Abstract
Purpose
To measure the 24-h intraocular pressure (IOP) by Icare PRO rebound in healthy and primary open-angle glaucoma (POAG) eyes and compare it with non-contact tonometry (NCT).
Methods
Thirty POAG patients, who were under IOP-lowering treatment, and 30 healthy subjects were included. Participants were hospitalized overnight for the 24-h IOP measurement. IOPs were measured by Icare PRO and NCT according to a standard protocol every 2 h during 24 h. The 24-h IOP curve and IOP-related parameters were compared between Icare PRO and NCT groups in POAG and healthy eyes.
Results
The IOPs measured by Icare PRO in habitual position increased notably at 22:00 in the normal group and at 20:00 in the POAG group, reached peak at 0:00, stayed high until 4:00, and then decreased in both groups (all p < 0.05). The POAG patients had higher mean 24-h IOP, peak IOP, IOP fluctuation, and greater IOP change from supine to sitting position in the nocturnal period than those in the normal subjects even after adjusting for eyes, age, gender, CCT, and axial length (all p < 0.05).
Conclusions
The Icare PRO provides a well-tolerated approach for 24-h IOP monitoring in habitual position. Twenty-four-hour IOP in habitual position is more sensitive for detecting high nocturnal IOP peaks and greater IOP fluctuation for POAG patients.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.

Introduction
Elevated intraocular pressure (IOP) is a major risk factor for progression of primary open-angle glaucoma (POAG), and IOP reduction is the only evidenced-based therapy to control the progression of glaucomatous optic neuropathy [1]. However, it is not rare that the visual field still progresses despite well-controlled IOP [2]. Glaucoma patients usually have their IOP monitored with single time measurement during office hours. However, nearly two-thirds of POAG patients experience high IOPs outside regular clinic hours [3], with peak IOPs most frequently occurring at night detected by 24-h IOP measurement [4, 5]. Therefore, the 24-h IOP measurement can provide a complete picture of an individual’s IOPs during a whole day, which is important for diagnosis and management of POAG.
Various devices are used for 24-h IOP measurement, such as Goldmann applanation tonometry (GAT), non-contact tonometry (NCT), and contact lens sensor (CLS). Although GAT is the gold standard for IOP measurement, multiple topical anesthesia and repeated applanation are needed for the procedure, which could potentially cause corneal abrasion. NCT is also a commonly used technique for 24-h IOP measurement [6,7,8]. However, it is taken in sitting position as well and fails to reflect the IOPs in natural body position over a 24-h period. The Triggerfish® (Sensimed AG, Lausanne, Switzerland) telemetric CLS is a technology for the continuous recording of IOP-related pattern with minimal disturbance to the wearer’s daily routine and sleep cycle, while the direct association between CLS output and IOP has not been established [9, 10]. Recently, a new developed Icare PRO rebound tonometry has been available for clinical practice [11]. It can be used without topical anesthesia and is particularly useful for measuring IOP in children [12]. Additionally, the Icare PRO includes a built-in inclination sensor that permits IOP to be measured on the subjects in supine position [13]. Previous research showed that Icare PRO offers excellent intradevice repeatability and inter-device reproducibility in the measurement of IOP [11, 13]. Therefore, the Icare PRO is potentially a good approach to measure IOPs over 24 h in habitual position, which could reflect IOPs in natural position better.
The present study aimed to measure the 24-h intraocular pressure by Icare PRO rebound and compare it with NCT in healthy and POAG eyes to identity the circadian IOP fluctuation pattern.
Methods
Participants
This study was approved by the Ethical Review Committee of Shanghai Jing’an District Bei Zhan Hospital (Number: 20170607003) and it adhered to the Declaration of Helsinki for Research Involving Human Participants. Written informed consent was obtained from each participant enrolled in the study.
