Abstract
Background
The cerebellum is a predilection site of pathology in progressive multiple sclerosis (PMS) patients, contributing to cognitive deficits. Aim of this study was to investigate lobular cerebellar functional connectivity (FC) in PMS patients in relation to cognition.
Methods
In this cross-sectional study, resting state fMRI analysis was carried out on 29 PMS patients (11 males, mean age 51.2 ± 11.9 years) and 22 age- and sex-matched healthy controls (HC) (11 males, mean age 49.6 ± 8.8 years). Data were analyzed with a seed-based approach, with four different seeds placed at the level of cerebellar Lobule VI, Crus I, Crus II and Lobule VIIb, accounting for cerebellar structural damage. Cognitive status was assessed with the BICAMS battery. Correlations between fMRI data and clinical variables were probed with the Spearman correlation coefficient.
Results
When testing FC differences between PMS and HC without taking into account cerebellar structural damage, PMS patients showed a reduction of FC between Crus II/Lobule VIIb and the right frontal pole (p = 0.001 and p = 0.002, respectively), with an increased FC between Lobule VIIb and the right precentral gyrus (p < 0.001). After controlling for structural damage, PMS patients still showed a reduced FC between Crus II and right frontal pole (p = 0.005), as well as an increased FC between Lobule VIIb and right precentral gyrus (p = 0.003), with the latter showing an inverse correlation with BVMT scores (r = − 0.393; p = 0.03).
Conclusion
PMS patients show cerebellar FC rearrangements that are partially independent from cerebellar structural damage, and are likely expression of a maladaptive functional rewiring.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The cerebellum is a complex structure, functionally integrated in several cognitive and behavioral circuits through substantial projections to cerebral association areas [1]. Although classifications of morphologic, phylogenetic and functional order can be applied to characterize the cerebellar structure [2], according to anatomical and functional studies two main domains can be topographically identified, with the anterior cerebellum involved in motor tasks and the posterior cerebellum responsible for cognitive functions [3]. An increasing body of evidence suggests that cerebellar damage impacts high order functions, contributing to cognitive dysfunctions in several psychiatric and neurological disorders [2, 4]. The cerebellum is a predilection site of pathology in patients with multiple sclerosis (MS) and contributes not only to the accumulation of physical disability [5] but also to the development of cognitive deficits in both the relapsing [6, 7] and progressive phase of the disease [7,8,9].
Resting-state functional MRI (RS-fMRI) is a non-invasive powerful tool that allows for the evaluation of functional connectivity (FC) between different brain regions when a subject is not performing any specific task. Previous RS-fMRI studies in MS patients have shown the presence of functional disruption in local [10] and long-range cerebellar FC not only within the cerebellar network [11,12,13] but also in the context of the sensory-motor and attentional/working memory networks [12, 14, 15]. Such FC rearrangements have been related to the presence of structural damage [10,11,12, 15, 16], and can be interpreted, according to the disease stage, as a sign of cortical plasticity and functional compensation, as a consequence of the exhaustion of such compensatory mechanisms or as a maladaptive rewiring of resting state networks [11, 12, 15, 17]. Although it is clear that clinically eloquent modifications of cerebellar FC occur in the posterior cerebellar lobe of patients with MS [10, 12, 14, 15] and that damage of specific cerebellar lobules differentially contributes to cognitive disability in all disease phenotypes [6, 8, 18, 19], no data is available about FC abnormalities of individual cerebellar lobules and the potential impact on the cognitive status in MS. In the present study we focused on the investigation of patients with progressive MS (PMS), more likely to experience cognitive deficits and cerebellar damage, to further understand the physiopathological bases of cognitive deficits in MS. Therefore, the aims of our study were: (i) to investigate cerebellar lobular FC taking into account the presence of cerebellar structural damage, and (ii) to test the relationship between FC rearrangements and cognitive performance.
Materials and methods
Participants
Thirty-five patients with PMS (mean age 50.7 ± 11.6 years; range 29–72 years; M/F:13/22) were prospectively enrolled at the University of Naples “Federico II” from October 2016 to February 2017 along with 22 healthy controls (HC) of comparable age and sex (mean age 49.6 ± 8.8 years; range 35–69 years; M/F:11/11).
