED; C, D) nerves at different background muscle activation levels. Data from less (LA) and more (MA) affected arms (n = 12 participants) are shown at left and right, respectively. Grey and black dots represent reflex amplitude at PRE and POST, respectively. Linear regression analyses were performed and Pearson r values were calculated for each best-fit line. Asterisk indicates significant linear correlation. Hash indicates significant difference between linear regression slopes. X-axis represents background ECR muscle activation. Y-axis represents cutaneous reflexes amplitudes
On the MA side, significant correlation was found in the early latency SR cutaneous reflexes during PRE and POST tests, and early latency MED cutaneous reflexes during PRE. A significantly decreased linear regression slope was found in the SR cutaneous response after training (t = 2.34, tcrit = 1.99, p = 0.02).
Cortical plasticity measurements—UBC
CSP duration decreased by 12% (p = 0.018) in the contralesional hemisphere (LA side) after training (Fig. 6). No visible CSP was elicited during baseline tests in the ipsilesional hemisphere. Percentage change in CSP and strength of the LA responder were not correlated (Pearson r = 0.374, n = 9).
Cortical silent period (CSP; A) on the ipsilesional (IL) side. Transcallosal inhibition (B) on both contralesional (CL) and IL sides. Grey and black bars represent PRE and POST tests results (n = 12). Each bar represents mean ± one standard error of the mean. Asterisk indicates significant difference (p < 0.05)
Increased normalized iSP-mean (iSP-mean/pre-stimulus) was noted (p = 0.023) with 1 and 3% changes in the contralesional and ipsilesional hemisphere, respectively, indicating reduced transcallosal inhibition (Fig. 6). However, no significant effects of Hemisphere, Time, or Hemisphere × Time interaction showed between PRE to POST and follow-up tests (n = 9, with three participants did not complete the follow-up TMS test). Correlation analysis showed no relationship between changes in normalized iSP-mean and strength for LA (Pearson r = 0.205, n = 9) or MA responders (Pearson r = 0.334, n = 6).
SICI and ICF were only generated in 4 participants during the PRE tests. No significant change occurred in either SICI or ICF after training. Results of the statistical analysis for strength and cortical plasticity are presented in Table 3.
Clinical measurements
Clinical function improved and motor impairment decreased after training. FM Upper Extremity score increased from 26.2 ± 20.6 to 28.7 ± 20.3 (mean ± standard deviation) after training (p = 0.001). Abb-WMFT rate increased from 37.7 ± 16.6 to 42.5 ± 18.8 in the LA arm (n = 24, p = 0.032), but there were no significant changes (from 8.7 ± 14.8 to 10.6 ± 16.8, p = 0.059) in the MA arm. The full WMFT rate (n = 12; all performed at the UBC) showed significant improvement from 28.1 ± 30.1 to 34.7 ± 33.1 (p = 0.004) on the MA side. Results of the statistical analysis for clinical measurements are presented in Table 4.
Discussion
Unilateral wrist extension strength training of the non-paretic, less affected side can improve muscle strength bilaterally in chronic stroke. Training-induced neural adaptation was found in spinal and cortical pathways on both sides. We show here that strength gains and neural adaptation can be induced by high-intensity strength training even in individuals with chronic stroke.
Cross-education and strength gains
Similar percentage gains in strength were seen between arms (~ 42 and ~ 35% in LA and MA). Dorsiflexion cross-education training after stroke showed similar results between legs (~ 34 and ~ 31% in LA and MA) (Dragert and Zehr 2013). Yet, in neurologically intact participants, cross-education strength gains on the untrained side is only ~ 8% on average (Munn et al. 2004) and ~ 9% for the upper limb (Manca et al. 2017). This suggests that unilateral training of the LA limb can not only be used to “boost” strength in the MA arm after stroke, and that relative gains are amplified as compared to non-stroke controls.
Training-induced strength gains were retained in both arms 5 weeks after training: 8 of the 17 LA and 3 of the 8 MA responders maintained their strength gains. Dragert and Zehr (2013) categorized 4 of the total sample of 19 (~ 21%) as non-responders (no strength gain on the trained side) after dorsiflexion training (Dragert and Zehr 2013), while here we categorized 7 of 24 (~ 29%) as non-responders. The slightly higher proportion of non-responders in the current study may suggest that a similar dose of cross-education in the arm does not induce as strong of an effect as in the leg for stroke participants. This could relate to differences in functional coupling between the arms compared to the legs. In a recent review by Halperin et al. (2015), the authors suggested non-local muscle fatigue is more likely to occur in the non-training lower limb muscles compared to the upper limb. In addition, the strength of neural coupling between the legs is stronger than the arms as seen in rhythmic locomotor tasks (Zehr et al. 2016). To fully utilize neural connections between arms, on-going high-intensity strength training should be applied in chronic stroke rehabilitation training.