The diagnostic criteria for POAG included (1) presence of glaucomatous optic disc damage and retinal nerve fiber layer (RNFL) thinning; (2) visual field defects confirmed on at least two visual field examinations; (3) open anterior chamber angles under gonioscopic examination; and (4) no history of ocular or systemic diseases causing optic nerve atrophy. All patients were medically treated with prostaglandin analogues (PGA).
The inclusion criteria for the normal subjects were as follows: open anterior chamber angle, normal-appearing optic nerve head, intact retinal nerve fiber layer, normal standard automated perimetry, and no records of elevated IOP greater than 21 mmHg.
Participants who had (1) incisional surgeries in either eye, including cataract surgeries, glaucoma surgeries, vitreoretinal surgeries, et al., (2) histories of eye diseases, such as uveitis, keratitis, and scleritis, (3) histories of trauma, or (4) refractive error greater than 6.0 diopter or lower than −6.0 diopter were excluded.
Participants underwent a complete ophthalmic examination, including a review of their medical histories, measurements of best-corrected visual acuity (BCVA), refractive status, slit-lamp biomicroscopy, IOP (Goldmann applanation tonometry), fundoscopy (Digital Retinal Camera CR-2 AF, Canon, Japan), gonioscopy, central corneal thickness (CCT) and axial length (Lenstar 900, HAAG-STREIT, AG, Swiss), retinal nerve fiber layer and ganglion cell complex thickness (RTvue OCT; Optovue Inc., Toledo, OH), and visual field (Octopus perimeter 101, HAAG-STREIT, AG, Swiss).
24-h IOP measurement by Icare PRO and NCT
Subjects were hospitalized to undergo 24-h IOP monitoring with an Icare PRO (iCare Finland Oy, Vantaa, Finland) and a non-contact tonometer (Full Auto Tonometer TX-F, Canon, Japan). All the IOP measurements were taken by the same well-trained operator (Dr. Zhaobin Fang). The IOPs of both eyes were measured every 2 h from 8:00 AM to 6:00 AM of the next day, specifically, at 8:00, 10:00, 12:00, 14:00, 16:00, 18:00, 20:00, and 22:00 (diurnal period IOP), and at 00:00, 2:00, 4:00, and 6:00 (nocturnal period IOP). Each subject was firstly measured IOP by Icare PRO followed by NCT from 6:00 to 22:00. During 0:00 to 4:00 time points, the IOPs were firstly measured in supine position by Icare PRO, then in sitting position by Icare PRO and NCT. For each time point, six consecutive measurements were performed for Icare PRO. The built-in software automatically discarded the highest and the lowest values, and the average IOP was calculated from the remaining four values. Three repeated measurements of NCT were taken at each time point and the average value was recorded. Measurements of different devices were separated by a 5-min interval.
Twenty-four-hour IOP curves were generated by using the IOP values at each time point. Mean IOP was calculated for the entire day, and separately for the diurnal period and the nocturnal period. Peak IOP and trough IOP were noted as the highest and lowest values among the 12 IOP values of the 24-h IOP records. IOP fluctuation was calculated by subtracting the trough IOP from the peak IOP.
Statistical analysis
All analyses were performed using SAS version 9.4 for Windows (SAS Institute) and R 3.4.3 (http://www.R-project.org). Numerical variables were shown as mean ± standard deviation. Reliability between Icare PRO and NCT was explored by intraclass correlation coefficient (ICC) and Bland-Altman plot. The differences between groups were analyzed by using mixed effect linear models for repeated measurements of two eyes and adjusted for age, sex, CCT, and AL. The exchangeable covariance structure was used to model the correlation of responses of two eyes from the same patients. Bonferroni test was used to do the post hoc analyses. Piecewise linear regression models and likelihood ratio tests were used to analyze the differences of changes at different time points. p < 0.05 was considered to be statistically significant.
