Abstract
Background
To evaluate antifungal chemotherapy in patients with fungal keratitis guided by in vivo confocal microscopy.
Methods
A total of 121 patients (121 eyes) with fungal keratitis were enrolled in this study. Confocal microscopy was performed in real time after topical and/or oral antifungal chemotherapy. Hyphal density and morphology, composition of inflammatory cells, and appearance of corneal stromal cells at the central and peripheral corneal lesions were recorded. Antifungal therapy discontinued at 1 week after hyphae and inflammatory cells could not be detected, and affected corneal stromal cells became visible.
Results
Successful outcomes were achieved in 110 patients (90.9%). By confocal microscopy, we observed the gradual decrease of hyphae-positive sites and hyphal density during the chemotherapy. The inflammatory cells reduced in number and heterogeneity, while corneal stromal cells recovered. The antifungal drugs were tapered according to the changes in hyphae, inflammatory cells, and corneal stromal cells. There was no fungal recurrence during the 2-month follow-up period. The other 11 patients (9.1%) had deteriorated infection within 1 week of antifungal therapy, and therefore were subjected to corneal transplantation.
Conclusions
In vivo confocal microscopy appears to be an effective approach to guide antifungal chemotherapy. It allows comprehensive evaluation of hyphae, inflammatory cells, and corneal stromal cells in real time, and provides valuable and objective information required in selecting and adjusting therapeutic regimens for the treatment of fungal keratitis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fungus is one of the most common pathogens of infectious keratopathy in Asia and Africa [1, 2]. The incidence of fungal keratitis has increased in China during the past decade [3]. Owing to a great variety of fungal pathogens, complex clinical manifestations, and limited antifungal medications, fungal keratitis is often difficult to control, and in some cases results in blindness, especially when the therapy is delayed [4]. Fungal keratitis has become the leading cause of penetrating keratoplasty among infectious keratitis in China [5].
Theoretically, fungal infections can be treated successfully with intensive topical medication in the early stage [6]. However, effective medications are still limited for the treatment of fungal keratitis. In China, only fluconazole, amphotericin B, and natamycin can be used in patients [7–9]. Most antifungal agents available today are fungistatic, and require a long course of therapy. However, toxicity of antifungal drugs to human cells, and severe adverse reactions, make them inappropriate for frequent and prolonged administration [10]. No reliable means has been available to distinguish the exact stages of fungal infection. Therefore, an effective method is needed to monitor the process of fungal keratitis and to guide the drug administration.
Confocal microscopy is a relatively new and non-invasive technique for the examination of normal or diseased corneas [11]. It has frequently been used in the diagnosis and monitoring of fungal keratitis [12–14]. In this study, we used confocal microscopy to evaluate the therapeutic effects of antifungal chemotherapy, and to guide the administration of antifungal medications.
Patients and methods
Patients
A total of 121 patients (121 eyes) were selected for this study from 738 patients who were diagnosed with fungal keratitis at the Shandong Eye Institute between January 2001 and May 2006. The inclusion criteria were that the inflammatory infiltrates were within the anterior 2/3 of the corneal stroma in depth and no more than 5 mm in diameter. These selected patients received antifungal chemotherapy only. Mean duration of the disease before antifungal treatment was 9.6 ± 4.5 days (range 6–14 months). They were 75 men and 46 women with a mean age of 36.4 ± 15.1 years (range 8–77 years).
Diagnosis and clinical examination
Hyphae and infiltrating inflammatory cells were detected in all patients by in vivo confocal microscopy. The size, depth, and morphology of the infiltrates were examined by slit lamp biomicroscopy. Corneal scrapings were collected for smears and culture. Hyphae were confirmed by microcopy after the samples were treated with 10% potassium hydroxide.
Drug treatment guided by in vivo confocal microscopy
Antifungal treatment
In the patients diagnosed with fungal keratitis, 0.5% fluconazole eye drops were given every half an hour, combined with 0.25% amphotercin B or 5% natamycin every 2 hours. Meanwhile, itraconazole was taken orally once a day for no more than 21 days to reinforce the therapeutic effects.
