Abstract
The Papez circuit is central to memory and emotional processes. However, little is known about its involvement in multiple sclerosis (MS). We aimed to investigate abnormalities of resting state (RS) effective connectivity (EC) between regions of the Papez circuit in MS and their relationship with cognitive performances. Sixty-two MS patients and 64 healthy controls (HC) underwent neuropsychological assessment, 3D T1-weighted, and RS functional MRI. RS EC analysis was performed using SPM12 and dynamic causal modeling. RS EC abnormalities were investigated using parametric empirical Bayes models and were correlated with cognitive scores. Compared to HC, MS patients showed (posterior probability > 0.95) higher EC between the right entorhinal cortex and right subiculum, and lower EC from the anterior cingulate cortex (ACC) to the posterior cingulate cortex (PCC), from left to right subiculum, from left anterior thalamus to ACC, and within ACC and PCC. Lower RS EC from the ACC to the PCC correlated with worse global cognitive scores (rho = 0.19; p = 0.03), worse visuospatial memory (rho = 0.19; p = 0.03) and worse semantic fluency (rho = 0.21; p = 0.02). Lower RS EC from the left to the right subiculum correlated with worse verbal memory (rho = 0.20; p = 0.02), lower RS EC within the ACC correlated with worse attention (rho = −0.19; p = 0.04) and more severe brain atrophy (rho = −0.26; p = 0.003). Higher EC from the right entorhinal cortex to right subiculum correlated with worse semantic fluency (rho = 0.21; p = 0.02). In conclusion, MS patients showed altered RS EC within the Papez circuit. Abnormal RS EC involving cingulate cortices and hippocampal formation contributed to explain cognitive deficits.
Similar content being viewed by others
Introduction
Cognitive impairment (CI) is common among multiple sclerosis (MS) patients, with a prevalence ranging from 35 to 91% [1]. Visual and verbal memory impairment is one of the most relevant cognitive symptoms in MS and occurs from the earliest stages of the disease [2], along with information processing speed (IPS) deficits [3].
The Papez circuit, first described by James Papez in 1937, is composed of interconnected cortical and subcortical structures and comprises, in each brain hemisphere: the hippocampus, the anterior thalamic nucleus, the mammillary body, and the cingulate cortex, originally included in the “grand lobe limbique” described by Paul Broca in 1878. A growing number of structures have been subsequently described as part of the limbic system, contributing mainly to emotional processes [4]. However, the Papez circuit has been continuously regarded as central to memory and cognitive functions [5].
Most of regions participating to the Papez circuit are hit by MS. For instance, the posterior cingulate cortex (PCC) and thalamus are among the first regions becoming atrophic in MS [5], and they are both relevant for cognition [6]. Hippocampal involvement is also evident from several studies [5]. Thalamic and hippocampal damage in MS derives both from direct effects of demyelination and damage to regions connected to these structures [5].
Resting state (RS) functional MRI (fMRI) has been extensively used to understand functional reorganization and correlates of cognitive performance in MS. RS fMRI studies highlighted the relevant role of the cingulate cortex [7], hippocampus [5], and thalamus [8] in explaining cognitive deficits in this condition. However, little interest has been given to investigate the whole Papez circuit.
The large majority of RS fMRI investigations in MS has analyzed functional connectivity (FC), namely the temporal association between spatially distinct, but functionally related brain regions [9]. By definition, RS FC analysis describes functional associations between regions (or networks), without investigating causal relationships between them. Conversely, effective connectivity (EC) aims at assessing directed causal influences that the activity of one region exerts on another one [10]. Dynamic causal modelling (DCM) is a framework proposed by Friston et al [11]. to build EC models among brain regions using fMRI data. Using this technique, which was applicable only to task-based fMRI data in its original formulation, EC abnormalities have been found in MS patients both during motor [12] and cognitive [13] fMRI tasks. The recent extension of DCM for cross-spectral densities [14] allowed to study causal connections also in task-free fMRI data, thus permitting a reliable EC assessment on RS fMRI data. Spectral DCM has been validated [15] and successfully applied to the study of different pathological conditions [16,17,18,19]. Therefore, the application of EC to a priori-defined networks of interest, such as the Papez circuit, is appealing, since it may help to identify MS-related connectivity abnormalities explaining global and circuit-specific cognitive deficits.
In this study, we wished to identify RS EC abnormalities within the Papez circuit in MS patients and to explore their correlation with cognitive deficits. To do this, we administered the Rao’s Brief Repeatable Battery of Neuropsychological Tests (BRB-N) and performed RS EC analysis by using spectral DCM. RS EC differences between groups were investigated using parametric empirical Bayes models (PEB) and correlated with global and domain-specific BRB-N z-scores.
Materials and methods
Ethic committee approval
The local ethical standards committee on human experimentation approved the study (IRCCS Ospedale San Raffaele, Milan, Italy). Written informed consent was obtained from all participants.