Results
A total of 60 eyes of 30 POAG patients and 60 eyes of 30 healthy subjects were enrolled in this study (S1. file). All the POAG patients were under IOP-lowering treatment (11 patients were treated with travoprost, 8 patients with bimatoprost, 7 patients with latanoprost, and 4 patients with tafluprost). The clinical characteristics of the two groups are shown in Table 1. The POAG group had longer axial length (p = 0.010) and thinner RNFL and GCC thickness (both p < 0.001) compared to healthy subjects. There were no statistically significant differences in gender (p = 0.796), age (p = 0.064), and CCT (p = 0.523) between two groups.
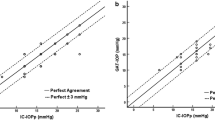
The inter-device agreement analysis was used to compare the consistence of the IOP values measured by Icare PRO and those by NCT. The intraclass correlation coefficient was 0.776 (95% confidence interval, CI: 0.738–0.810, p < 0.001) which was based on the measurements from 60 eyes in the normal group in sitting position at the diurnal time points. Additionally, the Bland-Altman analysis with linear regression was also assessed for the normal group and presented in Fig. 1. The diurnal IOP curve had no significant difference between NCT and Icare PRO in healthy subjects by piecewise linear regression model and likelihood ratio tests (p = 0.136). All those results showed good agreement between Icare PRO and NCT in sitting position during the diurnal period.
The 24-h IOP measurements of Icare PRO in habitual position and NCT in sitting position in normal and POAG groups are listed in Table 2 and the 24-h IOP curves are presented in Fig. 2. We statistically analyzed the differences of IOPs between Icare PRO and NCT in normal group, and found that the differences of IOPs at 25%, 50%, 75% percentiles were 0.1 mmHg, 1 mmHg, and 2.1 mmHg. In both groups of normal and POAG subjects, the mean differences of the IOPs (95% confidence interval) between Icare PRO in supine position and NCT in sitting position were more than 2.1 mmHg from 0:00 to 4:00 (Fig. 2 and Table 2).
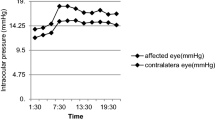
The 24-h IOP curves of normal and POAG groups by Icare PRO are shown in Fig. 3. The IOPs increased notably at night from 22:00 in the normal group [1.62 (1.30, 1.94), p < 0.001] and 20:00 in the POAG group [1.06 (0.77, 1.35), p < 0.001], reached peaks at 0:00 in both groups, stayed high until 4:00 in the morning [normal: 0.02 (− 0.21, 0.25), p < 0.001; glaucoma: 0.00 (− 0.29, 0.29), p < 0.001], and then decreased [normal: − 0.38 (− 0.51, −0.25), p = 0.024; POAG: − 0.45 (− 0.61, −0.30), p = 0.035]. The 24-h mean IOP (p = 0.034), nocturnal mean IOP (p = 0.028), peak IOP (p = 0.028), diurnal peak IOP (p = 0.004), nocturnal peak IOP (p = 0.042), 24-h IOP fluctuation (p = 0.014), and diurnal IOP fluctuation (p = 0.010) were all significantly higher in the glaucoma group compared with those in the normal group, after adjusting for eyes, age, gender, CCT, and axial length. No significant difference was found in other parameters (Table 3).
The 24-h IOP curves in normal and POAG groups by Icare PRO. The IOP ascended notably at night from 20:00 in the POAG group (p < 0.001) and from 22:00 in the normal group (p < 0.001), reached peaks at 0:00 in both groups, stayed high until 4:00 (normal: p < 0.001; glaucoma: p < 0.001), and then descended (normal: p = 0.024; POAG: p = 0.035)
The changes of IOPs from supine to sitting position measured by Icare PRO were greater in the POAG group than those in the normal group at 0:00 (2.63 ± 2.23 mmHg in POAG vs. 1.92 ± 1.20 in normal, p = 0.042) and 2:00 (2.53 ± 1.52 mmHg in POAG vs 1.76 ± 1.17 mmHg in normal, p = 0.001) after adjusting for eyes, age, gender, CCT, and axial length (Fig. 4 and Table 4). No significant difference was found at 4:00 between two groups (all p > 0.05).