Confocal microscopy examination
In vivo confocal microscopy (Confoscan 2.0, Nidek, Japan) was performed every week during the antifungal treatment, and a few more times after the completion of the medication. To ensure the fidelity of confocal microscopy, at least five different sites were chosen for examination, including the center of the ulcer as well as the superior, inferior, nasal, and temple sides of the infiltrates (Fig. 1). The density and morphology of the hyphae in corneal lesions, the number and configuration of inflammatory cells, and the appearance of corneal stromal cells in the central and peripheral corneal lesions were recorded.
Adjustment of antifungal treatment according to confocal microscopy
The status of fungal infections were evaluated, and the antifungal medications were adjusted as follows:
-
(1)
When the density of hyphae and inflammatory cells in corneal stroma decreased significantly, 0.5% fluconazole eye drops were tapered to once an hour, 0.25% amphotercin B and 5% natamycin to four times a day.
-
(2)
If the corneal epithelial defects were repaired, and hyphae disappeared, but inflammatory cells could still be detected by confocal microscopy, 0.1% diclofenac sodium eye drops were added four times a day.
-
(3)
If patients had a persistent epithelial defect, but neither hyphae nor inflammatory cells were detected by confocal microscopy, 0.25% amphotercin B and 5% natamycin discontinued, and recombinant human epidermal growth factor was added.
-
(4)
Once corneal epithelial defects were repaired, hyphae and inflammatory cells disappeared completely, and corneal stromal cells could be detected, 0.5% fluconazole eye drops were continued four times a day for an additional week.
If any patient was confirmed non-responsive to the drug treatment, and the infection worsened, corneal transplantation was performed to control the infection and preserve the vision. Such patients were withdrawn from the study.
Follow-up
All patients were followed up for 2 months after the drugs were withdrawn to ensure no recurrence of the fungal infection.
Results
Corneal scraping smears and cultures
Corneal scrapings were collected from 103 eyes. Eighty-five (82.5%) showed hyphae in 10% KOH wet mounts. With microbial cultures, fungus growth were observed in 64 eyes (62.1%), including Fusarium in 45 (70.3%), Aspergillus in seven (10.9%), Alternaria alternate in seven (10.9%), Penicillium in one (1.5%), and other fungal pathogens in four (6.2%).
Confocal microscopy examination
Before antifungal therapy, we found significant reflection at the center of corneal ulcers in all the patients by confocal microscopy. It was difficult to identify the tissue structure in most of them (Figs. 2 and 3). In a few cases, blur-shaped filaments could be seen. However, at the edge of the infiltrations, thin branching filaments which interlaced with each other could be identified clearly in the background. Some bright and obscure boundary inflammatory cells in various sizes could also be observed (Fig. 4a).
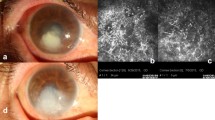
a Slit lamp microscopy shows an ulcer of fungal keratitis (4 mm × 5 mm), and branching hyphal infiltrate can be seen at the superior nasal side. b In vivo confocal microscopy shows a strong reflection at the center of the ulcer with a blurred border of filaments. At 1 week after antifungal treatment, the size of the infiltration is reduced (c); in vivo confocal microscopy shows a reduced reflection of the lesion and decreased filaments and inflammatory cells in number (d). At 2 weeks, the ulcer becomes smaller (e); hyphae and inflammatory cells in the center decrease in number (f). At 4 weeks, the corneal ulcer is healed, and new blood vessels grow into the cornea (g); trifle hyphae can be identified in the central infiltrate (h)
Slit lamp microscopic (a, c, e, g) and confocal microscopic (b, d, f, h) images of another fungal keratitis patient with antifungal chemotherapy. At 2 weeks after antifungal treatment, the hyphae disappear, but inflammatory cells remain visible (f). At 4 weeks, corneal ulcer is healed, and the cornea becomes transparent (g); there are few inflammatory cells but some corneal stromal cells (h)
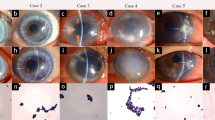
a Bright but opaque inflammatory cells in different sizes are identified in the fungal lesion. b Low reflecting white haze appear at 2 weeks after antifungal treatment. c, d The corneal epithelium looks glossy, and the corneal stromal cells are visible at 1 week after the completion of the antifungal treatment
At 1 week after antifungal treatment, 110 patients (90.5%) had significant improvement, with reduced infiltration area and oculus purulentus. Confocal microscopy showed that the reflection in the center of the corneal ulcers significantly weakened. Short filaments and inflammatory cells were visible in the ulcer center (Figs. 2 and 3). The density of hyphae decreased at the edge of the infiltration. Some hyphae began to break up into small pieces. The number of inflammatory cells reduced sharply.