Subjects
We consecutively enrolled 62 MS patients [20] and 64 healthy controls (HC) satisfying the following inclusion criteria: right handedness (Edinburgh Handedness Inventory pre-MS condition ≥33); no history of neurological (apart MS), orthopedic, or medical disorders; no psychiatric or severe mood disorders; no concomitant therapy with antidepressants, baclofen, psychoactive, or steroids; and no drug or alcohol abuse. In addition, MS-patients had to have absence of relapses and steroid treatment in the three months preceding study enrollment; stable treatment for MS for at least six months.
Clinical assessment
On the day of MRI acquisition, all study subjects underwent a complete neurological examination with Expanded Disability Status Scale (EDSS) score rating [21], and an extensive cognitive assessment using tests included in the BRB-N [22]. In particular, we used the Selective Reminding Test to assess verbal memory, the 10/36 Spatial Recall Test to assess visual memory, the Symbol Digit Modalities Test to assess IPS, the Paced Auditory Serial Addition Test 2” and 3” to assess attention, and the Word List Generation Test to assess verbal semantic fluency. Scores in each of the cognitive tests were normalized for age, sex, and education and transformed into z-scores, according to Italian normative values [23]. A global cognition z-score was obtained with a two-step approach: first, we averaged z-scores of tests belonging to the previous cognitive domains into domain z-scores; then, we averaged domain z-scores, obtaining the global cognition z-score [24]. For descriptive purpose, CI was defined as a score of 1.5 standard deviations below mean normative values in two or more tests of the BRB-N [25].
MRI acquisition
Using a 3.0 Tesla Philips Ingenia scanner (Philips Medical Systems, Eindhoven, The Netherlands), the following sequences of the brain were acquired from all subjects during a single session: (1) T2*-weighted single-shot echo planar imaging RS fMRI (repetition time [TR]/ echo time [TE] = 1560/35 ms; flip angle = 70°; matrix size = 96 × 96; field of view = 240 mm2; simultaneous multi-slice factor = 2; 320 sets of 48 axial slices, parallel to the anterior commissure-posterior commissure plane, with a thickness of 3 mm; duration = 8 min and 20 s); (2) 3D fluid-attenuated inversion recovery (FLAIR) turbo spin echo (TR/TE/inversion time [TI] = 4800/268/1650 ms; flip angle = variable; matrix size = 256 × 256; field of view = 256 × 256 mm2; echo train length [ETL] = 167; 192 contiguous sagittal slices, 1 mm thick); (3) 3D T2-weighted turbo spin echo (TR = 2500/330 ms; flip angle = variable; matrix size = 256 × 256; field of view = 256 × 256 mm2; ETL = 117; 192 contiguous sagittal slices, 1 mm thick); (4) 3D T1-weighted turbo field echo (TR/TE/TI = 7/3.2/1000 ms; flip angle = 8°; matrix size = 256 × 256; field of view = 256 × 256 mm2; 204 contiguous sagittal slices, 1 mm thick). During RS fMRI, subjects were instructed to keep their eyes closed, to remain motionless and not to think anything in particular. All subjects reported that they had not fallen asleep during scanning, according to a questionnaire delivered immediately after the MRI session.
RS fMRI pre-processing
RS fMRI data were pre-processed using the CONN toolbox (https://web.conn-toolbox.org/) and SPM12 (http://www.fil.ion.ucl.ac.uk/spm/software/spm12/) [26]. RS fMRI images were realigned to the mean of each session with a six-degree rigid-body transformation to correct for minor head movements. After rigid registration of realigned images to the lesion-filled 3D T1-weighted scan, RS fMRI images were normalized to the Montreal Neurological Institute (MNI) template using a standard affine transformation followed by non-linear warping. After detection of movement outliers (using the ART toolbox), images were smoothed with a 6 mm3 Gaussian filter. The five principal components derived from white matter and cerebrospinal fluid estimated with the anatomical component-based noise correction method (aCompCor) [27], and motion parameters with their first temporal derivatives were regressed out from RS fMRI time series as nuisance covariates. Outliers detected by the ART toolbox (if any) and spurious effects from the first two timepoints (to maximize magnetic equilibrium) were also regressed out from data. Finally, images were linearly detrended and band-pass filtered (0.01–0.1 Hz).
Structural MRI analysis
To calculate T2-hyperintense lesion volume (LV), focal lesions were identified using a fully automated approach using 3D FLAIR and T1-weighted sequences as input images [28]. On 3D T1-weighted lesion-filled images, normalized brain volume (NBV) was calculated using the FSL SIENAX software.