The IOP changes from supine to sitting position by Icare PRO in normal and POAG groups by box plots. Box plots showed that IOP changes from supine to sitting position by Icare PRO in normal and POAG groups at the three time points of 0:00, 2:00, and 4:00. The minimum, the 25th percentile, the median, the 75th percentile, the maximum, and the outliers (•) of IOPs were presented. The changes of IOPs by Icare PRO were greater in the POAG group than those of the normal group at 0:00 and 2:00 after adjusting for eyes, age, gender, CCT, and axial length (both * p < 0.05)
Discussion
The present study investigated 24-h IOP patterns by Icare RPO in healthy subjects and POAG patients, and compared it with NCT. Our results showed that there was a very good inter-device agreement of IOP measurements from Icare RPO and NCT in sitting position. Both normal and POAG eyes had higher IOPs measured by Icare RPO than those by NCT during nocturnal period due to different body positions. Additionally, The POAG eyes had significantly higher 24-hIOP level, greater IOP fluctuation, earlier IOP elevation in the nocturnal period in habitual position, and greater IOP change from supine to sitting position in the nocturnal period than those in the normal eyes even after adjusting for eyes, age, gender, CCT, and axial length.
It has been reported that Icare RPO has good inter-device agreement with other currently used devices [14,15,16,17,18]. Those studies demonstrated that there was good agreement among Icare PRO, Tonopen XL, NCT, and Goldmann applanation tonometer (GAT) in anesthetized children [14], in keratoconic eyes [15], and in healthy elder eyes [17, 18]. In our study, the agreement between Icare PRO and NCT was evaluated by ICC and Bland-Altman analysis with linear regression in sitting position during diurnal period. Additionally, the diurnal IOP curve measured by Icare PRO and NCT showed no significant difference in normal eyes by the piecewise linear regression model and likelihood ratio tests. These results were consistent with previous studies [13, 16,17,18].
Interestingly, the mean differences of IOPs between Icare PRO in supine position and NCT in sitting position were more than 2.1 mmHg from 0:00 to 4:00 for both normal and POAG groups. The results could be explained by the different body positions of two methods. The Icare PRO 24-h IOP curves were generated by measurements performed in habitual position, which was sitting position during the diurnal period and supine position during the nocturnal period. The NCT 24-h IOP curves were generated by measurements performed all in sitting position. Many studies had demonstrated that the IOP was higher in supine position compared to that in sitting position because of the elevated episcleral venous pressure [19,20,21,22]. Consistently, previous studies also reported that both POAG and normal-tension glaucoma (NTG) eyes showed a nocturnal acrophase pattern measured by a contact lens sensor [23]. Since Icare RPO is a position-independent rebound tonometer, it is able to be used to measure IOP in habitual position over 24 h, which reflects IOPs in patients’ habitual position better and easier.
The Advanced Glaucoma Intervention Study (AGIS) showed that the most important predictors for glaucomatous optic neuropathy progression are higher mean IOP and greater IOP fluctuation in the follow-up [24]. Besides, glaucomatous visual field progression has been demonstrated to be associated with nocturnal IOP elevation [25]. In our study, the POAG eyes, even under IOP-lowering treatment and with IOPs controlled within the normal range, had higher 24-h mean IOP and nocturnal mean IOP, greater IOP fluctuation, earlier IOP elevation in the nocturnal period in habitual position, and greater IOP change from supine to sitting position in the nocturnal period than those in normal eyes. These results were also in line with previous studies that used CLS for 24-h IOP-related parameters evaluation. Agnifili et al. and Tojo et al. reported greater 24-h IOP fluctuation in treated POAG and NTG eyes than in healthy controls [23, 26]. Mansouri et al. reported prolonged IOP peaks in 62.9–80% of POAG patients [27]. Furthermore, our results showed greater IOP variation in POAG eyes from supine to sitting position during nocturnal period compared with normal eyes, which was consistent with previous studies as well [28, 29]. Therefore, 24-h IOP in habitual position is more sensitive for detecting high nocturnal IOP peaks and large IOP fluctuation for POAG patients, which is potentially useful in the diagnosis, follow-up, and cause analysis for progression.