All patients enrolled had to fulfill the following inclusion criteria: age between 18 and 75 years, MS diagnosis according to the revised McDonald criteria [20] with a progressive phenotype [21] and an EDSS score ≤ 7.0. In particular, patients were classified as primary progressive (PP) when showing a clinical progression over a period of at least 1 year without prior exacerbations, while they were classified as secondary progressive (SP) when showing a clinical progression (also confirmed over a period of at least 1 year) after a conversion from a relapsing-remitting (RR) course. According to the same criteria [21], disease activity and progression over the year preceding the study visit were also evaluated. Exclusion criteria of the present study were: presence of other systemic conditions, alcohol or drug addiction, prior history of head trauma, clinical diagnosis of psychiatric disorders, and, for patients, clinical diagnosis of any neurological disorder other than MS.
Within 1 week from the MRI scan all patients underwent neuropsychological and clinical examination with evaluation of the Expanded Disability Status Scale (EDSS). The Brief International Cognitive Assessment of Multiple Sclerosis (BICAMS) battery, which includes the following tests: Symbol Digit Modalities Test (SDMT), Brief Visuospatial Memory Test—Revised (BVMT) and California Verbal Learning Test-II (CVLT), was also administered. Neuropsychological raw scores were then converted in z-scores according to [22], and used in the subsequent analyses.
The study was conducted in compliance with the ethical standard and approved by the “Carlo Romano” ethics committee for biomedical activities of the University of Naples “Federico II”. Written informed consent was obtained from all subjects according to the Declaration of Helsinki.
MRI data acquisition
All images were acquired on the same 3T MRI scanner (Siemens Trio, Siemens Medical Systems, Erlangen, Germany). The following sequences were acquired: a T2-weighted sequence (40 axial slices, TE = 63.0 ms, TR = 4500 ms, voxel size = 1.0 × 1.0 × 4.0 mm3) used for the quantification of the lesion volumes, a volumetric magnetization prepared rapid gradient-echo T1-weighted sequence (224 sagittal slices, TE = 2.47 ms, TR = 3000 ms, TI = 1000 ms, voxel size = 1.0 × 1.0 × 1.0 mm3) used for the volumetric analyses and a T2*-weighted echo-planar imaging sequence (30 axial slices, TE = 40 ms TR = 2500 ms, voxel size = 3 × 3 × 4 mm3, gap 1 mm, 200 time points) for the RS-fMRI analysis.
During the BOLD acquisition, subjects were asked to relax with eyes closed, without falling asleep, while laying supine in the MR scan with their head lightly fixed by straps and foam pads to minimize any possible head movement.
MRI data processing
T2-weighted hyperintense lesions were segmented for all MS patients by experienced observers using a semi-automatic segmentation technique (Jim 7, Xinapse Systems Ltd, Northants, UK), and both entire brain (LV) and the cerebellar (CLV) lesion volumes (according to [8]) were derived.
Cerebellar volumes were calculated on lesion-filled 3D T1-weighted images using the Spatially Unbiased Infratentorial Toolbox (SUIT) version 3.2, implemented in Statistical Parametric Mapping 12 (SPM12) (http://www.fil.ion.ucl.ac.uk/spm). A complete description of the method applied to obtain cerebellar volumes is available in [8]. For each subject, posterior cerebellar volumes were computed as the sum of their hemispheric and vermian portions for the following lobules: VI, Crus I, Crus II and VIIb [8], given their central role in cognition [3]. In addition, using the segmentation tool of SPM12, gray matter (GM), white matter (WM) and cerebrospinal fluid (CSF) volumes were obtained, and for each subject intracranial volume (ICV) was computed as their sum for normalization purposes.
Preprocessing of RS-fMRI was conducted as described in [23, 24] using the FC toolbox CONN (McGovern Institute for Brain Research, Massachusetts Institute of Technology, Cambridge; http://www.nitrc.org/projects/conn), which contains libraries for fMRI analysis based on SPM. Briefly, the following pre-processing steps were applied: removal of the first five time points, motion correction, slice timing correction, temporal despiking with a hyperbolic tangent squashing function followed by band-pass filtering (for f between 0.008 and 0.09 Hz) and a spatial smoothing using a 6-mm Gaussian kernel. From the motion correction procedure a computation of the mean displacement of the brain voxels was obtained as the root-mean-square (RMS) of the translations along the three axes [25], and those studies showing a mean relative RMS of 0.20 mm or higher, or with more than 2.0 mm displacement along or 2.0 degrees rotation around any axis were discarded from the analysis. In addition, a “scrubbing” procedure was applied to those time points (along with the preceding and the two following ones) showing a framewise differential of signal intensity > 9 z-values, to further suppress the effect of patient movements.