We also found strength gain only transferred at the trained position (wrist horizontal) during wrist extension in accordance with “specificity of training” (Sale and MacDougall 1981; Zhou 2000). Several studies show that different wrist and forearm positions affect grip strength and muscle activation (Mogk and Keir 2003; Richards et al. 1996; Terrell and Purswell 1976). Baldissera et al. found FCR H-reflex amplitudes decreased when the forearm position was changed from pronation to supination position. They suggested that the muscle afferent pathway (as assessed by the H-reflex) to FCR motor neurons is influenced by changes in afferent feedback accompanying forearm rotation (Baldissera et al. 2000). Zehr suggested that sensory feedback may be part of the ensemble signaling associated with the cross-education effect (Zehr 2006). Thus, it is possible that that changes in wrist position affected sensory feedback and muscle activation in both wrist extensors and flexors thus emphasizing task-specific transfer.
It is worth noting that the average post-lesion duration was 144 ± 72 months for the less affected side responders and 158 ± 66 months for the more affected side responders. The bilateral strength improvement found here further debunks the myth that stroke recovery plateaus 3- to 6-months after lesion, a concept commonly believed by many of those with stroke and often still taught to clinical professionals (Sun et al. 2015). Cross-education strength induced neural plasticity and clinical translation will be discussed in the following sections. The results from this study emphasize the idea that there is no time limit in stroke rehabilitation.
Spinal cord plasticity
Regression slopes between SR cutaneous reflex amplitudes and background EMG decreased with stronger inhibition in the MA arm indicating the excitability of cutaneous pathway was normalized to the LA side after training. Others have shown training-induced neural adaptation in spinal-mediated reflex pathways (Zehr 2002, 2006). Enhanced soleus H-reflexes were found in the untrained side in neurologically intact participants after dorsiflexion cross-education training (Dragert and Zehr 2011). Altered reciprocal inhibition amplitudes (suggesting increased sensitivity to descending voluntary commands) were found in untrained MA TA muscle after stroke (Dragert and Zehr 2013). Here, such correlation between reciprocal inhibition amplitude and background EMG was absent on the MA arm suggesting weaker excitability in the reciprocal inhibition pathway after stroke.
Altered SR (innervates dorsum of the hand) but not MED (innervates palm) cutaneous reflex amplitudes were found which may be related to sensory input from the mechanical action of the straps on the hand during wrist extension. Sensory input plays a critical role in the motor function recovery after stroke (Nudo et al. 2000; Celnik et al. 2007) and the excitabilities of cutaneous pathways can be altered through training (Zehr 2006). Studies in neurologically intact participants showed strength training with sensory electrical stimulation induced higher strength gains on the untrained side (Hortobagyi et al. 1999). In a short-term intervention study, unilateral voluntary contraction, sensory electrical stimulation or contraction combined with sensory stimulation produced altered amplitudes of H-reflexes and motor evoked potential on the contralateral sides differentially (Hortobagyi et al. 2003). These observations suggest enhanced sensory input modulates larger neural adaptation compared to performing voluntary contraction alone. Veldman et al. explored whether adding electrical stimulation to unilateral motor practice could amplify inter-limb transfer. Results suggested that outcomes from sensory electrical stimulation may depend on clinical status since the effects are much less (6%) in healthy compared to stroke participants 27% (Veldman et al. 2015). Further research is needed to understand whether enhanced sensory input could facilitate the cross-education effect of strength training after stroke.