One of the limitations of this study was the small sample size, since the subjects had to be hospitalized overnight for every 2 h’ IOP measurement. Additionally, although GAT is the gold standard for IOP measurement, multiple topical anesthesia and repeated applanation are needed for GAT in 24-h IOP measurements, which could potentially cause corneal abrasion. Therefore, NCT was chosen in this study. Furthermore, although patients do not need to sit up at night, the biological rhythm would still be disturbed since they need to be awakened repeatedly, even if only Icare PRO is used for IOP measurement. A continuous IOP tonometer is probably an even better solution, which has the advantage in no sleep interruption [30]. Although the contact lens sensor is an option, it still has difficulties in data interpretation. Further effort is needed for a better continuous IOP tonometer, which could deal with the 24-h IOP measurement ideally in the future. At last, we did not investigate short-time variability of Icare PRO in this study, although six consecutive measurements were performed for Icare PRO at each time point. The built-in software automatically discarded the highest and the lowest values, and the average IOP was calculated from the remaining four values according to the manufacturer’s instruction. The short-time variability of Icare pro Tonometer needs to be investigated in further studies.
In conclusion, the results of the present study demonstrated the agreement between Icare PRO and NCT in sitting position. The Icare PRO has the advantage of being able to measure IOP in habitual position over 24 h. The POAG eyes, even if under IOP-lowering treatment, still have higher 24-h IOP level, earlier IOP peaks, and greater IOP change from supine to sitting position in the nocturnal period than those in the normal eyes. The current study highlighted the importance of detecting nocturnal elevation of IOP and emphasized the clinical use of 24-h habitual position IOP measurement in glaucoma patients.
References
Kass MA, Heuer DK, Higginbotham EJ, Johnson CA, Keltner JL, Miller JP, Parrish RK 2nd, Wilson MR, Gordon MO (2002) The ocular hypertension treatment study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol 120:701–713; discussion 829-730. https://doi.org/10.1001/archopht.120.6.701
Collaborative Normal-Tension Glaucoma Study Group (1998) Comparison of glaucomatous progression between untreated patients with normal-tension glaucoma and patients with therapeutically reduced intraocular pressures. Am J Ophthalmol 126:487–497. https://doi.org/10.1016/s0002-9394(98)00223-2
Barkana Y, Anis S, Liebmann J, Tello C, Ritch R (2006) Clinical utility of intraocular pressure monitoring outside of normal office hours in patients with glaucoma. Arch Ophthalmol 124:793–797. https://doi.org/10.1001/archopht.124.6.793
Liu JH, Zhang X, Kripke DF, Weinreb RN (2003) Twenty-four-hour intraocular pressure pattern associated with early glaucomatous changes. Invest Ophthalmol Vis Sci 44:1586–1590. https://doi.org/10.1167/iovs.02-0666
Liu JH, Kripke DF, Hoffman RE, Twa MD, Loving RT, Rex KM, Gupta N, Weinreb RN (1998) Nocturnal elevation of intraocular pressure in young adults. Invest Ophthalmol Vis Sci 39:2707–2712
Deokule SP, Doshi A, Vizzeri G, Medeiros FA, Liu JH, Bowd C, Zangwill L, Weinreb RN (2009) Relationship of the 24-hour pattern of intraocular pressure with optic disc appearance in primary open-angle glaucoma. Ophthalmology 116:833–839. https://doi.org/10.1016/j.ophtha.2008.10.034