The infections in the other 11 patients (9.1%) worsened during this period with increasing oculus purulentus. By confocal microscopy, an enhancement of the central reflection was observed, as well as an increased load of filaments and a growing number of inflammatory cells at the edge, which supported the justification for corneal transplantation.
In the improved 110 patients, all the ulcers became smaller and clear at 2 weeks. The corneal epithelium recovered in 44 patients (40%), which was confirmed by fluorescein staining. The corneal edema alleviated, the branching hyphal infiltrates reduced, and the satellite lesions and endothelial plaques disappeared, although moderate infiltrates remained within the superficial stroma. According to the repeated confocal microscopic examinations, no hyphae or infiltrating cells were detected in 46 patients (41.8%), but thick striate opacity was still visible (Fig. 4b). In the other patients, there remained a few hyphae and inflammatory cells, which became similar in size and clearer than before. Fewer hyphae and infiltrating cells were found at the border of the infiltrates (Figs. 2 and 3). The corneal stromal cells became visible in the background (Fig. 4c,d).
The corneal ulcers healed within a mean time of 22 ± 4.3 days. After 4 weeks of drug therapy, the corneal epithelium appeared glossy. The corneal edema, branching hyphal infiltrates, satellite lesions, and endothelial plaques were completely cleared away. According to the confocal microscopy, trifle hyphae could be identified in the central infiltrates in 12 patients (10.9%), with a few infiltrating cells in 20 (18.2%) (Figs. 2 and 3). At the border of the infiltration, no hyphae or infiltrating cells were observed, whereas corneal stromal cells could be identified. A low reflecting white haze appeared in the background. The 12 patients with hyphae in the central infiltrates received further antifungal medications for 1 more week, when the hyphae vanished completely.
At 1 week after the cease of antifungal treatment, the affected corneas became transparency in 32 patients (29.1%), and thick or light corneal nebula remained in the other 78 (70.9%) (Fig. 5). By confocal microscopy, corneal stromal cells were found at the recovered corneas, occasionally with light opacity. There was thick corneal haze in the stroma in patients with corneal nebula.
Prognosis
All the 110 patients were followed up for no less than 2 months, and no recurrence of fungal keratitis was detected.
Discussion
Fungal keratitis is a chronic, smoldering, and intractable infection, sometimes requiring a long course of medical therapy [15]. What are the ideal criteria for judging the antifungal therapy, recovery of corneal epithelium, retraction of corneal lesion, or resolution of inflammation?
Previously, antifungal chemotherapy was always prolonged for thorough cure of fungal infection, when confocal microscopy worked only as a tool to find hyphae in the cornea lesion and to confirm the diagnosis. With a period of antifungal treatment, the corneal epithelial defects can recover, but the newly formed corneal epithelium usually has edema and opacity, which may be attributed to the remained corneal infiltrates and hyphae or toxicity of antifungal medicine. If it is caused by the former, further antifungal therapy is needed. Otherwise, there might be a high risk of recurrence. On the other hand, if the reason is drug toxicity, antifungal agents must be reduced or discontinued to allow the epithelium to recover. However, it is difficult to distinguish these two potential reasons merely by slit lamp. Cornea scrapings may destroy the newly formed epithelium, delay the healing of corneal ulcers, and even cause secondary infections. Moreover, the positive rate is rather low, for there might remain very few hyphae. Therefore, a more sensitive and reasonable approach is required.