Cortical plasticity
Significant decreases were found in TCI from both hemispheres and in CSP from the LA side after training. Reduced CSP on the LA side is similar to results after cross-education training in neurologically intact participants. Kidgell et al. found that 4 weeks of unilateral wrist flexion training decreased CSP duration significantly bilaterally which caused less inhibitory input to the motor neuron pool and increased net excitability of the corticospinal tract (Kidgell et al. 2015). Since the first 50 ms of the CSP duration is believed to be controlled by spinal mechanisms while the reductions after 100 ms are assumed to cause by supraspinal inhibition (Inghilleri et al. 1993) and the CSP duration seen in our participants was reduced from 131 to 115 ms, we assume that the training-induced reduction in CSP was primarily due to cortical factors. Although we found lack of correlation between the percentages of strength gain and changes in CSP and TCI measurements, previous studies showed that progressive decrease in CSP duration and stronger inhibition CL-iSP were associated with improvement in motor outcome for stroke participants (Brouwer and Schryburt-brown 2006; Classen et al. 1997; Harris-Love et al. 2016). Our group results here suggest CSP and TCI may play important role in increasing bilateral strength and motor function in stroke rehabilitation.
Paired-pulse TMS induced SICI has been used to examine GABAA mediated intracotical inhibition. People with stroke usually show deficient SICI modulation in the primary motor cortex due to the lesion (Harris-love et al. 2016; Shimizu et al. 2002). Here, we did not find decreased SICI which has been seen in other cross-education studies in neurologically intact individuals (Kidgell et al. 2015; Goodwill et al. 2012). This may due to the small sample size since SICI was only evoked in four participants. However, considering the strength gain at the POST test, lack of significant changes in SICI may indicate cross-education can utilize the intact cortical pathway inducing bilateral strength gain and without involving GABAA mediated inhibitory pathways.
Clinical translation
Training-induced neural plasticity was also reflected in clinical measurements. FM score increased 2.5 ± 3.1 points (mean ± standard deviation) with 4 participants showing ≥ 5 points increase suggesting reduced impairment in the MA arm and was maintained at follow-up. Although the minimal clinically important difference ranges from 4.25 to 7.25 (Page et al. 2012), the current study had more severe stroke participants with an average PRE FM score of 26.2 compared to 39.2 in the previous study (Page et al. 2012). In functional tests, abb-WMFT rate (n = 24) improved significantly in the LA arm. Full WMFT (n = 12, with 9 responders) performance time decreased by 1.5 s (standard deviation: 1.8 s) on the MA side, in the range of minimal clinically important difference of 1.5 to 2 s (Lin et al. 2009). This strength training protocol shows the potential to reduce impairment and improve motor function in the arm even for severely affected stroke participants. To induce clinically significant changes, higher training intensity and/or longer training durations may be required in stroke participants with severe impairment.
Summary
This study for the first time shows bilateral neuromuscular and strength gains in arm muscles can be induced in chronic stroke by training the less affected side only. Neural adaptations in spinal and cortical pathways demonstrate functional neural plasticity can occur even years after stroke using high-intensity training. These results further debunk the myth that stroke recovery plateaus 3- to 6-months after lesion and emphasize the idea that there is no time limit in stroke rehabilitation. However, response variability between participants suggests that to induce and maintain cross-education between arm muscles may require higher intensity and on-going training in stroke participants.
References
Ada L, Dorsch S, Canning CG (2006) Strengthening interventions increase strength and improve activity after stroke: a systematic review. Aust J Physiother 52(4):241–248. https://doi.org/10.1016/S0004-9514(06)70003-4
