Abstract
Purpose
To evaluate the efficacy of corneal cross-linking (CXL) as adjuvant therapy for the treatment of fungal ulcerative keratitis.
Methods
Forty-one patients with fungal ulcerative keratitis were recruited and assigned into two randomized controlled groups. These groups were treated with CXL combined with antifungal medications (CXL-M) or antifungal medications alone (M). The ulcers were assessed by slit-lamp biomicroscopy, slit-lamp images, in vivo confocal microscopy (IVCM), and anterior segment optical coherence tomography (AS-OCT). The patients were followed up before surgery/first visit (FV), 1 day after surgery, 1 and 2 weeks, and 1, 2, 3, 4, 5, and 6 months after surgery/FV.
Results
In the cured patients, the area of corneal ulcers, the duration of ulcer healing, the time to non-observed fungal hyphae by IVCM, the number of antifungal medications, the frequency of administered medications, and the maximum ulcer depth decreased significantly after CXL (all P < 0.05) compared with the M group. There were no significant differences in either corneal thickness or epithelial thickness of ulcers after healing between 5 and 6 months after surgery in the CXL-M group, while these were increased significantly at 6 months compared with 5 months after FV in the M group (both P < 0.05).
Conclusions
In our study, CXL accelerated healing of the fungal ulcers, shortened the treatment duration, and minimized the need for medications and surgery. It appears that CXL is an effective procedure and adjuvant therapy for managing fungal keratitis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fungal ulcerative keratitis is a destructive infectious corneal disease with a reported high incidence rate of 17–36% of infectious keratitis [1]; this disease is endemic in agricultural countries and may result in corneal opacity, blindness, and even loss of the eyeball [2]. Compared with bacterial ulcers, fungal ulcers tend to have a worse prognosis [3]. Since a fungal infection that is deep-seated in the corneal matrix can lead to the release of enzymes and an inflammatory immune reaction, the treatment of fungal keratitis remains a serious clinical health concern [4]. If corneal melting or perforation occurs due to therapy-resistant fungal ulcers, emergency keratoplasty may be necessary, although this procedure may lead to complications including relapse and graft rejection [5]. Currently, there are a variety of antifungal medications used for managing fungal keratitis, including polyenes, triazoles, and echinocandins, which may be associated with undesirable side effects such as hyperemia, chemosis, and delayed corneal reepithelialization process [6, 7]. Because of the increased drug-resistance to topical drugs and their poor permeability and common toxicity, conventional medications for fungal keratitis are still a challenge to ophthalmologists.
Corneal collagen cross-linking is a novel technique. Riboflavin (vitamin B2) produces singlet oxygen and hydroxyl radicals after ultraviolet A (UV-A) exposure [8]. With the formation of chemical bonds among stromal collagen fibrils, the biomechanical strength of the cornea is increased [9, 10]. Thus, several studies have reported that CXL is effective for treating keratoconus [11, 12], corneal ectasia [13], and bullous keratopathies [11, 14]. In addition, when activated by UV-A, riboflavin induces oxidative damage to the DNA/RNA of pathogens and suppresses their replication due to photochemical reactions [5, 15]. Since 2000, it has been used to inactivate pathogens in a variety of blood products, including platelet, red blood cell products, and plasma [16]. In 2008, the first clinical application of CXL was used to treat infectious keratitis [17]. After exposure to UV-A, the byproducts of riboflavin protect the cornea by destroying pathogens and enhancing collagen resistance to enzymatic degradation. In recent years, several studies have been conducted to examine the possibility of using CXL as an antifungal treatment [18,19,20]. However, the role CXL plays in antifungal therapy is still under evaluation.
In this article, we performed a therapeutic exploratory randomized clinical study to assess the efficacy of CXL as an adjuvant therapy to conventional medication for fungal ulcerative keratitis.