Confocal microscopy is a noninvasive technique for examining corneal injury and disease at the cellular level [16, 17], particularly in cases with deep seated infiltrations for which a routine microbiology test does not yield positive results [18]. It has proved to be more sensitive than culture in the diagnosis of experimentally induced Aspergillus fumigatus keratitis in rabbits [19]. In this study, the confocal microscopy made it possible to perform dynamic studies in real time. We performed multi-point and repeated confocal microscopy during the drug therapy for the treatment of fungal keratitis. To improve the detection rate, we first checked the corneal lesions for necrosis tissue by slit lamp microscopy and made a mark at the point mostly likely with hyphae, before confocal microscopy was employed. In patients with not too many hyphae and inflammatory cells, even if corneal epithelial defects had completely recovered, further antifungal treatment was given. If the patients had corneal epithelial edema and opacity, but no hyphae or inflammatory cells, we attributed it to the toxic action of antifungal medicine and reduced the dosage. In some patients, hyphae and infiltrating cells disappeared, and stromal cells could be detected, which indicates that the fungal infection was successfully treated, and the antifungal agents could be withdrawn. After 2 months of follow-up, no fungal keratitis recurred in any of these patients. This proves that the confocal microscopy is accurate and reliable in guiding the drug therapy.
However, confocal microscopy may not be useful in all patients with fungal keratitis. During the process of antifungal therapy, especially at the late stage, low levels of hyphae are difficult to identify by confocal microscopy. Therefore, in the current study, hyphae, inflammatory cells, and corneal stromal cells were considered together to make a comprehensive judgment.
Indeed, changes in hyphae, inflammatory cells, and corneal stromal cells could be identified by confocal microscopy. Before antifungal treatment, the center of corneal ulcers was filled with necrotic tissues, which presented a fierce reflection, with no clear tissue structure. The tissue images could be identified only after the infections were partially controlled. The images were more evident in the border of infiltrations than in the center. The hyphae and inflammatory cells could be detected, which is crucial for judging the improvement of fungal infections. Therefore, at least five points were selected at the center and border of the infiltrations to ensure reliable results in our study. By histopathological examination, neutrophils were found to be dominant among the inflammatory cells in fungal lesions. Plasmocytes and monocytes, bigger in size, could also be observed [20]. Thus, we conclude that the bigger cells detected by confocal microscopy were plasmocytes and monocytes, and the smaller ones were neutrophils. This explains the heterogeneous cell images in the early stage. After a period of effective antifungal treatment, plasmocytes and monocytes disappeared. That is why the cell images became uniform after the therapy. Therefore, morphological alterations of inflammatory cells can reflect the development of fungal keratitis to some extent. Fibroblast-like corneal stromal cells can transform into highly reflective myofibroblasts at the reparation phase of the inflammation [21, 22].
In summary, corneal ulcer healing does not mean a complete control of fungal keratitis. Sometimes, corneal epithelial edema and opacity are caused by the toxicity of antifungal agents, but not the hyphae. In vivo confocal microscopy appears to be a non-invasive, rapid, and effective approach to guide antifungal chemotherapy. It allows comprehensive evaluation of hyphae, inflammatory cells, and corneal stromal cells in real time, and provides valuable and objective information required in selecting and adjusting therapeutic regimens for the treatment of fungal keratitis.