Data sets were then normalized to the standard Montreal Neurological Institute (MNI) EPI template and resampled to a voxel size of 2 × 2 × 2 mm3. To evaluate the overall accuracy of the processing, the quality of the images was assessed by an experienced operator (MI).
For each subject, BOLD signal time course was calculated for lobules VI, Crus I, Crus II and VIIb using the Harvard-Oxford Atlas available in CONN [26], and the correlation map of the BOLD signal across the brain was generated, including in a General Linear Model (GLM) the time courses of WM and CSF signals, and the six parameters (translations and rotations along the X, Y and Z axes) of spatial transformation, as derived from the coregistration step.
Statistical analysis
Student’s t test was used to test group differences in terms of age, while χ2 test was used to test possible differences in terms of gender, with a significance level set for p < 0.05.
Group differences in terms of cerebellar volumes were tested via multivariate Generalized Linear Model (GLM), including ICV as a covariate of no interest to account for head size, with a significance level of p < 0.01, Bonferroni-corrected for multiple comparisons (0.05/5, as the number of tested variables, namely the total cerebellar and lobules VI, Crus I, Crus II and VIIb volumes). Differences in terms of FC maps were also probed voxel-wise over the whole brain using a GLM, including as confounding covariates age, sex and the average motion, to remove potential residual movement effects. This second-level analysis was also carried out including cerebellar structural metrics (namely, CLV and cerebellar lobule volumes) as additional confounding variables in the GLM, to test the impact of these metrics on cerebellar FC.
For all RS-fMRI analyses, both contrasts (HC > MS and HC < MS) were probed, and differences were considered significant for p < 0.0125 (0.05/4 as the number of tested seeds), corrected for the family-wise error at the cluster level.
To explore all possible relationships between cerebro-cerebellar abnormalities in FC and cognition, when significant differences emerged for the tested seeds after controlling for specific cerebellar atrophy and CLV, the first eigenvariate of each significant cluster was extracted in SPM12, corrected for age, sex, and RMS, and entered in a nonparametric correlation analysis with all available cognitive scores using the Spearman coefficient, with results considered significant for p < 0.05.
Analyses were carried out using Statistical Package for Social Science (SPSS Inc, v. 20.0, Chicago, Ill).
Results
The HC and MS groups were no different in terms of age (p = 0.68) and sex (p = 0.34). Of the 35 enrolled patients, 12 presented a PP course and 23 a SP course, with 10 subjects showing activity and 8 subjects showing disease progression in the year preceding the study visit.
Due to excessive movements during the RS-fMRI acquisition, six MS patients were excluded and a final population of 29 PMS patients was considered in all subsequent analyses. In the remaining 51 RS-fMRI datasets (29 PMS patients and 22 HC), no difference in terms of RMS was present between MS patients and HC (0.043 ± 0.036 vs 0.057 ± 0.044 in HC and MS respectively, p = 0.22).
Compared to HC, MS patients showed a decrease of total cerebellar volume (p = 0.001), as well as a decrease of all investigated cerebellar lobules (p ≤ 0.005).
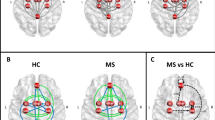
When testing FC of cerebellar lobules without taking into account cerebellar structural damage, MS patients showed a cluster of reduced FC between Crus II and right frontal pole (p = 0.001) and a similar cluster of reduced FC between Lobule VIIb and the right frontal pole (p = 0.002). In addition, MS patients showed increased FC between Lobule VIIb and a cluster located at the level of the right precentral gyrus, partly extending to the ipsilateral superior frontal gyrus (p < 0.001; Fig. 1a).
Results of the RS-fMRI analysis without taking into account (a) and after controlling (b) for cerebellar structural damage. Clusters of significant FC decrease in MS patients compared to HCs are shown in red, while clusters of significant FC increase are presented in blue. All results are superimposed on a standard 3D rendering of a brain volume in the Montreal Neurological Institute space (top–bottom: frontal view, lateral right view and upper view). RS-fMRI resting-state functional MRI, MS multiple sclerosis, HC healthy controls
When testing cerebellar lobular FC taking into account metrics of cerebellar structural damage, the FC between Crus II and right frontal pole (p = 0.005) remained significantly decreased, although to a lesser extent. Similarly, the cluster of increased FC between Lobule VIIb and right precentral gyrus was still significant (p = 0.003; Fig. 1b). No significant differences emerged for the other tested lobules.
