Abstract
We investigated modulation of inhibition of motor evoked potentials in the left forearm musculature attributable to changes in corticomotor excitability induced by passive rhythmic movement of the right limb. In the first experiment, eight healthy volunteers pre-activated their left flexor carpi radialis (FCR) in a simple isometric contraction (2.5–7.5% MVC) while their right hand underwent passive wrist flexion-extension. Transcranial magnetic (TMS) or electrical (TES) stimulation was applied to the right motor cortex and responses recorded from the test (left) limb in eight phases of the wrist flexion-extension cycle of the passively driven right limb. In half of the trials TMS conditioning was applied to the left motor cortex. The conditioning stimulus significantly inhibited TMS-evoked responses in the test FCR muscle, whereas TES-evoked responses did not appear to be inhibited. For TMS-evoked responses only, inhibition in the static pre-activated left FCR was modulated such that inhibition was greater when the right wrist was passively flexing than when it was extending. In the second experiment TMS was applied to the right motor cortex, contralateral to the test (left) limb, with the right hand either passively extending or flexing through the neutral position. Conditioning was applied to the left motor cortex at a range of intensities adjusted to threshold for flexion and extension movements. No difference was evident in the maximum magnitude of inhibition between the extension and flexion conditions. We propose there is an increased absolute threshold for recruitment and a decreased gain of inhibitory callosal pathways during extension phases of the wrist flexion-extension cycle.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It has been suggested that a potential role for transcallosal connections between Brodmann’s areas 3 and 4 may be to suppress co-activation of the ipsilateral motor cortex to facilitate bimanual cooperation during motor tasks (Schnitzler et al. 1996; Meyer et al. 1998; Reddy et al. 2000). Transcallosal pathways between homotopic regions of motor cortex have been identified in anatomical studies in monkeys (Jenny 1979; Jones et al. 1979). Although these connections are less abundant than for sensory or association cortices (Reddy et al. 2000), it is well established that the corpus callosum plays a pivotal role in coordinating motor planning and control (Preilowski 1972; Eliassen et al. 1999; Gazzaniga 2000).
The application of transcranial magnetic stimulation (TMS) and transcranial electrical stimulation (TES) has allowed the investigation of transcallosal connections in conscious human subjects. Ferbert et al. (1992) proposed a novel approach to study interhemispheric connections of the motor cortices using two magnetic stimulators. The effect of a conditioning stimulus, delivered over the motor cortex of one hemisphere, on the amplitude of the motor evoked potential (MEP) recorded in a distal hand muscle induced by a test stimulus over the opposite hemisphere was monitored. MEPs induced by the test stimulus were found to be inhibited when the conditioning-test interval was 6–30 ms. Ferbert et al. (1992) suggested that the inhibition was mediated transcallosally between homologous motor areas. This interhemispheric inhibition (IHI) was noted when the test muscle was both active and relaxed. Salerno and Georgesco (1996) and Hanajima et al. (2001) provided further evidence for the presence of IHI in distal hand muscles when two magnetic coils were employed with conditioning-test intervals of 11–15 ms. Gerloff et al. (1998) reported similar inhibition of a TMS-elicited test response in the flexor carpi radialis (FCR).
Recent studies have shown that passive flexion-extension of the wrist leads to marked modulations in corticomotor excitability of the FCR muscle (Carson et al. 2000). There is mounting evidence that at least some of the modulation noted is mediated supraspinally in response to movement-elicited afference (Lewis et al. 2001; Lewis and Byblow 2002). The extent to which this modulation may transfer between hemispheres is the topic of the experiments presented here. During passive movement of one limb, changes in the magnitude of inhibition noted in the contralateral static test limb may reflect differences in the number of recruited inhibitory callosal fibres, or the extent of inhibitory action of a given population of callosal fibres at a given phase of the passively driven hand. Alternatively, the efficacy of inhibition may solely reflect the excitability of the conditioned hemisphere (Ferbert et al. 1992).
In Experiment 1 we examined TMS- and TES-elicited responses delivered over the right motor cortex to isometrically activated left FCR and applied TMS over the left motor cortex at different phases of passive rhythmic flexion-extension movements of the right wrist. We hypothesized that inhibition would reflect callosal as opposed to sub-cortical mechanisms and that the modulation of inhibition would mirror the modulation of excitability in the corticomotor pathway of the passively moved limb. Both hypotheses were supported. In Experiment 2 we examined the magnitude of inhibition during passive hand extension compared with flexion after appropriate adjustments were made for test and conditioning stimulus intensity, relative to threshold in each movement direction. These results indicated that the excitability of the conditioned hemisphere contributed to the modulation of inhibition found in Experiment 1.