References
Srinivasan M, Gonzales CA, George C, Cevallos V, Mascarenhas JM, Asokan B, Wilkins J, Smolin G, Whitcher JP (1997) Epidemiology and aetiological diagnosis of corneal ulceration in Madurai, south India. Br J Ophthalmol 81:965–971
Leck AK, Thomas PA, Hagan M, Kaliamurthy J, Ackuaku E, John M, Newman MJ, Codjoe FS, Opintan JA, Kalavathy CM, Essuman V, Jesudasan CA, Johnson GJ (2002) Aetiology of suppurative corneal ulcers in Ghana and south India, and epidemiology of fungal keratitis. Br J Ophthalmol 86:1211–1215
Xie L, Dong X, Shi W (2001) Treatment of fungal keratitis by penetrating keratoplasty. Br J Ophthalmol 85:1070–1074
Dursun D, Fernandez V, Miller D, Alfonso EC (2003) Advanced fusarium keratitis progressing to endophthalmitis. Cornea 22:300–303
Xie L, Zhong W, Shi W, Sun S (2006) Spectrum of fungal keratitis in north China. Ophthalmology 113:1943–1948
Srinivasan M (2004) Fungal keratitis. Curr Opin Ophthalmol 15:321–327
Ganegoda N, Rao SK (2004) Antifungal therapy for keratomycoses. Expert Opin Pharmacother 5:865–874
Kalavathy CM, Parmar P, Kaliamurthy J, Philip VR, Ramalingam MD, Jesudasan CA, Thomas PA (2005) Comparison of topical itraconazole 1% with topical natamycin 5% for the treatment of filamentous fungal keratitis. Cornea 24:449–452
Thomas PA (2003) Fungal infections of the cornea. Eye 17:852–862
Thomas PA (2003) Current perspectives on ophthalmic mycoses. Clin Microbiol Rev 16:730–797
Chiou AG, Kaufman SC, Kaufman HE, Beuerman RW (2006) Clinical corneal confocal microscopy. Surv Ophthalmol 51:482–500
Florakis GJ, Moazami G, Schubert H, Koester CJ, Auran JD (1997) Scanning slit confocal microscopy of fungal keratitis. Arch Ophthalmol 115:1461–1463
Kaufman SC, Musch DC, Belin MW, Cohen EJ, Meisler DM, Reinhart WJ, Udell IJ, Van Meter WS (2004) Confocal microscopy: a report by the American Academy of Ophthalmology. Ophthalmology 111:396–406
Brasnu E, Bourcier T, Dupas B, Degorge S, Rodallec T, Laroche L, Borderie V, Baudouin C (2007) In vivo confocal microscopy in fungal keratitis. Br J Ophthalmol 91:588–591
Khanal B, Deb M, Panda A, Sethi HS (2005) Laboratory diagnosis in ulcerative keratitis. Ophthalmic Res 37:123–127
Cheng LL, Young AL, Wong AK, Law RW, Lam DS (2004) In vivo confocal microscopy of Thygeson’s superficial punctate keratitis. Clin Experiment Ophthalmol 32:325–327
Jalbert I, Stapleton F, Papas E, Sweeney DF, Coroneo M (2003) In vivo confocal microscopy of the human cornea. Br J Ophthalmol 87:225–236
Vaddavalli PK, Garg P, Sharma S, Thomas R, Rao GN (2006) Confocal microscopy for Nocardia keratitis. Ophthalmology 113:1645–1650
Avunduk AM, Beuerman RW, Varnell ED, Kaufman HE (2003) Confocal microscopy of Aspergillus fumigatus keratitis. Br J Ophthalmol 87:409–410
Fons A, Garcia-de-Lomas J, Nogueira JM, Buesa FJ, Gimeno C (1988) Histopathology of experimental Aspergillus fumigatus keratitis. Mycopathologia 101:129–131
Moilanen JA, Vesaluoma MH, Muller LJ, Tervo TM (2003) Long-term corneal morphology after PRK by in vivo confocal microscopy. Invest Ophthalmol Vis Sci 44:1064–1069
Patel S, McLaren J, Hodge D, Bourne W (2001) Normal human keratocyte density and corneal thickness measurement by using confocal microscopy in vivo. Invest Ophthalmol Vis Sci 42:333–339
Acknowledgements
This study was supported in part by the National Natural Science Foundation of China (30630063, 30271394), Department of Science and Technology of Shandong Province (2004GG2202154), and Qingdao Municipal Science and Technology Bureau (02KGYSH-01). The authors thank Ms. Ping Lin for her editorial assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shi, W., Li, S., Liu, M. et al. Antifungal chemotherapy for fungal keratitis guided by in vivo confocal microscopy. Graefes Arch Clin Exp Ophthalmol 246, 581–586 (2008). https://doi.org/10.1007/s00417-007-0719-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-007-0719-x