Finally, when testing for possible relationship between clinical scores and clusters of altered FC between cerebellum and cortex, we found an inverse correlation between the cluster of increased FC in MS at the level of the right precentral gyrus and BVMT scores (r = − 0.393; p = 0.03; Fig. 2).
Scatter plot of the correlation between clinical data and the cluster of increased functional connectivity in MS patients compared to HC (r = − 0.393; p = 0.03) between cerebellar Lobule VIIb and the right PCG (extending to the ipsilateral SFG). Linear fit (middle line) and 95%individual CIs (dashed lines) are shown. BVMT z-scores were calculated according to [22]. MS multiple sclerosis, HC healthy controls, PCG precentral gyrus, SFG superior frontal gyrus, BVMT brief visuospatial memory test
No other significant correlation emerged between the cluster of reduced FC at the level of the right frontal pole and the other clinical variables.
A list of demographic and clinical information of MS patients and HC analyzed in this study is reported in Table 1, while the results of the volumetric analysis are provided in Table 2 and results of the RS-fMRI analysis are showed in Table 3 and Fig. 1.
Discussion
Here, for the first time, we explored whole-brain FC of the individual cerebellar lobules involved in cognitive functions in PMS patients, demonstrating a functional rearrangement of the physiological connections between posterior cerebellum, frontal pole and areas involved in the task-positive and salience network [27,28,29]. Such reconfiguration of cerebellar FC seems to be somehow independent from cerebellar structural damage, as suggested by the results of our two-step analysis. This finding is not completely surprising, considering that structural damage occurring in different regions of the cerebellum-cortex circuits might play a different role in cerebellar FC rewiring. Specifically, in our population, the reduction in FC between lobule VIIb and the frontal pole is partially accounted for by cerebellar structural damage, while the decreased FC between Crus II and the frontal pole is still evident after correction for cerebellar atrophy and lesion volume. The latter finding might also be related to a lower degree of atrophy affecting Crus II, in line with the results of a recent work that showed a relative lower volume loss in Crus II of PMS patients compared with HCs [8].
Our results partially confirm and expand recent findings about dentate nucleus connectivity in relapsing-remitting and pediatric MS [11, 13]. In both populations, increased cerebellar FC with cortical frontoparietal regions characterized MS patients in comparison with controls [11, 13]. Since such FC increase was directly correlated with better clinical performance, it was interpreted as a compensatory adaptation. In our study, the presence of both increased and decreased cerebellar FC in patients compared to HC might reflect the more advanced disease stage of our population, and the consequence of a more diffuse and severe structural damage and/or the exhaustion of reserve capacity.
It has been recently suggested that cerebellar function shows similarities across different processing domains, with function prediction and error-based learning constituting the common mechanisms at the basis of cerebellar involvement in both motor and cognitive control [30]. In this functional frame, the posterior cerebellum participates in complex cognitive processes by means of partially overlapping connection to prefrontal and posterior-parietal cortex [28]. In particular, lobule VI and VIIb are involved in the selection of pertinent stimuli through their interaction with the salience network, while Crus I and Crus II are mostly involved in executive control, verbal working memory and language function, together with lobule VI. Furthermore, lobule VI is also involved in visuospatial processing, while lobule VIIb additionally participates in attention-demanding tasks and social cognition [29, 30]. Against this background, the inverse relationship we identified between increased FC in precentral gyrus and BVMT scores could be interpreted as an example of maladaptive functional reorganization, or, alternatively, could represent an attempt to maintain adequate cognitive performances, by increasing the connections between posterior cerebellum and areas involved in stimulus selection and level of attention [29]. Interestingly, the lack of other correlations between cerebellar FC changes and cognitive scores suggests that cerebellar connectivity impairment, although clinically relevant, it is not the only factor driving the development of cognitive impairment in PMS, but it has to be considered in a wider scenario where reconfiguration of different circuits controlling cognition, along with volume loss, contribute to the clinical expression of cognitive deficits in MS [8, 14,15,16].