Baldissera F, Bellani G, Cavallari P, Lalli S (2000) Changes in the excitability of the H-reflex in wrist flexors related to the prone or supine position of the forearm in man. Neurosci Lett 295:105–108. https://doi.org/10.1016/S0304-3940(00)01604-9
Barss TS, Klarner T, Pearcey GE, Sun Y, Zehr EP (2018) Time course “dose” of inter-limb strength transfer after hand grip training. J Appl Physiol (submitted)
Brouwer BJ, Schryburt-brown K (2006) Hand function and motor cortical output poststroke: are they related ? Arch Phys Med Rehabil 87:627–634. https://doi.org/10.1016/j.apmr.2006.02.006
Butefisch C, Hummelsheim H, Denzler P, Mauritz K-H (1995) Repetitive training of isolated movements improves the outcome of motor rehabilitation of the centrally paretic hand. J Neurol Sci 130:59–68
Celnik P, Hummel F, Harris-love M, Wolk R, Cohen G, L (2007) Somatosensory stimulation enhances the effects of training functional hand tasks in patients with chronic stroke. Arch Phys Med Rehabil 88:1369–1376. https://doi.org/10.1016/j.apmr.2007.08.001
Chen R, Tam A, Butefisch C, Corwell B, Ziemann U, Rothwell JC, Cohen LG (1998) Intracortical inhibition and facilitation in different representations of the human motor cortex. J Neurophysiol, 2870–2881
Classen J, Schnitzler A, Binkofski F, Werhahn KJ, Kim Y, Kessler KR, Benecke R (1997) The motor syndrome associated with exaggerated inhibition within the primary motor cortex of patients with hemiparetic stroke. Brain 120:605–619
Dragert K, Zehr EP (2011) Bilateral neuromuscular plasticity from unilateral training of the ankle dorsiflexors. Exp Brain Res 208(2):217–227. https://doi.org/10.1007/s00221-010-2472-3
Dragert K, Zehr EP (2013) High-intensity unilateral dorsiflexor resistance training results in bilateral neuromuscular plasticity after stroke. Exp Brain Res 225:93–104. https://doi.org/10.1007/s00221-012-3351-x
Farthing JP, Zehr EP (2014) Restoring symmetry: clinical applications of cross-education. Exerc Sport Sci Rev 42(2):70–75
Flansbjer UB, Miller M, Downham D, Lexell J (2008) Progressive resistance training after stroke: effects on muscle strength, muscle tone, gait performance and perceived participation. J Rehabil Med 40:42–48. https://doi.org/10.2340/16501977-0129
Flansbjer UB, Lexell J, Brogårdh C (2012) Long-term benefits of progressive resistance training in chronic stroke: a 4-year follow-up. J Rehabil Med 44:218–221. https://doi.org/10.2340/16501977-0936
Gladstone DJ, Danells CJ, Black SE (2002) The Fugl-Meyer assessment of motor recovery after stroke: a critical review of its measurement properties. Neurorehabil Neural Repair 16(3):232–240
Goodwill AM, Rearce JA, Kidgell JD (2012) Corticomotor plasticity following unilateral strength training. Neuroplast Exerc. https://doi.org/10.1002/mus.23316
Halperin I, Chapman DW, Behm DG (2015) Non-local muscle fatigue: effects and possible mechanisms. Eur J Appl Physiol 115:2031–2048. https://doi.org/10.1007/s00421-015-3249-y
Harris-love ML, Chan E, Dromerick AW, Cohen LG (2016) Neural substrates of motor recovery in severely impaired stroke patients with hand paralysis. Neurorehabil Neural Repair 30(4):328–338. https://doi.org/10.1177/1545968315594886
Hodics TM, Nakatsuka K, Upreti B, Alex A, Smith PS, Pezzullo JC (2012) Wolf motor function test for characterizing moderate to severe hemiparesis in stroke patients. Arch Phys Med Rehabil 93(11):1963–1967. https://doi.org/10.1016/j.apmr.2012.05.002
Hortobagy T, Lambert NJ, Hill JP (1997) Greater cross education following training with muscle lengthening than shortening. Med Sci Sport Exerc 29(1):107–112
Hortobagyi T, Scott K, Lambert J, Hamilton G, Tracy J (1999) Cross-education of muscle strength is greater with stimulated than voluntary contractions. Mot Control 3:205–219
Hortobagyi T, Taylor JL, Petersen NT, Russell G, Gandevia SC, Gandevia SC (2003) Changes in segmental and motor cortical output with contralateral muscle contractions and altered sensory inputs in humans. J Neurophysiol 90:2451–2459. https://doi.org/10.1152/jn.01001.2002
Hortobagyi T, Richardson SP, Lomarev M, Shamin E, Meunier S, Russman H, Hallett M (2011) Interhemispheric plasticity in humans. Med Sci Sport Exerc 43(7):1188–1199. https://doi.org/10.1249/MSS.0b013e31820a94b8.Interhemispheric