Materials and methods
Subjects
Eight subjects (five males and three females) volunteered for Experiment 1 (mean age 26; range 17–42 years). In Experiment 2, eight subjects (six males and two females) volunteered (mean age 29; range 17–53 years). All subjects were right handed and scored more than 50 on the laterality quotient of the Edinburgh Handedness Inventory (Oldfield 1971). Subjects were screened for contraindications to TMS and none had neurological impairments. Informed consent was obtained from all subjects and the experimental procedures were approved by the University of Auckland Human Subjects Ethics Committee in accordance with the Declaration of Helsinki.
Experimental apparatus
Subjects were seated in front of a manipulandum that generated passive flexion-extension movements of the right wrist joint (Lewis et al. 2001). Subjects sat comfortably with their right hand in the manipulandum and their left hand in a constrained hand-piece. The hands (thumbs uppermost) and forearms were supported and restrained with the wrist in a neutral posture (0°) and the elbows in a semi-flexed position.
Electromyographic activity of the left and right FCR muscles was recorded using 10-mm diameter Hydrospot Ag-AgCl electrodes (Physiometrix, USA). Standard techniques of skin preparation were employed and then electrodes were placed 2 cm apart on the belly of the FCR muscle. Triggering of EMG collection was initiated 30 ms prior to stimulus onset and, for each stimulus, 100 ms of data were collected. EMG signals were amplified (Grass Instruments P511) and bandpass filtered (30–1000 Hz). Signals were sampled at 4 kHz (Experiment 1) and 10 kHz (Experiment 2) using a MacLab A/D (ADInstruments, Castle Hill, NSW) acquisition system and stored to disc for further analysis.
Transcranial magnetic stimulation
Motor evoked potentials in the left and right FCR muscles were elicited by TMS to the right and left motor cortices, respectively. Two Magstim 200 stimulators (Magstim, Whitland, Dyfed) with BiStim unit and a figure-of-eight coil (70 mm each) were used to deliver magnetic stimuli.
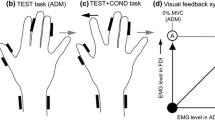
The subjects wore a tightly fitting cotton cap, with pre-marked grid locations, that was securely fastened to the head by Velcro straps. A neck support attached to a freestanding weight was used to maintain head position throughout testing. To determine the optimal site of stimulation over both the left and right hemispheres, the coil was systematically moved around the grid locations, with stimuli delivered at each until the sites eliciting the averaged MEP of the largest amplitude in the left (lFCR) and right (rFCR) FCR were located. These sites were defined as the ‘hotspots’. At each site the coil was positioned approximately perpendicular to the central sulcus at an angle to the midline and tangential to the scalp. All further testing was carried out with the test coil fixed in position over the right hemisphere by a freestanding device whilst the conditioning coil was held in position over the left hemisphere hotspot by the experimenter when required (see Fig. 1). The location of the test coil in relation to the right hemisphere hotspot was checked repeatedly throughout testing to ensure that the site and angle of stimulation remained constant.
A Schematic top view of the experimental set-up. Left FCR pre-activated at 5% MVC. Magnetic conditioning stimulus was applied to the left hemisphere 12 ms prior to the test stimulus applied over the right hemisphere. B Displacement of passive wrist (positive = flexion) and point of stimulation (▼) for the eight cycle phases
Experimental protocol
Experiment 1
The rest threshold (RTh) for the rFCR was determined as the minimum intensity at which no more than four of eight stimuli yielded a response of 50 μV. The conditioning-test interval for conditioned TMS was set at 12 ms for all subjects. Test stimulus intensity was adjusted to elicit ≈300 μV MEP amplitude in the pre-activated lFCR with the right hand static. It was verified that responses to TMS in lFCR were reduced when a conditioning pulse was applied to the left hemisphere. Initially the magnitude of inhibition was investigated in a static lFCR at rest (Fig. 2A). As can be seen, no increase in the magnitude of inhibition was noted when employing a conditioning stimulus beyond 120% RTh (CS120) and this intensity was adopted for the duration of the experiment. In conditioned trials the conditioning TMS pulse also served to elicit responses in rFCR.
Non-conditioned and conditioned responses were collected from pre-activated static lFCR while the right hand was passively moved by the manipulandum. Left FCR was maintained in a mild contraction between 2.5 and 7.5% of maximum isometric contraction in the presence of high-gain visual feedback to assist task performance. Subjects were instructed to maintain EMG silence in rFCR during all trials. During passive movement (0.6 Hz, amplitude 90°), stimuli were delivered in eight temporally equal phases of the right hand movement cycle corresponding to wrist joint angles of +43o, +18o, −18o, and −43o (Lewis et al. 2001). Thus, four flexing phases and four extending phases were examined. Responses were also collected with the lFCR pre-activated and the right hand at rest in a neutral posture.