Materials and methods
Patients with fungal ulcerative keratitis
Consecutive patients with fungal ulcerative keratitis were recruited at the Eye, Ear, Nose, and Throat Hospital from December 2015 to June 2017. All recruited patients were of Chinese ancestry and were randomized into two groups: a CXL combined with antifungal medications (CXL-M) group and an antifungal medication alone (M) group, which also served as the control group. The study protocol was approved by the hospital’s ethics committee and followed the tenets of the Declaration of Helsinki. Before inclusion, written informed consent was obtained from all subjects. All patients with corneal ulcers underwent smear and culture for pathogens and were examined simultaneously with in vivo confocal microscopy (IVCM) to help identify the pathogens. Based on their first presentation, the patients were prescribed a topical 5% natamycin eye drop (Natacyn, Alcon, Fort Worth, TX, USA) and a 1% voriconazole eye drop (Vfend IV, Pfizer Pharmaceuticals, New York, USA), with/without oral itraconazole (Pfizer, New York, NY, USA). Exclusion criteria included a perforated corneal ulcer, corneal melting or perforation resulting from any other causes, endophthalmitis, collagen vascular disease, corneal descemetocele, immune diseases, diabetes, and pregnancy.
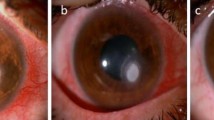
All enrolled eyes underwent a complete ophthalmologic examination before surgery first visit (FV), 1 day after surgery, 1 and 2 weeks after surgery/FV, and 1, 2, 3, 4, 5, and 6 months after surgery/FV. The following examinations were performed at the same time in 1 day, namely log MAR visual acuity, slit-lamp biomicroscopy, slit-lamp imaging, IVCM (HRT3-RCM; Heidelberg Engineering, GmbH, Heidelberg, Germany), and anterior segment optical coherence tomography (AS-OCT; RTVue Version 6.9 Optovue Inc., Fremont, CA, USA). Based on the slit-lamp images with a uniform scale plate (Fig. 1) and magnification, the area of corneal ulcers was analyzed with ImageJ software. Anterior sectional imaging of the cornea was acquired using an AS-OCT machine. For each eye, six AS-OCT cross-sectional images of the cornea were acquired at the angles of 0–180°, 30–210°, 60–240°, 90–270°, 120–300°, and 150–330°. After acquisition, the scanned images were processed by the AS-OCT software, and the following parameters were measured: the maximum depth of ulcer and the corneal thickness and epithelial thickness of ulcer after healing. Each examination and analysis was conducted by a masked operator uniformly. Disappearance of the inflammation reaction of anterior chamber and of circumcorneal congestion, and intact reepithelialization of corneal ulcer without infiltration were taken as clinical signs of the healing of the fungal ulcer [21], that is to say the patient is cured, in addition to non-observed fungal hyphae by multi-point and repeated confocal microscopy [22, 23].
Procedure
In the operating room, CXL was performed under sterile conditions at 9:00–11:00 a.m. on different days, by the same experienced ophthalmologist. The patient was placed in the supine position. A topical anesthetic (oxybuprocaine hydrochloride 0.4%, Santen Pharmaceutical Co., Ltd.) was then instilled three times. After inserting a lid speculum, the epithelium and the necrotic tissue of the ulcer were removed with a hockey knife; these tissues were then sent for pathogens culture. Riboflavin drops (Medio-Cross riboflavin/dextran solution, 0.1%) were instilled on the ulcer of corneal every 3 min for 30 min. The cornea was irradiated for 30 min using a Phoenix UV-A system (Peschke Meditrade GmbH, Huenenberg, Switzerland) at 365 nm with an irradiance of 3 mW/cm2 and a dose of 5.4 J/cm2. During the period of UV-A exposure, riboflavin was dropped onto the cornea every 1.5 min. The standard therapy was prescribed 2 h after CXL treatment and included topical 5% natamycin and 1% voriconazole eye drops administered q2h, from 6:00 a.m. to 10:00 p.m. Subsequently, the patients were evaluated, and the medication doses were tapered based on their clinical responses. The therapeutic regimen of each patient in the M group was adjusted based on their clinical presentation.
Statistical analysis
Data of log MAR visual acuity, area of the ulcer, the duration of ulcer healing, the time to non-observed fungal hyphae by IVCM, the number of antifungal medications, the frequency of medication administration, hypopyon, the maximum depth of the ulcer, and corneal thickness and epithelial thickness of the ulcer after healing were analyzed by independent-samples t test and the repeated measures analysis of variance (ANOVA) followed by the Dunnett post-hoc and Bonferroni analysis and the chi-square test. All data were considered to be statistically significantly different at P < 0.05. Quantitative values are summarized as the mean ± SD. SPSS software version 23.0 (SPSS, Chicago, IL, USA) was used for the data analyses.