RS EC analysis
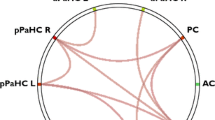
After RS fMRI pre-processing, RS EC analysis was performed by means of spectral DCM, as implemented in SPM12 (http://www.fil.ion.ucl.ac.uk/spm/software/spm12/) [14]. The model representing the Papez circuit (regions and connections among regions selected a priori, as shown in Fig. 1A) was constructed based on hypotheses derived from previous literature [4, 29] and was built by placing spheres (radius = 6 mm) in the following regions of interest (ROI): left and right entorhinal cortex (MNI coordinates: ±26, −1, −33) [30], left and right hippocampus, subiculum (MNI coordinates: ±27, −18, −16) [31], left and right anterior thalamic nuclei (MNI coordinates: ±4, −1, 0) [32]. For the anterior cingulate cortex (ACC) and PCC, bilateral masks of these two regions were built using the WFU PickAtlas toolbox (http://fmri.wfubmc.edu/software/PickAtlas), by forming a union mask of left and right ACC, and of left and right PCC, respectively. The first eigenvariate of RS fMRI time series was extracted from all these ROIs, after applying to time series a grey matter mask in the standard MNI space (available in SPM12 software) thresholded at 0.40, to avoid spurious contributions from white matter and cerebrospinal fluid. Intrinsic EC strengths between selected pairs of brain regions were then estimated using spectral DCM, as described in details elsewhere [14, 33]. Percentage variance explained by the models was calculated to evaluate goodness of model fitting.
A Model representing the Papez circuit. Connections supposed to be present in the model (and considered for estimation of effective connectivity strengths) are shown as grey arrows and divided in: (i) connections starting from the anterior cingulate and posterior cingulate cortex; (ii) connections starting from the left hemisphere; (iii) connections starting from the right hemisphere. B Dynamic causal model showing the results of the within-group analysis of effective connectivity. Within-group connections that resulted significant at the parametric empirical bayes analysis in healthy controls and patients with multiple sclerosis, separately, are shown as green arrows (for excitatory connections) and blue arrows (for inhibitory connections). C Dynamic causal model showing the results of the between-group analysis of effective connectivity. Significant between-groups differences of effective connectivity between controls and MS patients are reported as continuous arrows (for increased connections) and dashed arrows (for decreased connections); effect sizes are reported close to each arrow. MS multiple sclerosis; HC healthy controls; L left; R right; ACC anterior cingulate cortex; EC entorhinal cortex; aThal anterior thalami; Sub subiculum; PCC posterior cingulate cortex.
Statistical analysis
Brain T2 LV was square root-transformed. Demographic, clinical, neuropsychological, and structural MRI variables were compared between MS patients and HC using two-sample t-tests, Chi-square, Mann–Whitney tests, or MANCOVA models adjusted for age and sex, as appropriate.
Second-level analysis on DCM parameters was performed using PEB [34]. Unlike classical statistical models, PEB uses not only mean estimates of EC parameters from each subject, but also their estimated covariance (i.e., uncertainty) for statistical purposes. This methodology included Bayesian model reduction [35], in which Bayesian inversion was performed and reduced models were compared with the fully-connected model. In other words, connections between the eight nodes belonging to the Papez circuit were switched on and off in an iterative process to test their effect on free energy, which approximates log model evidence [36]. Parameters not contributing to the free energy were switched off by setting their prior mean and variance to zero. The parameters of the best 256 models from this iterative search procedure were then averaged, weighted by their model evidence (Bayesian Model Averaging) [37]. This methodology allowed us to determine: (a) the mean EC strength across subjects (group mean for HC and MS patients, separately), and (b) between-group difference in EC strengths between HC and MS. Results were reported in terms of effect size (ES) and posterior probability (Pp). Only effects having a Pp > 0 .95 were considered.
In MS patients, Spearman’s rank univariate correlations were run to assess correlations of clinical, BRB-N z-scores, and structural MRI variables with abnormal EC connections.
Results
Demographic, clinical, neuropsychological, and structural MRI characteristics
Table 1 summarizes the main demographic, clinical, neuropsychological, and structural MRI characteristics of the subjects. Twenty-eight (45%) of 62 MS patients had CI.
RS EC analysis: mean EC in each study group
Percentage variance explained by models was 44.2% (interquartile range = 35.4–52.5%), suggesting a good model inversion performance for each study subject. Figure 1B summarizes RS EC connections significantly present at PEB analysis in the Papez circuit in HC and MS patients, separately (Pp > 0.95). EC connections were interpreted as excitatory if the association coefficient estimated by DCM was positive, while they were interpreted as inhibitory if the association coefficient estimated by DCM was negative.
The Papez circuit was characterized by the presence, in both HC and MS patients, of strong evidence of inhibitory EC (Pp > 0.95): (1) within-regions (as imposed by default by spectral DCM for convergence of EC estimation); and (2) from the right entorhinal cortex to the ACC, PCC and left subiculum. Both groups also presented evidence of excitatory EC (Pp > 0.95): (1) from the ACC to the right anterior thalamus; (2) from the left entorhinal cortex to the right entorhinal cortex and vice versa, and (3) from the left subiculum to the right subiculum and vice versa.