In two subjects the effects of a magnetic conditioning stimulus on responses evoked by TES were examined. The electrical stimuli were delivered using a Digitimer D185 stimulator (Digitimer, Welwyn Garden City, Hertfordshire, UK). The cathode was fixed at the vertex and the anode fixed over the lFCR hotspot as determined by magnetic stimulation. TES intensities were chosen such that the amplitude of the lFCR responses to the test stimuli were matched to those elicited by magnetic stimulation.
In each 60-s trial, ten stimuli were delivered in a pseudo-random order, one in each of the cycle phases, plus one prior to and one following passive movement. Twelve trials were completed for both the non-conditioned and conditioned elements. In total, 24 passive movement trials were completed. For one subject, with TES, only six trials per condition were collected due to a lack of tolerance for the procedure.
Experiment 2
As in Experiment 1, subjects performed 5% MVC isometric wrist flexor contraction with the left hand, the conditioning-test interval was set at 12 ms, and passive movement of the right limb was performed as previous. Test stimulus intensity was adjusted to elicit a MEP amplitude of ≈400 μV in the pre-activated lFCR for each of the passive movement conditions of the right hand. Now, however, threshold was determined for the rFCR as the right hand passively extended or flexed through the neutral position (0°). The conditioning stimulus intensity was set to 100, 110, 120, 130, and 140% of the respective threshold determined for each movement direction. Conditioning stimulus intensities were pseudo-randomised within a right hand movement condition and presentation of movement conditions was randomised between subjects.
Data processing and analysis
Data were processed and analysed using custom-built routines housed on a SunSparc 5 workstation. The root mean square (r.m.s.) amplitude of EMG activity in rFCR 10 ms prior to the onset of the conditioning stimulus was determined and responses were removed from further analysis if r.m.s. amplitudes were greater than twice that obtained during static trials collected prior to passive movement of the right hand. Additionally, responses were removed from subsequent analysis if the r.m.s. amplitude of EMG activity in lFCR 10 ms prior to the onset of the conditioning stimulus was not within 2.5–7.5% MVC. The peak-to-peak MEP amplitudes of the remaining responses in each of the cycle phase and stimulus conditions were then averaged.
Statistical analysis
An α-level of 0.05 was adopted for establishing significance. A Huynh-Feldt correction factor was used for the RM-ANOVAs.
Experiment 1
For all analyses, data were pooled over the four flexing and the four extending phases and a planned contrast was made between them. A one-way repeated measures (RM) ANOVA was conducted on rFCR MEP amplitudes obtained during the conditioned passive movement trials. A two-way RM-ANOVA (stimulus condition × cycle phase) was used to confirm standardisation of the background EMG in the lFCR that preceded non-conditioned and conditioned responses collected during the eight cycle phases. Planned contrasts were again made as above. To investigate inhibition, a two-way RM-ANOVA (stimulus condition × cycle phase) was used to compare lFCR MEP amplitudes. To investigate modulation of the magnitude of inhibition in the lFCR a one-way RM-ANOVA was used to analyse the normalised responses as a percentage of non-conditioned responses (%NC).
Experiment 2
A paired t-test was used to determine if there was a difference between the flexion and extension threshold stimulus intensities. Response magnitude of rFCR was plotted against stimulus intensity normalised to rFCR threshold during flexion and extension, respectively. The data were fitted with the following three-parameter sigmoid function relating MEP amplitude to stimulus intensity:
where MEPmax is the maximum MEP defined by the function; m is the slope parameter of the function; s is stimulus intensity; and S50 is the stimulus intensity at which the MEP size is 50% of the maximal MEP amplitude (Carroll et al. 2001). An F-test was used to determine whether fitting a curve to data from each passive movement condition significantly improved the total variance accounted for compared with fitting a single curve to data from the extension and flexion passive movement conditions combined (Devanne et al. 2001).