Results
Forty-one eyes with fungal ulcerative keratitis were enrolled in the study. The data from before surgery/FV are listed in Table 1. Between the two groups at the initial presentation, there was no difference with regard to the age, gender, type of organisms isolated, area of the ulcer before surgery/FV, number of antifungal medications, frequency of medication administration, hypopyon, logMAR visual acuity, and maximum depth of the ulcer (all P > 0.05). The causative pathogens Aspergillus or Fusarium were found in approximately 2/3 of all patients. However, we found fungal hyphae in corneal ulcers of all patients evaluated with IVCM before surgery/FV. There were 18 eyes in the CXL-M group and 14 eyes in the M group that were cured after their respective treatments. In the cured patients, the time to non-observed fungal hyphae by IVCM in the CXL-M group was 1.29 ± 0.38 months, which was less than that in the M group (2.14 ± 0.28 months; P = 0.037). As shown in Table 2, at 1 week after CXL/FV, the number of antifungal medications used in the CXL-M group was 2, which was lower than that in the M group (2.29 ± 0.47; P = 0.014). In addition, in the CXL-M group, the number of antifungal medications decreased again at 3 months after surgery compared with the M group (P = 0.037). The frequency of medication administration in the CXL-M group was significantly lower than that in the M group from 1 week to 3 months after surgery/FV (all P < 0.05). After the ulcers healed, the log MAR visual acuity was obviously increased in both groups (both P < 0.05); however, there was no difference between the two groups (P = 0.826).
The analysis of the areas of corneal ulcers in the cured patients, based on slit-lamp images, indicated that the areas of ulcers before surgery in the CXL-M group (14.93 ± 1.17 mm2) were larger than those in the M group (8.22 ± 1.99 mm2) (P = 0.027) (Fig. 2). However, 1 week after CXL/FV, we found that there was no significant difference between the two groups (CXL-M 10.79 ± 1.55 mm2; M 8.38 ± 1.34 mm2; P = 0.495). During this time, the areas of the corneal ulcers decreased significantly from 14.93 ± 1.17 mm2 to 10.79 ± 1.55 mm2 in the CXL-M group (P = 0.001). At both 2 and 3 months after CXL/FV, the areas of the corneal ulcers were both smaller in the CXL-M group than those in the M group (both P < 0.05). In the CXL-M group, there was no difference in the areas of the corneal ulcers between 1 day after CXL and before surgery (P = 0.297). However, compared with 1 day after CXL, the areas of the corneal ulcers decrease at all the other follow-up times after CXL (all P < 0.05). In the CXL-M group, the time to healing of the ulcers of 1.30 ± 0.93 months was shorter than that in the M group (2.21 ± 1.35 months; P = 0.036).
Area of corneal ulcer in the cured patients. +There were significant differences in the area of corneal ulcers at all other follow-up periods after CXL, compared with that at 1 day after surgery (P < 0.05 for all). *There were significant differences in the areas of corneal ulcer between the CXL-M group and the M group (P < 0.05). n/n′ = number of cured patients in the CXL-M group/number of cured patients in the M group
In the cured patients, it was found that there was no significant difference in the maximum depth of the ulcers between the two groups before surgery/FV (P = 0.789) (Table 3). At both 2 weeks and 1 month after CXL/FV, the maximum depths of the ulcers in the CXL-M group were 37.62 ± 4.79 μm and 16.5 ± 3.04 μm, which were shallower than those in the M group (68.57 ± 5.52 μm, P = 0.049; 52.72 ± 3.6 μm, P = 0.036, respectively). Reepithelialization and healing of the corneal ulcers in the two groups were both completed at 5 months after CXL/FV. Therefore, the AS-OCT data indicated that there were no significant differences in the corneal thicknesses of ulcers after healing between 5 and 6 months after CXL in the CXL-M group (5 months 529.96 ± 3.43 μm; 6 months 518.71 ± 5.08 μm; P = 0.229); however, in the M group, the corneal thicknesses of ulcers after healing were increased at 6 months compared with those at 5 months after FV (5 months 606.09 ± 4.31 μm, 6 months 653.19 ± 7.66 μm, P = 0.044). Furthermore, there was no significant difference in epithelial thicknesses after healing between 5 and 6 months after CXL in the CXL-M group (5 months 42.06 ± 1.86 μm, 6 months 43.71 ± 1.22 μm, P = 0.347); however, the epithelial thicknesses after healing were increased at 6 months compared with those at 5 months after FV in the M group (5 months 45.35 ± 0.95 μm, 6 months 54.39 ± 3.48 μm, P = 0.038).