Seibold LK, DeWitt PE, Kroehl ME, Kahook MY (2017) The 24-hour effects of brinzolamide/brimonidine fixed combination and timolol on intraocular pressure and ocular perfusion pressure. J Ocul Pharmacol Ther 33:161–169. https://doi.org/10.1089/jop.2016.0141
Sit AJ, Liu JH, Weinreb RN (2006) Asymmetry of right versus left intraocular pressures over 24 hours in glaucoma patients. Ophthalmology 113:425–430. https://doi.org/10.1016/j.ophtha.2005.10.003
Cutolo CA, De Moraes CG, Liebmann JM, Mansouri K, Traverso CE, Ritch R, Triggerfish C (2019) The effect of therapeutic IOP-lowering interventions on the 24-hour ocular dimensional profile recorded with a sensing contact lens. J Glaucoma 28:252–257. https://doi.org/10.1097/IJG.0000000000001185
De Moraes CG, Mansouri K, Liebmann JM, Ritch R, Triggerfish C (2018) Association between 24-hour intraocular pressure monitored with contact lens sensor and visual field progression in older adults with glaucoma. JAMA Ophthalmol 136:779–785. https://doi.org/10.1001/jamaophthalmol.2018.1746
Sahin A, Basmak H, Niyaz L, Yildirim N (2007) Reproducibility and tolerability of the ICare rebound tonometer in school children. J Glaucoma 16:185–188. https://doi.org/10.1097/IJG.0b013e31802fc6bc
Grigorian F, Grigorian AP, Olitsky SE (2012) The use of the iCare tonometer reduced the need for anesthesia to measure intraocular pressure in children. J AAPOS 16:508–510. https://doi.org/10.1016/j.jaapos.2012.07.004
Nakakura S, Mori E, Yamamoto M, Tsushima Y, Tabuchi H, Kiuchi Y (2015) Intradevice and interdevice agreement between a rebound tonometer, Icare PRO, and the Tonopen XL and Kowa hand-held applanation tonometer when used in the sitting and supine position. J Glaucoma 24:515–521. https://doi.org/10.1097/IJG.0000000000000016
Serafino M, Villani E, Lembo A, Rabbiolo G, Specchia C, Trivedi RH, Nucci P (2020) A comparison of Icare PRO and Perkins tonometers in anesthetized children. Int Ophthalmol 40:19–29. https://doi.org/10.1007/s10792-019-01143-3
Bilgec MD, Atalay E, Sozer O, Gursoy H, Bilgin M, Yildirim N (2020) The influence of corneal geometrical and biomechanical properties on tonometry readings in keratoconic eyes. Int Ophthalmol 40:849–857. https://doi.org/10.1007/s10792-019-01248-9
Chen M, Zhang L, Xu J, Chen X, Gu Y, Ren Y, Wang K (2019) Comparability of three intraocular pressure measurement: iCare pro rebound, non-contact and Goldmann applanation tonometry in different IOP group. BMC Ophthalmol 19:225. https://doi.org/10.1186/s12886-019-1236-5
Kato Y, Nakakura S, Matsuo N, Yoshitomi K, Handa M, Tabuchi H, Kiuchi Y (2018) Agreement among Goldmann applanation tonometer, iCare, and Icare PRO rebound tonometers; non-contact tonometer; and Tonopen XL in healthy elderly subjects. Int Ophthalmol 38:687–696. https://doi.org/10.1007/s10792-017-0518-2
Guler M, Bilak S, Bilgin B, Simsek A, Capkin M, Hakim Reyhan A (2015) Comparison of intraocular pressure measurements obtained by Icare PRO rebound tonometer, Tomey FT-1000 noncontact tonometer, and Goldmann applanation tonometer in healthy subjects. J Glaucoma 24:613–618. https://doi.org/10.1097/IJG.0000000000000132
Linden C, Qvarlander S, Johannesson G, Johansson E, Ostlund F, Malm J, Eklund A (2018) Normal-tension glaucoma has normal intracranial pressure: a prospective study of intracranial pressure and intraocular pressure in different body positions. Ophthalmology 125:361–368. https://doi.org/10.1016/j.ophtha.2017.09.022
Fang SY, Wan Abdul Halim WH, Mat Baki M, Din NM (2018) Effect of prolonged supine position on the intraocular pressure in patients with obstructive sleep apnea syndrome. Graefes Arch Clin Exp Ophthalmol 256:783–790. https://doi.org/10.1007/s00417-018-3919-7