A two-way RM-ANOVA (movement condition × stimulus intensity) was conducted on rFCR MEP amplitudes. Planned contrasts were made between the extension and flexion conditions at each of the stimulus intensities. A two-way RM-ANOVA (movement condition × stimulus intensity) was used to confirm standardisation of background EMG in the lFCR. In order to determine if test MEP amplitudes were matched between the extension and flexion movement conditions, a t-test was conducted on lFCR non-conditioned MEP amplitudes. To investigate the inhibitory effect in lFCR, conditioned MEP amplitudes (%NC) were plotted against conditioning stimulus intensity (normalised to rFCR threshold) for the extension and flexion conditions. Data were fitted with the following adjusted three-parameter sigmoid function:
where MEP amplitude is conditioned MEP amplitude (%NC); MEPmin is the minimum MEP defined by the function; m is the slope parameter of the function; cs is the conditioning stimulus intensity; and S50 is the stimulus intensity at which the MEP amplitude is halfway between unity and MEPmin. Equation 2 is a variation of Eq. 1 in order to describe the inhibitory effect of a magnetic conditioning stimulus applied to the contralateral hemisphere. Thus, the slope parameter is negative. Additionally, as the equation describes the inhibitory effect of the conditioning stimulus, it is assumed that the maximum normalised MEP amplitude (conditioned/non-conditioned) will be unity. An F-test was used to determine if differences existed between extension and flexion conditions. A two-way RM-ANOVA (movement condition × conditioning intensity) was used to analyse the MEP amplitudes of lFCR. Finally, the conditioning stimulus intensities during flexion and extension, respectively, which approximated 120% rFCR rest threshold, were identified and analysed using a one-way RM-ANOVA in order to investigate the magnitude of inhibition in lFCR MEPs at conditioning stimulus intensities comparable to those in Experiment 1.
Results
Experiment 1
Figure 2A depicts typical EMG traces collected from lFCR reflecting recruitment of inhibition as the result of a conditioning stimulus applied to the left hemisphere in one subject with both limbs at rest. Inhibition reached maximum in the lFCR by CS120 confirming the protocol in the forearm, as well as the adequacy of conditioning and test stimulus intensity ranges used in the present experiment (based on previous studies using intrinsic hand muscles). Table 1 reports the group means of left and right FCR MEP amplitudes for each of the eight phases of passive movement. Static values are also reported.
Representative EMG traces obtained from the left FCR of one subject with both hands at rest. A EMG traces in left FCR following a test magnetic pulse administered to the right hemisphere only (NC), and conditioning of the test pulse applied to the left hemisphere at 100% (CS100), 120% (CS120), and 130% (CS130) of rest threshold. Conditioning-test interval = 12 ms. B EMG traces in left FCR following a test magnetic pulse applied to the right hemisphere only during the F+43 cycle phase of the right hand (FlexNC), transcranial magnetic conditioning of the test pulse administered to the left hemisphere during the F+43 cycle phase of the right hand (FlexConTMS), a test magnetic pulse applied to the right hemisphere only during the E+43 cycle phase of the right hand (ExtNC), and transcranial magnetic conditioning of the test pulse administered to the left hemisphere during the E+43 cycle phase of the right hand (ExtConTMS). Left FCR pre-activated at 5% MVC. Conditioning-test interval = 12 ms. C EMG traces in left FCR following an electric test stimulus applied to the right hemisphere only during the F+43 cycle phase of the right hand (FlexNC), magnetic conditioning of the test pulse administered to the left hemisphere during the F+43 cycle phase of the right hand (FlexConTES), an electric stimulus applied to the right hemisphere only during the E+43 cycle phase of the right hand (ExtNC), and magnetic conditioning of the test pulse administered to the left hemisphere during the E+43 cycle phase of the right hand (ExtConTES). Left FCR pre-activated at 5% MVC. Conditioning-test interval = 12 ms
Right limb: MEP amplitude from TMS
During passive movement one subject had inappropriate pre-trigger EMG across all phases and was not included in the analysis. Right FCR MEP amplitudes (normalised to MEPmax) are depicted in Fig. 3A. A main effect of phase was evident for the group (F (7,42)= 4.119, P<0.01). The planned comparison revealed that the four extending phases (phases E+43 to E−43) had a significantly lower MEP amplitude than the four flexing phases (phases F−43 to F+43) (P<0.01).
Experiment 1. A TMS-elicited MEP amplitude of right FCR obtained during passive movement of the right hand at 0.6 Hz. Static values are also shown. Group mean with MEP normalised to individual subject’s maximum MEP and left hand pre-activated. Bars represent one S.E.M. **p<0.01 B Left conditioned FCR MEP amplitude (normalised to non-conditioned) during pre-activation with passive movement of the right hand at 0.6 Hz. Group averages elicited with TMS as the test pulse over the right hemisphere. Bars represent one S.E.M. Conditioning-test interval = 12 ms. *p<0.05
Left limb: MEP amplitude from TMS
There was no statistically significant difference between the r.m.s. amplitudes of EMG activity in the period prior to the stimulus for non-conditioned and conditioned trials (F (1,6)<1, P=0.703), and no interaction between stimulus condition and phase (F (7,42)<1, P=0.972). While an effect of phase was evident (F (7,42)=3.882, P<0.01), the planned contrast revealed that r.m.s. amplitude of pre-stimulus EMG activity did not differ significantly between the extending phases and the flexing phases (P=0.083).