Complications
In the CXL-M group, hypopyon occurred in one eye 1 day after CXL and was resolved 2 weeks after CXL. However, in the M group, hypopyon increased in two eyes 1 week after FV. Among these eyes, the hypopyon was resolved in one eye 1 month after FV, and the other eye subsequently suffered a corneal perforation. There were nine eyes that were followed up for corneal perforations out of all the patients (of the three eyes in the CXL-M group, the failure rate was 14.29%, and of the six eyes in the M group, the failure rate was 30.00%; P = 0.402), necessitating emergency keratoplasty procedures.
Discussion
Fungal keratitis is a severe corneal infection appearing as an ulcerative lesion that requires treatment with antifungal medications. Because fungi are eukaryotes and many potential targets for therapy are also found in human cells, severe adverse reactions make antifungal drugs inappropriate for frequent and prolonged administration [22, 24]. It was reported that about 31% of fungal keratitis would be unresponsive to antifungal agents; some may even get more severe in the process of drug application due to few species of antifungal agents, slow response to therapy, and inadequate corneal penetration [25]. Although emergency keratoplasty can be performed to rescue severe fungal ulcers, relapse and graft rejection are still common [26]. Thus, several studies have reported that CXL has been applied to treat fungal keratitis as a novel option that is supplementary to conventional therapy and have demonstrated that the combination of CXL and antifungal medications has benefits for successfully treating fungal keratitis [17, 20, 27, 28]; furthermore, the increased riboflavin concentration can increase the efficiency of CXL [28]. Nevertheless, the evidence that CXL has excellent antifungal efficacy and can halt the progression of corneal melting in humans remains limited.
The results of our study indicated that, in the cured patients, CXL appeared to minimize the need for medications and surgery, accelerate the healing of fungal ulcers, and shorten the duration of treatment (Figs. 3 and 4). It seems that multiple mechanisms are involved in the treatment of fungal keratitis with CXL. Fungus infection in the cornea not only activates the immune response to release inflammatory mediators, such as IL-6, IL-8, and MCP-1 [29], but also increases the activity of enzymes, such as pepsin, trypsin, and collagenase, which digest corneal collagen and lead to corneal melting and perforation [30, 31]. Spoerl et al. [32] found that CXL can improve the resistance of collagen to enzymatic digestion. CXL increases the interlinking of chemical bonds to change the structure of corneal collagen fibers, thus blocking the interaction between enzymes and their target sites [33]. More importantly, CXL not only inactivates or eradicates pathogens by damaging ribonucleic acids but also has a direct cytotoxic effect on inflammatory cells, reducing the inflammatory reaction associated with the immune response [34]. As another potential mechanism in the process, CXL can induce keratocyte apoptosis in the anterior part of the cornea, decreasing the corneal opacity after CXL for the transformation of activated keratocytes into fibroblasts [33].
Representative images of the corneal ulcer of a representative patient and the corresponding IVCM in the CXL-M group at all follow-up periods. Within 1 month after CXL, the healing of the fungal ulcer had continuously accelerated, and the reepithelialization of the corneal ulcer was completed at 2 months after CXL. There were no fungal hyphae observed on IVCM at 2 weeks after CXL. The white arrow shows the fungal hyphae
Representative images of the corneal ulcer of a representative patient and its corresponding IVCM in the M group at all follow-up periods. The reepithelialization and healing of the corneal ulcer were completed at 4 months after FV. There were no fungal hyphae observed on IVCM at 4 months after FV. The white arrow shows the fungal hypha
Riboflavin is an essential component of living cells and tissues, which is a natural compound. UV-A radiation with riboflavin may induce DNA/RNA damage through various mechanisms. First, there is a direct interaction in the production of radical intermediates with DNA/RNA via electron transfer. Second, there is an energy transfer from activated riboflavin to O2 (molecular oxygen) to produce the powerful oxidant 1O2, which is the main reactive substance in UV-A-induced DNA/RNA damage within cells [35]. Additionally, there is an electron transfer from activated riboflavin to O2 to form O2−, followed by dismutation to H2O2, which can cause DNA/RNA damage in the presence of metal ions, such as Cu (copper) ions. The oxidant effect of UV-A combined with riboflavin is the major pathway of cellular damage, which is involved in pathogen eradication in corneal ulcers [36].