HC also exhibited strong evidence of inhibitory EC (Pp > 0.95): (1) from the left entorhinal cortex to the left anterior thalamus, PCC and right subiculum; (2) from the right entorhinal cortex to the right anterior thalamus; (3) from the left thalamus to ACC and PCC; and (4) from the right anterior thalamus to the right subiculum. Finally, HC presented excitatory EC (Pp > 0.95) from the right anterior thalamus to the PCC.
MS patients exhibited excitatory EC connections (Pp > 0.95): (1) from the ACC to the left anterior thalamus; (2) from the left and right subiculum to the ACC, and (3) from the right anterior thalamus to the ACC; and (4) from the right subiculum to the right entorhinal cortex. MS also presented evidence of inhibitory EC (Pp > 0.95): (1) from the left entorhinal cortex to the ACC; (2) from the PCC to the left anterior thalamus; and (3) from the right entorhinal cortex to the right subiculum.
RS EC analysis: between-group comparison
The results of the comparison between HC and MS patients, performed using PEB, are shown in Fig. 1C. Within the Papez circuit, MS patients showed higher inhibitory EC vs HC (Pp > 0.95) from the right entorhinal cortex to the right subiculum (ES = 0.076) and higher excitatory EC from the right subiculum to the right entorhinal cortex (ES = −0.049).
MS patients also showed lower inhibitory EC within the ACC (ES = −0.131) and PCC (ES = −0.112), as well as from the left anterior thalamus to the ACC (ES = −0.063). Finally, they showed lower excitatory EC from the ACC to the PCC (ES = 0.101) and from the left to the right subiculum (ES = 0.054).
Correlation analysis
Results of correlation analysis of RS EC abnormalities with cognitive z-scores and structural MRI measures in MS patients are summarized in Table 2.
A lower RS EC from the ACC to the PCC was correlated with worse global cognitive scores (rho = 0.19; p = 0.03), worse visuospatial memory (rho = 0.19; p = 0.03) and worse semantic fluency (rho = 0.21; p = 0.02). Moreover, a lower RS EC from the left to the right subiculum was correlated with worse verbal memory scores (rho = 0.20; p = 0.02), and a lower inhibitory RS EC within the ACC was correlated with worse attention scores (rho = −0.19; p = 0.04) and with lower NBV (rho = −0.26; p = 0.003).
Finally, higher inhibitory EC from the right entorhinal cortex to the right subiculum was associated with worse semantic fluency scores (rho = 0.21, p = 0.02).
No correlation was found between RS EC abnormalities within the Papez circuit and IPS, EDSS, disease duration, and T2-hyperintense LV.
Discussion
In this cross-sectional study, we implemented a RS EC analysis to investigate causal connectivity abnormalities of the Papez circuit in MS patients, a pioneering investigation topic in this condition. Then, we explored the correlation of these abnormalities with cognitive performance. We selected such an analysis because EC models of RS fMRI data, built with spectral DCM, are able to assess the causal influence of a given brain area on another one. As a consequence, they are particularly useful to investigate causal connectivity between definite sets of regions, such as the Papez circuit, and their reliability has been demonstrated in estimating causal connections also in absence of external stimulations during fMRI acquisition [14, 15].
The Papez circuit has been historically regarded as vital for memory and emotional processing. Although a role of this network in emotion is still recognized, its relevance to learning abilities and episodic memory has been demonstrated in the last decades [29]. While the Papez circuit was thought to be a one-way circular network linearly linking the hippocampus, mammillary bodies, anterior thalamic nuclei, cingulate cortex, and parahippocampal region, today there is growing knowledge of reciprocal connections between some of its structures, many of them non-linearly connecting different regions of the circuit [4, 29]. Moreover, structures belonging to the Papez circuit are embedded into wider cerebral networks, so their activity and possible dysfunction appear to be relevant for the entire functioning of the brain, potentially impacting on a broad range of cognitive functions. In summary, the Papez circuit is likely to be more complex than originally thought, and studies analysing its role in MS are likely to be useful to explain some of the cognitive manifestations of this condition.
In line with previous literature, the prevalence of CI in our patients was 45% [1]. As expected, given the prevalence of CI in our patients and the role of the Papez circuit for cognition, we found abnormalities in RS EC of the Papez circuit in MS patients compared to HC. First, our analysis revealed a lower RS EC within ACC and PCC in the Papez circuit of MS patients vs HC. Both ACC and PCC are functional hubs of several RS networks and they belong to the default mode network. The default mode network is strongly affected by MS, showing a reduced average network FC [38]. The reduction in RS FC of its anterior component, the ACC, whose activity is associated with alertness and attention [39], was identified as a key biomarker of the accumulation of cognitive deficits in progressive MS patients [39]. The PCC is also a critical hub of the human connectome and its high blood flow and oxygen consumption probably makes it more vulnerable to pathology [40]. In addition, a lower inhibitory EC within the ACC and PCC might be explained by their proximity to the corpus callosum, which is consistently affected by MS [41].