Figure 2B depicts representative EMG traces obtained from the lFCR of one subject at selected flexion-extension cycle phases with TMS as the test pulse. Note the suppression of MEP amplitude in the ConTMS traces compared with the NC traces. Left FCR MEP amplitude in the conditioned trials was significantly lower than amplitudes in the non-conditioned trials (F (1,6)=18.241, P<0.01). An interaction between conditioning and phase approached significance (F (7,42)=1.937, P=0.088) and the planned contrast revealed test responses during extension were significantly lower than during flexion (P<0.01). Conditioned MEP amplitudes (%NC) from TMS are presented in Fig. 3B. A main effect of phase was not evident (F (7,42)=1.677, P=0.141), but planned contrasts revealed that inhibition was significantly greater in flexing phases than in extending phases (P<0.01).
Left limb: MEP amplitude from TES
Figure 2C depicts representative EMG traces obtained from the lFCR of one subject at selected flexion-extension cycle phases of the passively driven right hand using TES as the test pulse. Note the lack of suppression of MEP amplitude in the ConTES traces compared with the NC traces, which contrasts with the TMS-elicited NC traces depicted in Fig. 2B. The second subject tested with TES revealed the same pattern of results (lack of MEP suppression).
Experiment 2
Right limb: threshold, S-R curve and MEP amplitude
Group means of rFCR threshold stimulus intensity (% maximum stimulator output; MSO) were 38±7% for flexion and 51±11% for extension (P<0.01). Best-fit parameters and r2 values estimated for the passive extension and flexion conditions of the right hand, and for the extension and flexion data sets combined, are presented in Table 2. Fitting a curve to data from the extension and flexion conditions individually significantly improved the total variance accounted for compared with fitting a single curve to data from the two passive movement conditions combined (F (1,76)=11.246, P<0.01).
Group averaged rFCR MEP amplitude is depicted in Fig. 4A. Main effects of movement condition (F (1,7)=7.177, P<0.05) and stimulus intensity (F (4,28)=9.341, P<0.01), and an interaction between movement condition and stimulus intensity were present (F (4,28)=5.092, P<0.01). Specifically, MEP amplitude was significantly lower during extension than during flexion at stimulus intensities of 130% (P<0.01) and 140% (P<0.01) of adjusted rFCR motor threshold.
Experiment 2 group data. Pre-activated left FCR maintained isometric contraction at 2.5 – 7.5% of MVC. Stimulus intensity expressed as a percentage of right FCR corticomotor threshold for the given movement condition. A Right FCR non-conditioned MEP amplitude for the two passive movement conditions of the right hand—extension through neutral and flexion through neutral. Estimated stimulus-response curves; solid line (extension), dotted line (flexion). Bars represent one S.E.M. **p<0.01 contrast between extension and flexion group mean values. B Left FCR conditioned MEP amplitude normalised to non-conditioned MEP amplitude for the extension and flexion movement conditions of the right hand. Estimated stimulus-response curves; solid line (extension), dotted line (flexion). Bars represent one S.E.M. *p<0.05 contrast between extension and flexion group mean values
Left limb: S-R curves and MEP amplitude
Best-fit parameters and r2 values are presented in Table 3. Fitting a curve to data from the extension and flexion passive movement conditions individually significantly improved the total variance accounted for, compared with fitting a single curve to data from the extension and flexion passive movement conditions combined (F (1,76)=6.478, P<0.01).
Analysis of r.m.s. amplitude of EMG activity in the period prior to the stimulus revealed no effect of movement condition (F (1,7)<1, P=0.592), no effect of stimulus intensity (F (5,35)<1, P=0.564), and no interaction between movement condition and stimulus intensity (F (5,35)=1.275, P=0.306). Group means of lFCR non-conditioned MEP amplitude did not differ significantly (P=0.076) between extension (0.543 ±0.250 mV) and flexion (0.410±0.158 mV).
A main effect of conditioning stimulus intensity was evident for non-normalised lFCR MEP amplitudes in both extension (F (5,35)=6.859, P<0.01) and flexion (F (5,35)=3.673, P<0.05). MEP amplitude in the extension conditioned trials was significantly inhibited below the non-conditioned values at conditioning stimulus intensities of 110% (P<0.01), 120% (P<0.01), 130% (P<0.01), and 140% (P<0.01) of rFCR corticomotor threshold during passive wrist extension. MEP amplitude in the flexion conditioned trials was significantly inhibited below the non-conditioned values at conditioning stimulus intensities of 130% (P<0.05) and 140% (P<0.05) of rFCR corticomotor threshold during passive wrist flexion.