Inghilleri M, Berardelli A, G Cruccu, M Manfredi (1993) Silent period evoked by transcranial stimulation of the human cortex and cervicomedullary junction. J Physiol 466:521–534
Kaupp C, Pearcey GE, Klarner T, Sun Y, Cullen H, Barss TS, Zehr EP (2018) Rhythmic arm cycling training improves walking and neurological integrity in chronic stroke—the arms can give legs a helping hand in rehabilitation. J Neurophysiol 119:1095–1112. https://doi.org/10.1152/jn.00570.2017
Kidgell DJ, Frazer AK, Rantalainen T, Ruotsalainen I, Ahtiainen J, Avela J, Howatson G (2015) Increased cross-education of muscle strenght and reduced corticospinal inhibition following eccentric strength training. Neuroscience 300:566–575. https://doi.org/10.1016/j.neuroscience.2015.05.057
Kido A, Tanaka N, Stein RB (2004) Spinal reciprocal inhibition in human locomotion. J Appl Physiol 96:1969–1977. https://doi.org/10.1152/japplphysiol.01060.2003
Klarner T, Barss T, Sun Y, Kaupp C, Beattie S, Zehr EP (2014) Reliability of multiple baseline measures for locomotor retraining after stroke. Replace, repair, restore, relieve-bridging clinical and engineering solutions in neurorehabilitation. Biosyst Biorobotic 7:479–486. https://doi.org/10.1007/978-3-319-08072-7
Klarner T, Barss TS, Sun Y, Kaupp C, Loadman PM, Zehr EP (2016a) Exploiting interlimb arm and leg connections for walking rehabilitation: a training intervention in stroke. Neural Plast 2016:1517968. https://doi.org/10.1155/2016/1517968
Klarner T, Barss TS, Sun Y, Kaupp C, Loadman PM, Zehr EP (2016b) Long-term plasticity in reflex excitability induced by five weeks of arm and leg cycling training after stroke. Brain Sci 6:54. https://doi.org/10.3390/brainsci6040054
Kujirai T, Carramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A, Marsden CD (1993) Corticocortical inhibition in human motor cortex. J Physiol 471:501–519
Latella C, Kidgell DJ, Pearce AJ (2012) Reduction in corticospinal inhibition in the trained and untrained limb following unilateral leg strength training. Eur J Appl Physiol 112:3097–3107. https://doi.org/10.1007/s00421-011-2289-1
Lee M, Carroll TJ (2007) Cross education possible mechanisms for the contralateral effects of unilateral resistance training. Sports Med 37(1):1–14
Lin K, Hsieh Y, Wu C, Chen C, Jang Y, Liu J (2009) Minimal detectable change and clinically important difference of the Wolf Motor Function Test in stroke patients. Rehabil Neural Repair 23(5):429–434
A Manca, D Dragone, Z Dvir, F Deriu (2017) Cross-education of muscular strength following unilateral resistance training: a meta-analysis. Eur J Appl Physiol 117(11):2335–2354. https://doi.org/10.1007/s00421-017-3720-z
Mang CS, Borich MR, Brodie SM, Brown KE, Snow NJ, Wadden KP, Boyd LA (2015) Clinical Neurophysiology Diffusion imaging and transcranial magnetic stimulation assessment of transcallosal pathways in chronic stroke. Clin Neurophysiol 126(10):1959–1971. https://doi.org/10.1016/j.clinph.2014.12.018
Mogk J, Keir P (2003) The effects of posture on forearm muscle loading during gripping. Ergonomics 46(9):956–975. https://doi.org/10.1080/0014013031000107595
Munn J, Herbert RD, Gandevia SC (2004) Contralateral effects of unilateral resistance training: a meta-analysis. J Appl Physiol 96:1861–1866. https://doi.org/10.1152/japplphysiol.00541.2003
Nudo RJ, Friel KM, Delia SW (2000) Role of sensory deficits in motor impairments after injury to primary motor cortex. Neuropharmacology 39:733–742
Page SJ, Fulk GD, Boyne P, Page SJ, Fulk GD, Boyne P (2012) Clinical importance differences for the upper-extremity Fugl-Meyer scale in people with minimal to moderate impairment due to chronic stroke. Phys Ther 92(6):791–798. https://doi.org/10.2522/ptj.20110009
Patten C, Lexell J, Brown HE (2004) Weakness and strength training in persons with poststroke hemiplegia: rationale, method, and efficacy. J Rehabil Res Dev 41(3):293–312
Richards LG, Olson B, Palmiter-Thomas P (1996) How forearm position affects grip strength. Am J Occup Therapy 50(2):133–138
Sale D, MacDougall D (1981) Specificity in strength training:a review for the coach and athlete. Can J Sport Sci 6(2):87–92
Scripture E, Smith TL, Brown EM (1894) On education of muscular control and power. Stud Yale Psychol Lab 2:114–119