Although all efforts have been made to account for the impact of atrophy and focal lesions on cerebellar FC, a limitation of the present study is the lack cerebellar damage assessment at microstructural level. A further limitation can be found in the small number of subjects falling into each group when categorizing patients according to the disease course (PP/SP), activity (active/inactive) and progression status (progressive/stable), which prevented us from conducting any subgroup comparison. For these reasons, further explorations are warranted in future studies.
In conclusion, our study shows that FC rearrangements occur in PMS patients, somehow independently from cerebellar macroscopic structural damage and that they are likely to be an expression of a maladaptive functional rearrangement rather than a compensatory mechanism. Taken together, these results further expand the current knowledge of cerebellar damage in MS, confirming its role in the complex pathophysiology of cognitive deficits.
References
Buckner RL (2013) The cerebellum and cognitive function: 25 years of insight from anatomy and neuroimaging. Neuron 80(3):807–815. https://doi.org/10.1016/j.neuron.2013.10.044
Mormina E, Petracca M, Bommarito G, Piaggio N, Cocozza S, Inglese M (2017) Cerebellum and neurodegenerative diseases: beyond conventional magnetic resonance imaging. World J Radiol 9(10):371–388. https://doi.org/10.4329/wjr.v9.i10.371
Stoodley CJ, Schmahmann JD (2010) Evidence for topographic organization in the cerebellum of motor control versus cognitive and affective processing. Cortex 46(7):831–844. https://doi.org/10.1016/j.cortex.2009.11.008
Koziol LF, Budding D, Andreasen N, D’Arrigo S, Bulgheroni S, Imamizu H, Ito M, Manto M, Marvel C, Parker K, Pezzulo G, Ramnani N, Riva D, Schmahmann J, Vandervert L, Yamazaki T (2014) Consensus paper: the cerebellum’s role in movement and cognition. Cerebellum 13(1):151–177. https://doi.org/10.1007/s12311-013-0511-x
Inglese M, Petracca M, Mormina E, Achiron A, Straus-Farber R, Miron S, Fabian M, Krieger S, Miller A, Lublin F, Sormani MP (2017) Cerebellar volume as imaging outcome in progressive multiple sclerosis. PLoS One 12(4):e0176519. https://doi.org/10.1371/journal.pone.0176519
Moroso A, Ruet A, Lamargue-Hamel D, Munsch F, Deloire M, Coupe P, Ouallet JC, Planche V, Moscufo N, Meier DS, Tourdias T, Guttmann CR, Dousset V, Brochet B (2017) Posterior lobules of the cerebellum and information processing speed at various stages of multiple sclerosis. J Neurol Neurosurg Psychiatry 88(2):146–151. https://doi.org/10.1136/jnnp-2016-313867
Weier K, Penner IK, Magon S, Amann M, Naegelin Y, Andelova M, Derfuss T, Stippich C, Radue EW, Kappos L, Sprenger T (2014) Cerebellar abnormalities contribute to disability including cognitive impairment in multiple sclerosis. PLoS One 9(1):e86916. https://doi.org/10.1371/journal.pone.0086916
Cocozza S, Petracca M, Mormina E, Buyukturkoglu K, Podranski K, Heinig MM, Pontillo G, Russo C, Tedeschi E, Russo CV, Costabile T, Lanzillo R, Harel A, Klineova S, Miller A, Brunetti A, Morra VB, Lublin F, Inglese M (2017) Cerebellar lobule atrophy and disability in progressive MS. J Neurol Neurosurg Psychiatry 88(12):1065–1072. https://doi.org/10.1136/jnnp-2017-316448
D’Ambrosio A, Pagani E, Riccitelli GC, Colombo B, Rodegher M, Falini A, Comi G, Filippi M, Rocca MA (2017) Cerebellar contribution to motor and cognitive performance in multiple sclerosis: an MRI sub-regional volumetric analysis. Mult Scler 23(9):1194–1203. https://doi.org/10.1177/1352458516674567
Dogonowski AM, Andersen KW, Madsen KH, Sorensen PS, Paulson OB, Blinkenberg M, Siebner HR (2014) Multiple sclerosis impairs regional functional connectivity in the cerebellum. Neuroimage Clin 4:130–138. https://doi.org/10.1016/j.nicl.2013.11.005