We also found a reduced excitatory RS EC from the ACC to the PCC in MS patients compared to HC. This abnormality is likely to reflect damage to long-range white matter tracts connecting these areas, mainly the cingulum [41]. Indeed, long-range white matter tracts are known to be consistently more affected than short-range connections by lesions and microstructural degeneration in MS [42]. The structural integrity of these long-range connections is hypothesized to subserve higher cognitive functions, which require global integration in the form of cortico-cortical connections [42], and to influence brain functional connectivity.
The loss of inhibitory RS EC from the right anterior thalamus to the ACC in MS patients vs HC is of special interest, particularly considering that thalamic RS FC is generally increased in MS patients, as documented by several studies [8, 43]. However, this is not the first study showing increased synchronous FC between thalamus and ACC, but at the same time decreased thalamo-frontal EC [43]. Taken together, these two findings suggests a loss of efficacy in the thalamic modulation on the ACC in MS patients: despite the increased synchronous activation of these two regions, the ability of the thalamus to modulate functional activity of the ACC is lower in MS patients than in HC.
The RS EC from the left to the right subiculum, found to be lower in MS patients compared to HC, is in line with previous research reporting reduced FC of the left hippocampus with its interhemispheric homologue in MS patients without memory disturbances [44], suggesting that dysregulation of hippocampal activity is relevant in these patients and could even precede memory impairment.
Finally, we found an increased RS EC between the right subiculum and the ipsilateral entorhinal cortex in MS patients compared to HC. Different patterns of fMRI abnormalities of the hippocampus are reported in the literature and they are documented even before the development of measurable structural damage [5]. In particular, an increased RS FC of the hippocampus has been observed in MS patients with cognitive dysfunctions [45], while cognitively impaired patients displayed less functional activation during task-based acquisition [45, 46]. The abnormalities of the hippocampal formation’s EC found in our study are confirmative of the presence of functional dysregulation of this region in MS.
The analysis of correlation showed that a lower EC within the ACC was related with worse attentive functions in MS patients. This result was expected, given the known relevance of cortical frontal regions for executive abilities [47]. Interestingly, lower EC within ACC also correlated with brain atrophy. These correlations suggest a complex interplay between functional dysregulation in core hubs, neurodegeneration and cognitive symptoms in MS.
Then, we found that a lower RS EC strength from the ACC to the PCC in MS patients correlated with lower global cognition, lower visuospatial memory, and lower semantic fluency. This result reinforces the notion that impaired long-range EC is crucial to explain cognitive dysfunction in MS [42]. The correlation with global cognitive performance supports the known importance of this connection for higher cognitive functions [48]. Several neuroimaging studies also reported the relevance of fronto-parietal areas for episodic memory and executive functions, with a prominent role of ACC activation in verbal fluency tasks [49].
In MS patients, reduced excitatory RS EC from the left to the right subiculum correlated with lower verbal memory domain score. This result is in line with a vast literature identifying the hippocampi and their interhemispheric functional connections as core mechanism for the encoding and recollection of new memories even in healthy subjects [50], and with the previously discussed evidence confirming the dysregulation of hippocampus functional connectivity in MS [44].
Finally, we found a correlation between higher inhibitory RS EC from the right entorhinal cortex to the right subiculum and lower semantic fluency score in MS patients. Although the involvement of the right hemisphere in a verbal task seems to be counterintuitive, recent evidence reports a crucial functional involvement of both the left and the right hippocampus in semantic fluency tasks [51]. Thus, our results suggest a non-lateralized contribution of the hippocampal formation to the semantic fluency ability.