In an in vitro study, it has been shown that the UV-A absorption in CXL can be calculated according to the Beer-Lambert law [37]. The cytotoxic irradiance level after CXL has been shown to be approximately tenfold lower compared with treatment with UV-A alone, while riboflavin increases the UV-A absorption to 95% in the cornea, compared with 25–35% without it [38]. The permeation of riboflavin into the corneal matrix requires a certain amount of time. Nevertheless, the concentration gradient of riboflavin through the cornea still became flattered with time, even after 30 min [39]. With a surface irradiance of 3 mW/cm2, cell loss is only found in the anterior 250–300 μm of the corneal stroma in humans [40]. For improving the permeability of riboflavin into the corneal matrix, the principal step is debridement, which is to remove the epithelium and necrotic tissues of corneal ulcers, resulting in a thinning of the corneal thickness. Meanwhile, it was speculated that debridement might be beneficial to the temporary improvement of penetration of antifungal medications after CXL. As a photosensitizer and blocker, riboflavin can prevent damage from UV-A to the underlying tissues, including the endothelium. In our study, the minimum corneal thickness of the ulcers was > 350 μm, which is thicker than the minimal safe thickness of 330 μm that has been reported for the CXL procedure [41, 42]. After the ulcers had healed, there were no lesions observed in the corneal endothelium. In the cured patients, the areas of corneal ulcers in the CXL-M group were larger than those in the M group before surgery/FV, while we did not find any expansion in corneal ulcers 1 day after CXL in the CXL-M group. In addition, the areas of corneal ulcers in the CXL-M group decreased to the same extent as those in the M group 1 week after surgery/FV. As reported, CXL can accelerate the process of ulcer reepithelialization and relieve symptoms, such as pain, lacrimation, foreign body sensation, and burning sensation [6, 43].
We also found that the corneal thickness of ulcers after healing was closer to normal and remained stable in the CXL-M group, whereas it was thicker than 600 μm and appeared to be increasing in the M group. Given the high frequency of administration of antifungal eye drops in the M group, the toxicity of topical medications may be one of the major causes of postponed ulcer healing. With a high frequency of administration of topical antifungal medications, the corneal epithelium becomes thicker and more opaque, and the surface of the cornea becomes rougher and more irregular after ulcer healing [44].
Although CXL has the capability of strengthening corneal collagen, its safety must be investigated. There is still a risk of requiring surgical debridement with the CXL procedure. Surgical debridement has no standardized protocol. The aim of debridement is to remove epithelial and necrotic tissues of corneal ulcers, including toxic fragments, pathogens, inflammatory cells, and substances that may cause further damage to the cornea [45, 46]. In addition, if the ulcerative infiltration is deep-seated in the cornea, debridement has the possibility of thinning the corneal ulcer and can even lead to perforation [47]. Consequently, further research is expected to indicate the role debridement play in this study. In our study, we found that advanced corneal ulcers melted after CXL in three eyes, which ultimately required emergency keratoplasty procedures. In terms of the minimum corneal thickness of ulcers before surgery, although the ulcers in these three eyes were thicker than 350 μm, the corneal infiltration and melting nearly reached a depth of the posterior 1/3 of the corneal stroma, which was much deeper than other ulcers in the cured eyes in the CXL-M group. Thus, we speculated that surgical damage might have played a major role in accelerating corneal ulcer melting and perforation, even though CXL strengthened corneal collagen, which alone was sufficient to halt the progression of the disease. In addition, when a corneal ulcer is relatively too thin due to debridement, phototoxicity from UV-A exposure may increase the risk of corneal endothelial lesions, resulting in the acceleration of perforation of the corneal ulcer [48]. If riboflavin traverses the cornea, it enters the anterior chamber [39]. It is still unknown whether the endothelium acts as a diffusion barrier to riboflavin. Overall, CXL may be perilous for recalcitrant fungal ulcers with deep-seated infiltration and melting, especially in those involving the posterior 1/3 of the corneal stroma.