Conditioned lFCR MEP amplitudes (%NC) are presented in Fig. 4B. A main effect of movement condition was evident (F (1,7)=6.822, P<0.05). No interaction was found between movement condition and conditioning stimulus intensity (F (4,28)<1). Planned contrasts revealed significantly lower normalised MEP amplitudes in the extension compared with the flexion passive movement condition at conditioning stimulus intensities of 100% (P<0.05), 110% (P<0.05), 120% (P<0.05), and 130% (P<0.05), but not at 140% (P>0.1) of rFCR motor threshold.
Static rFCR threshold was found to be 42% MSO when group mean data were considered. Therefore, 120% static rFCR threshold was determined to be 50% MSO. In the extension passive movement condition, the group mean extension threshold conditioning stimulus intensity chosen for comparison was 51% MSO (100% rFCR extension threshold). In the flexion passive movement condition, the group mean flexion threshold conditioning stimulus intensity chosen for comparison was 49% MSO (130% rFCR flexion threshold). No significant difference was found between these means (F (1,7)<1).
Discussion
Interhemispheric inhibition during passive contralateral movement
Experiment 1 served to further confirm the findings of Gerloff et al. (1998) who reported conditioning-induced inhibition in the FCR muscle when suprathreshold TMS was applied over the hemisphere opposite that to which the test stimulus was applied (Figs. 2A, B and 3B). Previous dual-coil TMS studies of this nature have investigated the inhibitory phenomenon in distal hand muscles (Ferbert et al. 1992; Ugawa et al. 1993; Meyer et al. 1995; Salerno and Georgesco 1996; Schnitzler et al. 1996; Di Lazzaro et al. 1999; Hanajima et al. 2001) and attribute the effect to IHI mediated by callosal pathways.
In order to provide further evidence that inhibition in the lFCR originated at the cortical level, the effect of a magnetic conditioning stimulus on an electrical test stimulus was investigated in two subjects. Whereas TMS activates corticospinal neurons transynaptically, anodal TES predominantly activates pyramidal axons directly (Day et al. 1989; Chen 2000). From inspection of Fig. 2C, it appears that TES responses were not inhibited although TMS-evoked responses were inhibited (Figs. 2B and 3B). These findings are in agreement with the findings of Ferbert et al. (1992) who found no significant inhibition of a response to an anodal electric stimulus when preceded by a magnetic conditioning stimulus applied to the opposite hemisphere. Thus, in support of previous studies, it seems likely that the inhibition arising from the conditioning stimulus occurred predominantly at the cortical level. Such inhibition is presumed to arise between homotopic representations of FCR via the corpus callosum, as suggested by previous authors (Meyer and Roricht 1996).
Non-conditioned FCR MEP amplitude decreased in the pre-activated target FCR during passive wrist extension of the contralateral limb compared with passive wrist flexion and static values. It is unlikely that voluntary drive was modulated since r.m.s. EMG in the period prior to the stimulus was not significantly different between extending and flexing phases. Thus, membrane potential, and consequently the postsynaptic state, was constant. As a result, the modulation of non-conditioned responses in lFCR can be inferred to have arisen due to changes in presynaptic mechanisms (Stein 1995). The larger non-conditioned MEP amplitude in the lFCR during flexion of the right hand suggests a relatively greater facilitatory effect of the contralateral hemisphere on the corticospinal neurons associated with the muscle of interest during that movement condition as compared with during extension. Furthermore, there was evidence of modulation of (normalised) conditioned responses obtained from the lFCR when evoked by TMS. It is our contention that this finding provides further evidence for a cortical contribution to the changes in excitability of the hemisphere contralateral to a passively driven limb (e.g. Lewis et al. 2001; Lewis and Byblow 2002). The larger non-conditioned MEP amplitudes yet greater inhibitory effect of the conditioning stimulus during flexion phases of the right hand, as compared with extension, may suggest an inhibition of facilitation during flexion. That is, a greater callosally-mediated inhibitory effect may be necessary in order to suppress inappropriate activation of the contralateral homologous musculature during flexion.