Malihi M, Sit AJ (2012) Effect of head and body position on intraocular pressure. Ophthalmology 119:987–991. https://doi.org/10.1016/j.ophtha.2011.11.024
Linder BJ, Trick GL, Wolf ML (1988) Altering body position affects intraocular pressure and visual function. Invest Ophthalmol Vis Sci 29:1492–1497
Agnifili L, Mastropasqua R, Frezzotti P, Fasanella V, Motolese I, Pedrotti E, Di Iorio A, Mattei PA, Motolese E, Mastropasqua L (2015) Circadian intraocular pressure patterns in healthy subjects, primary open angle and normal tension glaucoma patients with a contact lens sensor. Acta Ophthalmol 93:e14–e21. https://doi.org/10.1111/aos.12408
Nouri-Mahdavi K, Hoffman D, Coleman AL, Liu G, Li G, Gaasterland D, Caprioli J, Advanced Glaucoma Intervention S (2004) Predictive factors for glaucomatous visual field progression in the Advanced Glaucoma Intervention study. Ophthalmology 111:1627–1635. https://doi.org/10.1016/j.ophtha.2004.02.017
Sung KR, Lee S, Park SB, Choi J, Kim ST, Yun SC, Kang SY, Cho JW, Kook MS (2009) Twenty-four hour ocular perfusion pressure fluctuation and risk of normal-tension glaucoma progression. Invest Ophthalmol Vis Sci 50:5266–5274. https://doi.org/10.1167/iovs.09-3716
Tojo N, Abe S, Ishida M, Yagou T, Hayashi A (2017) The fluctuation of intraocular pressure measured by a contact lens sensor in normal-tension glaucoma patients and nonglaucoma subjects. J Glaucoma 26:195–200. https://doi.org/10.1097/IJG.0000000000000517
Mansouri K, Liu JH, Weinreb RN, Tafreshi A, Medeiros FA (2012) Analysis of continuous 24-hour intraocular pressure patterns in glaucoma. Invest Ophthalmol Vis Sci 53:8050–8056. https://doi.org/10.1167/iovs.12-10569
Grippo TM, Liu JH, Zebardast N, Arnold TB, Moore GH, Weinreb RN (2013) Twenty-four-hour pattern of intraocular pressure in untreated patients with ocular hypertension. Invest Ophthalmol Vis Sci 54:512–517. https://doi.org/10.1167/iovs.12-10709
Hirooka K, Shiraga F (2003) Relationship between postural change of the intraocular pressure and visual field loss in primary open-angle glaucoma. J Glaucoma 12:379–382. https://doi.org/10.1097/00061198-200308000-00015
Wasilewicz R, Varidel T, Simon-Zoula S, Schlund M, Cerboni S, Mansouri K (2020) First-in-human continuous 24-hour measurement of intraocular pressure and ocular pulsation using a novel contact lens sensor. Br J Ophthalmol. https://doi.org/10.1136/bjophthalmol-2019-315276
Funding
This study was funded by the Shanghai Jing’an District Health Commission (2016MS12, 2017QN03, 2019MS12), the State Program of National Natural Science Foundation of China (81570887, 81870692), the State Key Program of National Natural Science Foundation of China (81430007), the subject of major projects of National Natural Science Foundation of China (81790641), the Shanghai Committee of Science and Technology, China (17410712500), and the top priority of clinical medicine center of Shanghai (2017ZZ01020). The sponsor or funding organization had no role in the design or conduct of this research.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(XLSX 33 kb)
Rights and permissions
About this article
Cite this article
Fang, Z., Wang, X., Qiu, S. et al. 24-h intraocular pressure patterns measured by Icare PRO rebound in habitual position of open-angle glaucoma eyes. Graefes Arch Clin Exp Ophthalmol 259, 2327–2335 (2021). https://doi.org/10.1007/s00417-021-05192-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-021-05192-2