In summary, CXL appears to be an effective and promising adjuvant therapy for the management of fungal keratitis. Our study is only preliminary research, due to the undiversified concentration of riboflavin, the small number of subjects, and the relatively short follow-up time. To establish CXL as adjuvant therapy for fungal keratitis, further prospective randomized studies with a larger number of enrolled patients and a longer follow-up may provide support. Furthermore, additional research evaluating the depth of corneal infiltration and the type of fungal species involved is needed to draw further conclusions.
References
Kernt M, Kampik A (2009) Intracameral voriconazole: in vitro safety for human ocular cells. Toxicology 258:84–93. https://doi.org/10.1016/j.tox.2009.01.008
Wu J, Zhang WS, Zhao J, Zhou HY (2016) Review of clinical and basic approaches of fungal keratitis. Int J Ophthalmol 9:1676–1683. https://doi.org/10.18240/ijo.2016.11.23
Prajna NV, Srinivasan M, Lalitha P, Krishnan T, Rajaraman R, Ravindran M, Mascarenhas J, Oldenburg CE, Ray KJ, McLeod SD, Acharya NR, Lietman TM (2013) Differences in clinical outcomes in keratitis due to fungus and bacteria. JAMA Ophthalmol 131:1088–1089. https://doi.org/10.1001/jamaophthalmol.2013.1612
Fini ME, Cook JR, Mohan R (1998) Proteolytic mechanisms in corneal ulceration and repair. Arch Dermatol Res 290 Suppl:S12–S23
Anwar HM, El-Danasoury AM, Hashem AN (2011) Corneal collagen crosslinking in the treatment of infectious keratitis. Clin Ophthalmol 5:1277–1280. https://doi.org/10.2147/OPTH.S24532
Martins SA, Combs JC, Noguera G, Camacho W, Wittmann P, Walther R, Cano M, Dick J, Behrens A (2008) Antimicrobial efficacy of riboflavin/UVA combination (365 nm) in vitro for bacterial and fungal isolates: a potential new treatment for infectious keratitis. Invest Ophthalmol Vis Sci 49:3402–3408. https://doi.org/10.1167/iovs.07-1592
Kimakura M, Usui T, Yokoo S, Nakagawa S, Yamagami S, Amano S (2014) Toxicity of topical antifungal agents to stratified human cultivated corneal epithelial sheets. J Ocul Pharmacol Ther 30:810–814. https://doi.org/10.1089/jop.2014.0044
Baier J, Maisch T, Maier M, Engel E, Landthaler M, Baumler W (2006) Singlet oxygen generation by UVA light exposure of endogenous photosensitizers. Biophys J 91:1452–1459. https://doi.org/10.1529/biophysj.106.082388
Garg P, Das S, Roy A (2017) Collagen cross-linking for microbial keratitis. Middle East Afr J Ophthalmol 24:18–23. https://doi.org/10.4103/meajo.MEAJO_305_16
Kymionis GD, Mikropoulos DG, Portaliou DM, Voudouragkaki IC, Kozobolis VP, Konstas AG (2013) An overview of corneal collagen cross-linking (CXL). Adv Ther 30:858–869. https://doi.org/10.1007/s12325-013-0065-9
Alhayek A, Lu PR (2015) Corneal collagen crosslinking in keratoconus and other eye disease. Int J Ophthalmol 8:407–418. https://doi.org/10.3980/j.issn.2222-3959.2015.02.35
O'Brart DP, Patel P, Lascaratos G, Wagh VK, Tam C, Lee J, O'Brart NA (2015) Corneal cross-linking to halt the progression of keratoconus and corneal ectasia: seven-year follow-up. Am J Ophthalmol 160:1154–1163. https://doi.org/10.1016/j.ajo.2015.08.023
Li G, Fan ZJ, Peng XJ (2012) Corneal collagen crosslinking for corneal ectasia of post-LASIK: one-year results. Int J Ophthalmol 5:190–195. https://doi.org/10.3980/j.issn.2222-3959.2012.02.15
Sharma N, Roy S, Maharana PK, Sehra SV, Sinha R, Tandon R, Titiyal JS, Vajpayee RB (2014) Outcomes of corneal collagen crosslinking in pseudophakic bullous keratopathy. Cornea 33:243–246. https://doi.org/10.1097/ICO.0000000000000004