The decreased inhibitory effect of the conditioning stimulus during extension is presumably a result of reduced callosally mediated inhibitory effect as suggested by the lack of inhibition of TES-evoked conditioned test responses. There are two mechanisms through which a decrease in callosally mediated inhibition could occur. Firstly, the modulation of inhibition noted in the test limb may be the result of varying temporal summation altering the efficacy of the inhibitory action of a given population of inhibitory callosal fibres connecting homotopic muscle representations of the involved musculature. Thus, the number of inhibitory callosal fibres recruited by a given conditioning stimulus would not change during passive movement but rather the effective action of the population would change. Alternatively, the modulation of inhibition in the test limb may reflect a change in spatial summation, or the number of inhibitory callosal fibres recruited at a given cycle phase of the passively driven hand. This may occur as a result of changes in cortical excitability in the left (conditioned) hemisphere as the result of passive movement of the right limb, i.e. the rFCR representation may be more excitable during passive flexion than during passive extension (Lewis et al. 2001). Therefore, in Experiment 1 the changes in inhibition noted in the lFCR between extension and flexion phases of the contralateral passive limb may reflect changes in the relative strength of the conditioning stimulus, thereby recruiting disparate numbers of inhibitory callosal fibres. To differentiate between these possibilities, Experiment 2 examined the magnitude of inhibition as the passively driven hand flexed versus extended through the same neutral position. Since test stimulus intensity affects the amount of inhibition induced by a magnetic stimulus of constant intensity applied to the opposite hemisphere (Ferbert et al. 1992), response amplitude was matched between extension and flexion passive movement conditions of the right hand. From Fig. 4, the absolute conditioning stimulus intensity appears to be the major contributor in determining the magnitude of inhibition. There was no difference in inhibition between the extension and flexion conditions when considering absolute conditioning stimulus intensities comparable to those employed in Experiment 1. The conditioning stimulus intensity utilised in Experiment 1 was likely to have elicited lFCR responses from the rapidly changing portion of the stimulus-response profiles for lFCR (see Fig. 4B). This finding may have been the basis for the comparatively less inhibition in lFCR during extension phases compared with flexion phases, i.e. the conditioning stimulus intensity was at a level sufficient to elicit maximum inhibition during flexion but not extension.
Modulation of interhemispheric inhibition
The magnitude of inhibition when the inhibitory effect is maximal for a given passive movement condition could be considered a measure of the efficacy of the inhibitory action of callosal fibres connecting homotopic muscle representations of the target musculature. In Experiment 2 a greater magnitude of inhibition was noted in extension compared with flexion phases of passive movement at conditioning stimulus intensities close to rFCR threshold for extension or flexion, respectively. This difference was abolished when conditioning stimulus intensity was 140% of rFCR threshold, suggesting that the maximum amount of inhibition able to be elicited is comparable between the extension and flexion conditions. Additionally, the estimated MEPmin parameter obtained from Eq. 2 (see Table 3) did not differ between the extension and flexion conditions. Thus, it is unlikely that differences in the magnitude of inhibition in lFCR between passive extension and flexion noted in Experiment 1 were due to a modulation of the efficacy of the inhibitory action of callosal fibres.
In Experiment 2 the onset of inhibition in lFCR occurred earlier in the passive extension compared with passive flexion when conditioning stimulus intensity was expressed as a percentage of rFCR threshold. The absolute conditioning stimulus intensity at which inhibition was first noted was greater during extension than during flexion. Therefore, the absolute threshold for the onset of inhibition was greater during extension than flexion. Additionally, the estimated slope parameter was shallower in the extension condition (see Table 3). This effect probably reflects the gain of the inhibitory callosal pathway which is dictated by the respective corticomotoneuronal networks of the flexor muscle in the conditioned hemisphere (Devanne et al. 1997).
Conclusion
It has been suggested that the regulation of IHI might serve to prevent the advent of mirror movements in intact individuals. In adults with callosal abnormalities, and who express mirror movements, there is an indication of ineffective IHI (Rothwell et al. 1991). During passive wrist flexion, a marked disinhibition of the passively driven FCR muscle representation may serve to increase the IHI effect passing transcallosally to the homologous muscle representation from tonic levels. This mechanism may act to suppress co-activation of the homologous muscle representation in the contralateral motor cortex when the limbs are moved independently.