Our study is not without limitations. First, even if mammillary bodies were described as part of the Papez circuit by anatomical studies [4, 29], they have not been included in our RS FC model: this subthalamic region is particularly small, and its functional contribution to EC is therefore difficult to disentangle from systematic signal contamination from cerebrospinal fluid. Second, even if we applied a grey matter mask when extracting RS fMRI time series from our regions of interest, we cannot fully exclude some spurious contribution from non-grey matter tissue, especially in the subiculum, which is a small hippocampal region that might be not always fully discernible on our scans. Third, while our models explained a non-trivial amount of variance of RS fMRI data—namely, 44.2% on average—one might ask why this was not higher, particularly given that the data were analysed in the frequency domain (i.e., the RS fMRI time series were summarized by their complex cross-spectra, increasing efficiency). A potential explanation was our focus on subcortical structures, which typically enable a lower signal-to-noise ratio (SNR) than cortical structures in RS fMRI. In particular, it is well-known that the hippocampus is particularly susceptible to distortion from air/tissue and bone/tissue interfaces, which would likely have reduced SNR in the entorhinal cortex and the subiculum. To evaluate this hypothesis, future RS fMRI studies of the Papez circuit may consider scanning for longer to increase SNR, and test whether MRI sequences optimized for imaging the hippocampus can increase the variance explained [52]. Fourth, although we focused only on Papez circuit regions, these structures are embedded in the larger limbic system [4] and many of them, as discussed, are part of more extended functional and structural networks. It is therefore possible that their relevance for cognition is not limited to their reciprocal causal connections. Fifth, connections between regions of the Papez circuit were defined as excitatory and inhibitory purely basing on the signs of EC associations derived from DCM estimation. A study of cortical excitability using electrophysiological techniques is needed for a correct interpretation of these findings. Sixth, strength of correlations between abnormal EC connections and neuropsychological scores was not particularly high. However, this is an expected result, since cognitive performance in MS is likely to depend also from several other brain regions that were not included in the present analysis. Finally, future work is required to investigate structural MRI damage, both regarding cortical and subcortical atrophy and white matter tract integrity, which could underlie abnormalities of causal functional connections identified in our results.
To conclude, this was one of the first works unveiling abnormal causal connectivity among brain hubs of the Papez circuit in MS patients, correlated in part with performance at cognitive tasks often affected by MS. In particular, the dysregulation of the cingulate cortices and the hippocampal formation shows a clear correlation with global cognition, attention, verbal and visuospatial memory and semantic fluency.
References
Ruano L, Portaccio E, Goretti B, Niccolai C, Severo M, Patti F, et al. Age and disability drive cognitive impairment in multiple sclerosis across disease subtypes. Mult Scler J. 2017;23:1258–67.
Feuillet L, Reuter F, Audoin B, Malikova I, Barrau K, Cherif AA, et al. Early cognitive impairment in patients with clinically isolated syndrome suggestive of multiple sclerosis. Mult Scler. 2007;13:124–7.
Benedict RHB, Amato MP, DeLuca J, Geurts JJG. Cognitive impairment in multiple sclerosis: clinical management, MRI, and therapeutic avenues. Lancet Neurol. 2020;19:860–71.
Catani M, Dell’acqua F, Thiebaut de Schotten M. A revised limbic system model for memory, emotion, and behaviour. Neurosci Biobehav Rev. 2013;37:1724–37.
Eshaghi A, Marinescu RV, Young AL, Firth NC, Prados F, Jorge Cardoso M, et al. Progression of regional grey matter atrophy in multiple sclerosis. Brain 2018;141:1665–77.
Bisecco A, Capuano R, Caiazzo G, d’Ambrosio A, Docimo R, Cirillo M, et al. Regional changes in thalamic shape and volume are related to cognitive performance in multiple sclerosis. Mult Scler. 2021;27:134–8.
Parisi L, Rocca MA, Valsasina P, Panicari L, Mattioli F, Filippi M. Cognitive rehabilitation correlates with the functional connectivity of the anterior cingulate cortex in patients with multiple sclerosis. Brain Imaging Behav. 2014;8:387–93.
Schoonheim MM, Hulst HE, Brandt RB, Strik M, Wink AM, Uitdehaag BM, et al. Thalamus structure and function determine severity of cognitive impairment in multiple sclerosis. Neurology 2015;84:776–83.
Rocca MA, De Meo E, Filippi M. Functional MRI in investigating cognitive impairment in multiple sclerosis. Acta Neurol Scand. 2016;134:39–46.
Stephan KE, Friston KJ. Analyzing effective connectivity with functional magnetic resonance imaging. Wiley Interdiscip Rev Cogn Sci. 2010;1:446–59.
Friston KJ, Harrison L, Penny W. Dynamic causal modelling. Neuroimage 2003;19:1273–302.
Rocca MA, Absinta M, Valsasina P, Ciccarelli O, Marino S, Rovira A, et al. Abnormal connectivity of the sensorimotor network in patients with MS: a multicenter fMRI study. Hum Brain Mapp. 2009;30:2412–25.
Rocca MA, Valsasina P, Ceccarelli A, Absinta M, Ghezzi A, Riccitelli G, et al. Structural and functional MRI correlates of Stroop control in benign MS. Hum Brain Mapp. 2009;30:276–90.
Friston KJ, Kahan J, Biswal B, Razi AA. DCM for resting state fMRI. Neuroimage 2014;94:396–407.
Razi A, Kahan J, Rees G, Friston KJ. Construct validation of a DCM for resting state fMRI. Neuroimage 2015;106:1–14.
Benhamou E, Marshall CR, Russell LL, Hardy CJD, Bond RL, Sivasathiaseelan H, et al. The neurophysiological architecture of semantic dementia: spectral dynamic causal modelling of a neurodegenerative proteinopathy. Sci Rep. 2020;10:16321.