Alshehri JM, Caballero-Lima D, Hillarby MC, Shawcross SG, Brahma A, Carley F, Read ND, Radhakrishnan H (2016) Evaluation of corneal cross-linking for treatment of fungal keratitis: using confocal laser scanning microscopy on an ex vivo human corneal model. Invest Ophthalmol Vis Sci 57:6367–6373. https://doi.org/10.1167/iovs.16-20110
Goodrich RP (2000) The use of riboflavin for the inactivation of pathogens in blood products. Vox Sang 78(Suppl 2):211–215
Iseli HP, Thiel MA, Hafezi F, Kampmeier J, Seiler T (2008) Ultraviolet A/riboflavin corneal cross-linking for infectious keratitis associated with corneal melts. Cornea 27:590–594. https://doi.org/10.1097/ICO.0b013e318169d698
Igal V, Pikkel Igal YS, Pikkel YY (2017) Corneal cross-linking as a treatment for fungal keratitis associated with corneal melting. Case Rep Ophthalmol 8:148–151. https://doi.org/10.1159/000456537
Shetty R, Nagaraja H, Jayadev C, Shivanna Y, Kugar T (2014) Collagen crosslinking in the management of advanced non-resolving microbial keratitis. Br J Ophthalmol 98:1033–1035. https://doi.org/10.1136/bjophthalmol-2014-304944
Li Z, Jhanji V, Tao X, Yu H, Chen W, Mu G (2013) Riboflavin/ultravoilet light-mediated crosslinking for fungal keratitis. Br J Ophthalmol 97:669–671. https://doi.org/10.1136/bjophthalmol-2012-302518
Mohan M, Panda A, Gupta SK (1989) Management of human keratomycosis with miconazole. Aust N Z J Ophthalmol 17:295–297
Shi W, Li S, Liu M, Jin H, Xie L (2008) Antifungal chemotherapy for fungal keratitis guided by in vivo confocal microscopy. Graefes Arch Clin Exp Ophthalmol 246:581–586. https://doi.org/10.1007/s00417-007-0719-x
Das S, Samant M, Garg P, Vaddavalli PK, Vemuganti GK (2009) Role of confocal microscopy in deep fungal keratitis. Cornea 28:11–13. https://doi.org/10.1097/ICO.0b013e318181cff7
Campoy S, Adrio JL (2017) Antifungals. Biochem Pharmacol 133:86–96. https://doi.org/10.1016/j.bcp.2016.11.019
Wang JY, Wang DQ, Qi XL, Cheng J, Xie LX (2018) Modified ulcer debridement in the treatment of the superficial fungal infection of the cornea. Int J Ophthalmol 11:223–229. https://doi.org/10.18240/ijo.2018.02.07
Xie L, Zhai H, Shi W (2007) Penetrating keratoplasty for corneal perforations in fungal keratitis. Cornea 26:158–162. https://doi.org/10.1097/01.ico.0000248381.24519.0d
Price MO, Tenkman LR, Schrier A, Fairchild KM, Trokel SL, Price FW, Jr. (2012) Photoactivated riboflavin treatment of infectious keratitis using collagen cross-linking technology. J Refract Surg 28: 706–713 DOI https://doi.org/10.3928/1081597X-20120921-06
Ozdemir HB, Kalkanci A, Bilgihan K, Gocun PU, Ogut B, Karakurt F, Erdogan M (2018) Comparison of corneal collagen cross-linking (PACK-CXL) and voriconazole treatments in experimental fungal keratitis. Acta Ophthalmol. https://doi.org/10.1111/aos.13829
Kimura K, Orita T, Nomi N, Fujitsu Y, Nishida T, Sonoda KH (2012) Identification of common secreted factors in human corneal fibroblasts exposed to LPS, poly(I:C), or zymosan. Exp Eye Res 96:157–162. https://doi.org/10.1016/j.exer.2011.10.015
Vajpayee RB, Shafi SN, Maharana PK, Sharma N, Jhanji V (2015) Evaluation of corneal collagen cross-linking as an additional therapy in mycotic keratitis. Clin Exp Ophthalmol 43:103–107. https://doi.org/10.1111/ceo.12399
Papaioannou L, Miligkos M, Papathanassiou M (2016) Corneal collagen cross-linking for infectious keratitis: a systematic review and meta-analysis. Cornea 35:62–71. https://doi.org/10.1097/ICO.0000000000000644