References
Carroll TJ, Riek S, Carson RG (2001) Reliability of the input-output properties of the cortico-spinal pathway obtained from transcranial magnetic and electrical stimulation. J Neurosci Methods 112:193–202
Carson RG, Byblow WD, Riek S, Lewis GN, Stinear JW (2000) Passive movement alters the transmission of corticospinal input to upper limb motoneurones. Abstracts for 30th Annual Meeting of Society for Neuroscience 1231
Chen R (2000) Studies of human motor physiology with transcranial magnetic stimulation. Muscle Nerve [Supp 9]: S26–S32
Day BL, Dressler D, Maertens De Noordhout A, Marsden CD, Nakashima K, Rothwell JC, Thompson PD (1989) Electric and magnetic stimulation of human motor cortex: surface EMG and single motor unit responses. J Physiol 412:449–473
Devanne H, Lavoie BA, Capaday C (1997) Input-output properties and gain changes in the human corticospinal pathway. Exp Brain Res 114:329–338
Devanne H, Cohen L, Kouchtir-Devanne N, Capaday C (2001) Integrated motor cortical control of task-related muscles during pointing in humans. J Neurophysiol 87:3006–3017
Di Lazzaro V, Oliviero A, Profice P, Insola A, Mazzone P, Tonali P, Rothwell JC (1999) Direct demonstration of interhemispheric inhibition of the human motor cortex produced by transcranial magnetic stimulation. Exp Brain Res 124:520–524
Eliassen JC, Baynes K, Gazzaniga MS (1999) Direction information coordinated via the posterior third of the corpus callosum during bimanual movements. Exp Brain Res 128:573–577
Ferbert A, Priori A, Rothwell JC, Day BL, Colebatch JG, Marsden CD (1992) Interhemispheric inhibition of the human motor cortex. J Physiol 453:525–546
Gazzaniga MS (2000) Cerebral specialization and interhemispheric communication: does the corpus callosum enable the human condition? Brain 123:1293–1326
Gerloff C, Cohen LG, Floeter MK, Chen R, Corwell B, Hallett M (1998) Inhibitory influence of the ipsilateral motor cortex on responses to stimulation of the human cortex and pyramidal tract. J Physiol 510:249–259
Hanajima R, Ugawa Y, Machii K, Mochizuki H, Terao Y, Enomoto H, Furubayashi T, Shiio Y, Uesugi H, Kanazawa I (2001) Interhemispheric facilitation of the hand motor area in humans. J Physiol 531:849–859
Jenny AB (1979) Commisural projections of the cortical hand motor area in monkeys. J Comp Neurol 188:137–146
Jones EG, Coulter JD, Wise SP (1979) Commisural columns in the sensory-motor cortex of monkeys. J Comp Neurol 188:113–136
Lewis GN, Byblow WD (2002) Modulations in corticomotor excitability during passive limb movement. Is there a cortical influence? Brain Res 943:263–275
Lewis GN, Byblow WD, Carson RG (2001) Phasic modulation of corticomotor excitability during passive movement of the upper limb: effects of movement frequency and muscle specificity. Brain Res 900:282–294
Meyer BU, Roricht S (1996) Callosally and corticospinally mediated motor responses induced by transcranial magnetic stimulation in man originate from the same motor cortex region. J Physiol 491P:119
Meyer BU, Roricht S, Grafin von Einsiedel H, Kruggel F, Weindl A (1995) Inhibitory and excitatory interhemispheric transfers between motor cortical areas in normal humans and patients with abnormalities of the corpus callosum. Brain 118:429–440
Meyer BU, Roricht S, Woiciechowsky C (1998) Topography of fibers in the human corpus callosum mediating interhemispheric inhibition between the motor cortices. Ann Neurol 43:360–369
Oldfield RC (1971) The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9:97–113
Preilowski B (1972) Possible contribution of the anterior forebrain commisures to bilateral motor coordination. Neuropsychologia 10:267–277
Reddy H, Lassonde M, Bemasconi A, Matthews PM, Andermann F, Amold DL (2000) An fMRI study of the lateralization of motor cortex activation in acallosal patients. Neuroreport 11:2409–2413
Rothwell JC, Colebatch J, Britton TC, Priori A, Thompson PD, Day BL, Marsden CD (1991) Physiological studies in a patient with mirror movements and agenesis of the corpus callosum. J Physiol 438:34P
Salerno A, Georgesco M (1996) Interhemispheric facilitation and inhibition studied in man with double magnetic stimulation. Electroencephalograph Clin Neurophysiol 101:395–403
Schnitzler A, Kessler KR, Benecke R (1996) Transcallosally mediated inhibition of interneurons within human primary motor cortex. Exp Brain Res 112:381–391
Stein RB (1995) Presynaptic inhibition in humans. Prog Neurobiol 47:533–544
Ugawa Y, Hanajima R, Kanazawa I (1993) Interhemispheric facilitation of the hand area of the human motor cortex. Neurosci Lett 160:153–155
Acknowledgements
The authors would like to thank Gwyn Lewis, Steve McMillan, Vanessa Lim, and Melody Polych for assistance with data collection, and Richard Carson, Gwyn Lewis and Jim Stinear, and two anonymous reviewers for helpful comments on this manuscript. S.W. was supported by a Faculty of Science Study Award, University of Auckland and a Sport and Exercise Science Departmental Scholarship, University of Auckland. This research was supported by a Staff Research Grant to W.B.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Warbrooke, S.A., Byblow, W.D. Modulation of interhemispheric inhibition during passive movement of the upper limb reflects changes in motor cortical excitability. Exp Brain Res 156, 11–19 (2004). https://doi.org/10.1007/s00221-003-1760-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-003-1760-6