Fridgeirsson EA, Figee M, Luigjes J, van den Munckhof P, Schuurman PR, van Wingen G, et al. Deep brain stimulation modulates directional limbic connectivity in obsessive-compulsive disorder. Brain. 2020;143:1603–12.
Ray D, Bezmaternykh D, Mel’nikov M, Friston KJ, Das M. Altered effective connectivity in sensorimotor cortices is a signature of severity and clinical course in depression. Proc Natl Acad Sci USA. 2021;118:e2105730118.
Uscatescu LC, Kronbichler L, Stelzig-Scholer R, Pearce BG, Said-Yurekli S, Reich LA, et al. Effective connectivity of the hippocampus can differentiate patients with schizophrenia from healthy controls: a spectral DCM approach. Brain Topogr. 2021;34:762–78.
Thompson AJ, Banwell BL, Barkhof F, Carroll WM, Coetzee T, Comi G, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018;17:162–73.
Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–9.
Rao S A manual for the Brief Repeatable Battery of Neuropsychological Tests in multiple sclerosis. In: Wisconsin MCo, editor. Milwaukee, WI 1990.
Amato MP, Portaccio E, Goretti B, Zipoli V, Ricchiuti L, De Caro MF, et al. The Rao’s Brief Repeatable Battery and Stroop Test: normative values with age, education and gender corrections in an Italian population. Mult Scler. 2006;12:787–93.
Sepulcre J, Vanotti S, Hernandez R, Sandoval G, Caceres F, Garcea O, et al. Cognitive impairment in patients with multiple sclerosis using the Brief Repeatable Battery-Neuropsychology test. Mult Scler. 2006;12:187–95.
Amato MP, Morra VB, Falautano M, Ghezzi A, Goretti B, Patti F, et al. Cognitive assessment in multiple sclerosis-an Italian consensus. Neurol Sci. 2018;39:1317–24.
Behzadi Y, Restom K, Liau J, Liu TT. A component-based noise correction method (CompCor) for BOLD and perfusion-based fMRI. Neuroimage 2007;37:90–101.
Whitfield-Gabrieli S, Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012;2:125–41.
Valverde S, Cabezas M, Roura E, Gonzalez-Villa S, Pareto D, Vilanova JC, et al. Improving automated multiple sclerosis lesion segmentation with a cascaded 3D convolutional neural network approach. Neuroimage 2017;155:159–68.
Bubb EJ, Kinnavane L, Aggleton JP. Hippocampal–diencephalic–cingulate networks for memory and emotion: An anatomical guide. Brain Neurosci Adv. 2017;1:2398212817723443.
Konishi K, Joober R, Poirier J, MacDonald K, Chakravarty M, Patel R, et al. Healthy versus Entorhinal Cortical Atrophy identification in asymptomatic APOE4 carriers at risk for Alzheimer’s disease. J Alzheimers Dis. 2018;61:1493–507.
Robinson JL, Barron DS, Kirby LA, Bottenhorn KL, Hill AC, Murphy JE, et al. Neurofunctional topography of the human hippocampus. Hum Brain Mapp. 2015;36:5018–37.
Bergsland N, Zivadinov R, Dwyer MG, Weinstock-Guttman B, Benedict RH. Localized atrophy of the thalamus and slowed cognitive processing speed in MS patients. Mult Scler. 2016;22:1327–36.
Zeidman P, Jafarian A, Corbin N, Seghier ML, Razi A, Price CJ, et al. A guide to group effective connectivity analysis, part 1: First level analysis with DCM for fMRI. Neuroimage 2019;200:174–90.
Zeidman P, Jafarian A, Seghier ML, Litvak V, Cagnan H, Price CJ, et al. A guide to group effective connectivity analysis, part 2: Second level analysis with PEB. Neuroimage 2019;200:12–25.
Friston K, Penny W. Post hoc Bayesian model selection. Neuroimage 2011;56:2089–99.
Friston KJ, Litvak V, Oswal A, Razi A, Stephan KE, van Wijk BCM, et al. Bayesian model reduction and empirical Bayes for group (DCM) studies. Neuroimage 2016;128:413–31.
Penny WD, Stephan KE, Daunizeau J, Rosa MJ, Friston KJ, Schofield TM, et al. Comparing families of dynamic causal models. PLoS Comput Biol. 2010;6:e1000709.
Rocca MA, Valsasina P, Leavitt VM, Rodegher M, Radaelli M, Riccitelli GC, et al. Functional network connectivity abnormalities in multiple sclerosis: Correlations with disability and cognitive impairment. Mult Scler J. 2018;24:459–71.
Rocca MA, Valsasina P, Absinta M, Riccitelli G, Rodegher ME, Misci P, et al. Default-mode network dysfunction and cognitive impairment in progressive MS. Neurology 2010;74:1252–9.