Spoerl E, Wollensak G, Seiler T (2004) Increased resistance of crosslinked cornea against enzymatic digestion. Curr Eye Res 29:35–40. https://doi.org/10.1080/02713680490513182
Hovakimyan M, Guthoff RF, Stachs O (2012) Collagen cross-linking: current status and future directions. J Ophthalmol 2012:406850. https://doi.org/10.1155/2012/406850
Wang F (2008) UVA/riboflavin-induced apoptosis in mouse cornea. Ophthalmologica 222:369–372. https://doi.org/10.1159/000151247
Pouget JP, Douki T, Richard MJ, Cadet J (2000) DNA damage induced in cells by gamma and UVA radiation as measured by HPLC/GC-MS and HPLC-EC and Comet assay. Chem Res Toxicol 13:541–549
Hiraku Y, Ito K, Hirakawa K, Kawanishi S (2007) Photosensitized DNA damage and its protection via a novel mechanism. Photochem Photobiol 83:205–212. https://doi.org/10.1562/2006-03-09-IR-840
Wollensak G, Spoerl E, Reber F, Seiler T (2004) Keratocyte cytotoxicity of riboflavin/UVA-treatment in vitro. Eye (Lond) 18:718–722. https://doi.org/10.1038/sj.eye.6700751
Wollensak G, Sporl E, Reber F, Pillunat L, Funk R (2003) Corneal endothelial cytotoxicity of riboflavin/UVA treatment in vitro. Ophthalmic Res 35:324–328 DOI 74071
Spoerl E, Mrochen M, Sliney D, Trokel S, Seiler T (2007) Safety of UVA-riboflavin cross-linking of the cornea. Cornea 26:385–389. https://doi.org/10.1097/ICO.0b013e3180334f78
Mazzotta C, Balestrazzi A, Traversi C, Baiocchi S, Caporossi T, Tommasi C, Caporossi A (2007) Treatment of progressive keratoconus by riboflavin-UVA-induced cross-linking of corneal collagen: ultrastructural analysis by Heidelberg Retinal Tomograph II in vivo confocal microscopy in humans. Cornea 26:390–397. https://doi.org/10.1097/ICO.0b013e318030df5a
Hafezi F (2011) Limitation of collagen cross-linking with hypoosmolar riboflavin solution: failure in an extremely thin cornea. Cornea 30:917–919. https://doi.org/10.1097/ICO.0b013e31820143d1
Raiskup F, Spoerl E (2013) Corneal crosslinking with riboflavin and ultraviolet A. Part II. Clinical indications and results. Ocul Surf 11:93–108. https://doi.org/10.1016/j.jtos.2013.01.003
Rahman MR, Johnson GJ, Husain R, Howlader SA, Minassian DC (1998) Randomised trial of 0.2% chlorhexidine gluconate and 2.5% natamycin for fungal keratitis in Bangladesh. Br J Ophthalmol 82:919–925
Foster CS, Lass JH, Moran-Wallace K, Giovanoni R (1981) Ocular toxicity of topical antifungal agents. Arch Ophthalmol 99:1081–1084
Allan BD, Dart JK (1995) Strategies for the management of microbial keratitis. Br J Ophthalmol 79:777–786
Zloty P, Villavicencio O, Belin MW (2013) Aggressive debridement improves outcome of fungal keratitis. Asia Pac J Ophthalmol (Phila) 2:217–220. https://doi.org/10.1097/APO.0b013e3182993f4b
McGrath LA, Lee GA (2014) Techniques, indications and complications of corneal debridement. Surv Ophthalmol 59:47–63. https://doi.org/10.1016/j.survophthal.2013.03.004
Chan E, Snibson GR, Sullivan L (2014) Treatment of infectious keratitis with riboflavin and ultraviolet-A irradiation. J Cataract Refract Surg 40:1919–1925. https://doi.org/10.1016/j.jcrs.2014.09.001
Funding
This work was supported by grants from the National Natural Science Foundation of China (No. 81300759).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Anji Wei and Kaidi Wang are the co-first authors.
Rights and permissions
About this article
Cite this article
Wei, A., Wang, K., Wang, Y. et al. Evaluation of corneal cross-linking as adjuvant therapy for the management of fungal keratitis. Graefes Arch Clin Exp Ophthalmol 257, 1443–1452 (2019). https://doi.org/10.1007/s00417-019-04314-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-019-04314-1