Cirillo S, Rocca MA, Ghezzi A, Valsasina P, Moiola L, Veggiotti P, Amato MP, Comi G, Falini A, Filippi M (2016) Abnormal cerebellar functional MRI connectivity in patients with paediatric multiple sclerosis. Mult Scler 22(3):292–301. https://doi.org/10.1177/1352458515592191
Rocca MA, Valsasina P, Leavitt VM, Rodegher M, Radaelli M, Riccitelli GC, Martinelli V, Martinelli-Boneschi F, Falini A, Comi G, Filippi M (2017) Functional network connectivity abnormalities in multiple sclerosis: correlations with disability and cognitive impairment. Mult Scler. https://doi.org/10.1177/1352458517699875
Sbardella E, Upadhyay N, Tona F, Prosperini L, De Giglio L, Petsas N, Pozzilli C, Pantano P (2017) Dentate nucleus connectivity in adult patients with multiple sclerosis: functional changes at rest and correlation with clinical features. Mult Scler 23(4):546–555. https://doi.org/10.1177/1352458516657438
Loitfelder M, Filippi M, Rocca M, Valsasina P, Ropele S, Jehna M, Fuchs S, Schmidt R, Neuper C, Fazekas F, Enzinger C (2012) Abnormalities of resting state functional connectivity are related to sustained attention deficits in MS. PLoS One 7(8):e42862. https://doi.org/10.1371/journal.pone.0042862
Petracca M, Saiote C, Bender HA, Arias F, Farrell C, Magioncalda P, Martino M, Miller A, Northoff G, Lublin F, Inglese M (2017) Synchronization and variability imbalance underlie cognitive impairment in primary-progressive multiple sclerosis. Sci Rep 7:46411. https://doi.org/10.1038/srep46411
Rocca MA, Bonnet MC, Meani A, Valsasina P, Colombo B, Comi G, Filippi M (2012) Differential cerebellar functional interactions during an interference task across multiple sclerosis phenotypes. Radiology 265(3):864–873. https://doi.org/10.1148/radiol.12120216
Schoonheim MM, Meijer KA, Geurts JJ (2015) Network collapse and cognitive impairment in multiple sclerosis. Front Neurol 6:82. https://doi.org/10.3389/fneur.2015.00082
Grothe M, Lotze M, Langner S, Dressel A (2017) Impairments in walking ability, dexterity, and cognitive function in multiple sclerosis are associated with different regional cerebellar gray matter loss. Cerebellum 16(5–6):945–950. https://doi.org/10.1007/s12311-017-0871-8
Moroso A, Ruet A, Lamargue-Hamel D, Munsch F, Deloire M, Coupe P, Charre-Morin J, Saubusse A, Ouallet JC, Planche V, Tourdias T, Dousset V, Brochet B (2017) Microstructural analyses of the posterior cerebellar lobules in relapsing-onset multiple sclerosis and their implication in cognitive impairment. PLoS One 12(8):e0182479. https://doi.org/10.1371/journal.pone.0182479
Polman CH, Reingold SC, Banwell B, Clanet M, Cohen JA, Filippi M, Fujihara K, Havrdova E, Hutchinson M, Kappos L, Lublin FD, Montalban X, O’Connor P, Sandberg-Wollheim M, Thompson AJ, Waubant E, Weinshenker B, Wolinsky JS (2011) Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 69(2):292–302. https://doi.org/10.1002/ana.22366
Lublin FD, Reingold SC, Cohen JA, Cutter GR, Sorensen PS, Thompson AJ, Wolinsky JS, Balcer LJ, Banwell B, Barkhof F, Bebo B Jr, Calabresi PA, Clanet M, Comi G, Fox RJ, Freedman MS, Goodman AD, Inglese M, Kappos L, Kieseier BC, Lincoln JA, Lubetzki C, Miller AE, Montalban X, O’Connor PW, Petkau J, Pozzilli C, Rudick RA, Sormani MP, Stuve O, Waubant E, Polman CH (2014) Defining the clinical course of multiple sclerosis: the 2013 revisions. Neurology 83(3):278–286. https://doi.org/10.1212/WNL.0000000000000560
Goretti B, Niccolai C, Hakiki B, Sturchio A, Falautano M, Minacapelli E, Martinelli V, Incerti C, Nocentini U, Murgia M, Fenu G, Cocco E, Marrosu MG, Garofalo E, Ambra FI, Maddestra M, Consalvo M, Viterbo RG, Trojano M, Losignore NA, Zimatore GB, Pietrolongo E, Lugaresi A, Langdon D, Portaccio E, Amato MP (2014) The Brief International Cognitive Assessment for Multiple Sclerosis (BICAMS): normative values with gender, age and education corrections in the Italian population. BMC Neurol 14:171. https://doi.org/10.1186/s12883-014-0171-6