Crossley NA, Mechelli A, Scott J, Carletti F, Fox PT, McGuire P, et al. The hubs of the human connectome are generally implicated in the anatomy of brain disorders (vol 137, 2382, 2014). Brain 2015;138:E374–E.
Storelli L, Pagani E, Preziosa P, Filippi M, Rocca MA. Measurement of white matter fiber-bundle cross-section in multiple sclerosis using diffusion-weighted imaging. Mult Scler J. 2021;27:818–26.
Meijer KA, Steenwijk MD, Douw L, Schoonheim MM, Geurts JJG. Long-range connections are more severe y damaged and relevant for cognition in multiple sclerosis. Brain 2020;143:150–60.
Hidalgo de la Cruz M, Valsasina P, Mesaros S, Meani A, Ivanovic J, Martinovic V, et al. Clinical predictivity of thalamic sub-regional connectivity in clinically isolated syndrome: a 7-year study. Mol Psychiatr. 2021;26:2163–74.
Roosendaal SD, Hulst HE, Vrenken H, Feenstra HEM, Castelijns JA, Pouwels PJW, et al. Structural and functional hippocampal changes in multiple sclerosis patients with intact memory function. Radiology 2010;255:595–604.
Hulst HE, Schoonheim MM, Van Geest Q, Uitdehaag BMJ, Barkhof F, Geurts JJG. Memory impairment in multiple sclerosis: Relevance of hippocampal activation and hippocampal connectivity. Mult Scler J. 2015;21:1705–12.
Hulst HE, Schoonheim MM, Roosendaal SD, Popescu V, Schweren LJS, van der Werf YD, et al. Functional adaptive changes within the hippocampal memory system of patients with multiple sclerosis. Hum Brain Mapp. 2012;33:2268–80.
Mansouri FA, Tanaka K, Buckley MJ. Conflict-induced behavioural adjustment: a clue to the executive functions of the prefrontal cortex. Nat Rev Neurosci. 2009;10:141–52.
Loitfelder M, Filippi M, Rocca M, Valsasina P, Ropele S, Jehna M, et al. Abnormalities of Resting State Functional Connectivity Are Related to Sustained Attention Deficits in MS. Plos One. 2012;7.
Wagner S, Sebastian A, Lieb K, Tuscher O, Tadic A. A coordinate-based ALE functional MRI meta-analysis of brain activation during verbal fluency tasks in healthy control subjects. BMC Neurosci. 2014;15:19.
Wang L, Negreira A, La Violette P, Bakkour A, Sperling RA, Dickerson BC. Intrinsic interhemispheric hippocampal functional connectivity predicts individual differences in memory performance ability. Hippocampus 2010;20:345–51.
Glikmann-Johnston Y, Oren N, Hendler T, Shapira-Lichter I. Distinct functional connectivity of the hippocampus during semantic and phonemic fluency. Neuropsychologia 2015;69:39–49.
Weiskopf N, Hutton C, Josephs O, Deichmann R. Optimal EPI parameters for reduction of susceptibility-induced BOLD sensitivity losses: A whole-brain analysis at 3 T and 1.5 T. NeuroImage 2006;33:493–504.
Author information
Authors and Affiliations
Contributions
OM and RB contributed to the conception of the study, acquisition, analysis, and interpretation of data, drafting the text, and preparing the figures. PV contributed to the acquisition, analysis, and interpretation of MRI data and revising the manuscript. MAR and MF contributed to the conception of the study, drafting and revising the text, acting as the study supervisors. All the authors gave their approval to the current version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
OM and RB have nothing to disclose. PV received speaker honoraria from Biogen Idec. MAR received speaker honoraria from Bayer, Biogen, Bristol Myers Squibb, Celgene, Genzyme, Merck Serono, Novartis, Roche, and Teva, and receives research support from the MS Society of Canada and Fondazione Italiana Sclerosi Multipla. MF is Editor-in-Chief of the Journal of Neurology, Associate Editor of Human Brain Mapping, Associate Editor of Radiology, and Associate Editor of Neurological Sciences; received compensation for consulting services and/or speaking activities from Alexion, Almirall, Bayer, Biogen, Celgene, Eli Lilly, Genzyme, Merck-Serono, Novartis, Roche, Sanofi, Takeda, and Teva Pharmaceutical Industries; and receives research support from Biogen Idec, Merck-Serono, Novartis, Roche, Teva Pharmaceutical Industries, Italian Ministry of Health, Fondazione Italiana Sclerosi Multipla, and ARiSLA (Fondazione Italiana di Ricerca per la SLA).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Marchesi, O., Bonacchi, R., Valsasina, P. et al. Resting state effective connectivity abnormalities of the Papez circuit and cognitive performance in multiple sclerosis. Mol Psychiatry 27, 3913–3919 (2022). https://doi.org/10.1038/s41380-022-01625-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41380-022-01625-4
- Springer Nature Limited