Shimizu T, Hosaki A, Hino T, Sato M, Komori T, Hirai S, Rossini PM (2002) Motor cortical disinhibition in the unaffected hemisphere after unilateral cortical stroke. Brain 125:1896–1907
Sun Y, Boots J, Zehr EP (2015) The lingering effects of a busted myth- false time limits in stroke rehabilitation. Appl Physiol Nutr Metab 40(8):858–861
Terrell R, Purswell JL (1976) The influence of forearm and wrist orientation on static grip strength as a design criterion for hand tools. In: Proceedings of the human factors and ergonomics society annual meeting, vol 20, No 1, pp 28–32
Thompson AK, Estabrooks KL, Chong S, Stein RB (2008) Spinal reflexes in ankle flexor and extensor muscles after chronic central nervous system lesions and functional electrical stimulation. Neurorehabil Neural Repair 23(2):133–143. https://doi.org/10.1177/1545968308321067
Urbin MA, Harris-love ML, Carter AR, Lang CE (2015) High-intensity, unilateral resistance training of a non-paretic muscle group increases active range of motion in a severely paretic upper extremity muscle group after stroke. Front Neurol. https://doi.org/10.3389/fneur.2015.00119
Veldman MP, I Zijdewind, Solnik S, NA Maffiuletti, KMM Berghuis, M Javet, T Hortobagyi (2015) Direct and crossed effects of somatosensory electrical stimulation on motor learning and neuronal plasticity in humans. Eur J Appl Physiol 115:2505–2519. https://doi.org/10.1007/s00421-015-3248-z
Winstein CJ, Rose DK, Tan SM, Lewthwaite R, Chui HC, Azen SP (2004) A randomized controlled comparison of upper-extremity rehabilitation strategies in acute stroke: a pilot study of immediate and long-term outcomes. Arch Phys Med Rehabil 85:620–628. https://doi.org/10.1016/j.apmr.2003.06.027
Wolf SL, Catlin PA, Ellis M, Archer AL, Morgan B, Piacentino A (2001) Assessing Wolf motor function test as outcome measure for research in patients after stroke. Stroke 32:1635–1640
Yang Y-R, Wang R-Y, Lin K-H, Chu M-Y, Chan R-C (2006) Task-oriented progressive resistance strength training improves muscle strength and functional performance in individuals with stroke. Clin Rehabil 20:860–870
Yue GH, Cole KJ (1992) Strength increases from the motor program: comparison of training with maximal voluntary and imagined muscle contractions. J Neurophysiol 67(5):1114–1123
Zehr EP (2002) Considerations for use of the Hoffmann reflex in exercise studies. Eur J Appl Physiol 86:455–468. https://doi.org/10.1007/s00421-002-0577-5
Zehr EP (2006) Training-induced adaptive plasticity in human somatosensory reflex pathways. J Appl Physiol 101:1783–1794. https://doi.org/10.1152/japplphysiol.00540.2006
Zehr EP (2011) Evidence-based risk assessment and recommendations for physical activity clearance: stroke and spinal cord injury. Appl Physiol Nutr Metab 36:S214-231. https://doi.org/10.1139/h11-060
Zehr EP, Kido A (2001) Neural control of rhythmic, cyclical human arm movement: task dependency, nerve specificity and phase modulation of cutaneous reflexes. J Physiol 537(3):1033–1045. https://doi.org/10.1111/j.1469-7793.2001.01033.x
Zehr EP, Barss TS, Dragert K, Frigon A, Vasudevan EV, Haridas C, Komiyama T (2016) Neuromechanical interactions between the limbs during human locomotion: an evolutionary perspective with translation to rehabilitation. Exp Brain Res 234(11):3059–3081. https://doi.org/10.1007/s00221-016-4715-4
Zhou S (2000) Chronic neural adaptations to unilateral exercise mechanisms of cross education. Exerc Sport Sci Rev 28(4):177–184
Acknowledgements
Dr. E.Paul Zehr’s research was supported by funding from the Heart and Stroke Foundation (British Columbia and Yukon). Yao Sun was supported by a Focus on Stroke doctoral award from the Heart and Stroke Foundation of Canada. Data collection at UBC was funded by an operating grant from the Canadian Institutes of Health Research operating Grant (MOP-106651) awarded to Dr. Lara Boyd. The authors also wish to acknowledge Matt Jensen’s contribution in designing, making and instrumenting the training devices.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Sun, Y., Ledwell, N.M.H., Boyd, L.A. et al. Unilateral wrist extension training after stroke improves strength and neural plasticity in both arms. Exp Brain Res 236, 2009–2021 (2018). https://doi.org/10.1007/s00221-018-5275-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-018-5275-6