Cocozza S, Pisani A, Olivo G, Sacca F, Ugga L, Riccio E, Migliaccio S, Brescia Morra V, Brunetti A, Quarantelli M, Tedeschi E (2017) Alterations of functional connectivity of the motor cortex in Fabry disease: an RS-fMRI study. Neurology 88(19):1822–1829. https://doi.org/10.1212/WNL.0000000000003913
Cocozza S, Pontillo G, Quarantelli M, Sacca F, Riccio E, Costabile T, Olivo G, Brescia Morra V, Pisani A, Brunetti A, Tedeschi E (2018) Default mode network modifications in Fabry disease: a resting-state fMRI study with structural correlations. Hum Brain Mapp. https://doi.org/10.1002/hbm.23949
Van Dijk KR, Sabuncu MR, Buckner RL (2012) The influence of head motion on intrinsic functional connectivity MRI. Neuroimage 59(1):431–438. https://doi.org/10.1016/j.neuroimage.2011.07.044
Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ (2006) An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 31(3):968–980. https://doi.org/10.1016/j.neuroimage.2006.01.021
Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME (2005) The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A 102(27):9673–9678. https://doi.org/10.1073/pnas.0504136102
O’Reilly JX, Beckmann CF, Tomassini V, Ramnani N, Johansen-Berg H (2010) Distinct and overlapping functional zones in the cerebellum defined by resting state functional connectivity. Cereb Cortex 20(4):953–965. https://doi.org/10.1093/cercor/bhp157
Sang L, Qin W, Liu Y, Han W, Zhang Y, Jiang T, Yu C (2012) Resting-state functional connectivity of the vermal and hemispheric subregions of the cerebellum with both the cerebral cortical networks and subcortical structures. Neuroimage 61(4):1213–1225. https://doi.org/10.1016/j.neuroimage.2012.04.011
Sokolov AA, Miall RC, Ivry RB (2017) the cerebellum: adaptive prediction for movement and cognition. Trends Cogn Sci 21(5):313–332. https://doi.org/10.1016/j.tics.2017.02.005
Acknowledgements
This study was supported in part by grant NMSS (RG 5120A3/1) to MI.
Funding
SC reports fees for speaking from Genzyme. ET received fees for speaking from Scientific Press, ArsEducandi and Shire Italy. RL personal fees from Merck Serono, Biogen, Novartis, Almirall, Genzyme and TEVA. VBM reports personal fees from Novartis, Biogen, Genzyme, TEVA, Almirall, Bayer and Merck. MI grants from Novartis Pharmaceuticals, National Multiple Sclerosis Society, Noto Foundation, NIH and TEVA Neuroscience. This study was supported in part by grant NMSS (RG 5120A3/1) to MI.
Author information
Authors and Affiliations
Contributions
SC Study conception and design; Analysis and interpretation. GP Acquisition of data; Analysis and interpretation. CR Acquisition of data; Analysis and interpretation. CVR Acquisition of data; Analysis and interpretation. TC Acquisition of data; Analysis and interpretation. AP Acquisition of data; Analysis and interpretation. ET Acquisition of data; Critical Revision. RL Acquisition of data; Critical Revision. VBM Analysis and interpretation; Critical Revision. AB Critical Revision; Study Supervision. MI Study conception and design; Critical Revision; Study Supervision. MP Study conception and design; Critical Revision; Study Supervision.
Corresponding author
Ethics declarations
Conflicts of interest
All authors declare no conflict of interest.
Ethical approval
The study was conducted in compliance with ethical standard and approved by the “Carlo Romano” ethics committee for biomedical activities of the University of Naples “Federico II”.
Informed consent
Written informed consent was obtained from all subjects according to the Declaration of Helsinki.
Rights and permissions
About this article
Cite this article
Cocozza, S., Pontillo, G., Russo, C. et al. Cerebellum and cognition in progressive MS patients: functional changes beyond atrophy?. J Neurol 265, 2260–2266 (2018). https://doi.org/10.1007/s00415-018-8985-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-018-8985-6