Abstract
Cognitive disorders occur in up to 65 % of multiple sclerosis (MS) patients; they have been correlated with different MRI measures of brain tissue damage, whole and regional brain atrophy. The hippocampal involvement has been poorly investigated in cognitively impaired (CI) MS patients. The objective of this study is to analyze and compare brain tissue abnormalities, including hippocampal atrophy, in relapsing–remitting MS (RRMS) patients with and without cognitive deficits, and to investigate their role in determining cognitive impairment in MS. Forty-six RRMS patients [20 CI and 26 cognitively preserved (CP)] and 25 age, sex and education-matched healthy controls (HCs) underwent neuropsychological evaluation and 3-Tesla anatomical MRI. T2 lesion load (T2-LL) was computed with a semiautomatic method, gray matter volume and white matter volume were estimated using SIENAX. Hippocampal volume (HV) was obtained by manual segmentation. Brain tissues volumes were compared among groups and correlated with cognitive performances. Compared to HCs, RRMS patients had significant atrophy of WM, GM, left and right Hippocampus (p < 0.001). Compared to CP, CI RRMS patients showed higher T2-LL (p = 0.02) and WM atrophy (p = 0.01). In the whole RRMS group, several cognitive tests correlated with brain tissue abnormalities (T2-LL, WM and GM atrophy); only verbal memory performances correlated with left hippocampal atrophy. Our results emphasize the role of T2-LL and WM atrophy in determining clinically evident cognitive impairment in MS patients and provide evidence that GM and hippocampal atrophy occur in MS patients regardless of cognitive status.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cognitive disorders (CD) are frequently reported in Multiple Sclerosis (MS) occurring in 40–65 % of patients [1]. They start early in the disease, generally worsen over time and are partially independent of physical disability [1]. The most frequent deficits are found in processing speed, working memory, new learning, visual and verbal memory [2]. Cognitive impairment deeply impacts on quality of life and therapy adherence in MS patients; the definition of its pathogenic mechanisms and the identification of markers useful to monitor its evolution might contribute to identify pharmacologic and rehabilitative strategies.
CD have been associated with different magnetic resonance imaging (MRI) measures of brain tissue damage such as T2 lesion load (T2-LL) [3], whole brain [4], ventricular and cortical volumes [5], corpus callosum size [6] and cortical lesions [7]. The clinical relevance of regional cortical volume, including hippocampal volume, has recently been explored [8–11]; since the Hippocampus is known to play a critical role in visual and verbal memory [12], frequently compromised in MS [2], it represents an important target to study memory disorders in MS.
Indeed, histo-pathologic, morphological and functional MRI studies have recently reported hippocampal involvement in MS patients showing hippocampal demyelination, atrophy, and functional abnormalities of the cingulated-hippocampus network [13–17]. Moreover, in MS patients, hippocampal atrophy has been correlated with neuropsychological scores [8–11].
Despite the role of hippocampus in cognitive domains compromised in MS patients, the manner in which hippocampal atrophy affects cognitive function in MS is not well established.
To better understand the pathophysiological basis of CD in MS, we performed, an extensive evaluation of cognitive status and MRI measures of brain tissue damage, including hippocampal volume (HV), in MS patients with and without cognitive impairment and in healthy controls (HCs); we also looked at the correlations between brain tissue volumes and cognitive performances.
Methods
Study population
We studied 46 patients with relapsing–remitting MS (RRMS) according to revised McDonald criteria [18] and 25 HCs matched for sex, age and education.
MS patients were recruited among those referred to our outpatient MS clinic according to the following inclusion criteria: age 18–60 years (mean 39.6 ± 7.7; range 18–57); absence of relapses and steroid therapy for at least 3 months prior to the study; absence of significant anxiety or depression as assessed, respectively, by the State Trait Anxiety Inventory [19] and the Chicago Multiscale Depression Inventory [20].
Patients and HCs taking psychoactive drugs or substances that might interfere with neuropsychological examination (NPE) were excluded. Other exclusion criteria were claustrophobia and all contraindications for undergoing MRI investigation.
The study was approved by local Ethic Committee and a signed informed consent was obtained by all participants according to the Declaration of Helsinki.
Clinical and neuropsychological assessment
All participating subjects underwent neurological evaluation (NE) and NPE; in MS patients, disability was assessed by the expanded disability status scale (EDSS) [21]. Both NE and NPE took place the same day of MRI scan; NPE was performed using the version A of the Rao’s brief repeatable battery (BRB), which incorporates subtests of verbal memory acquisition and delayed recall [selective reminding test (SRT) and SRT-delayed recall (SRT-D)], visuo-spatial memory acquisition and delayed recall [10/36 spatial recall test (10/36-SPART) and 10/36-SPART-delayed recall (10/36-SPART-D)], concentration, sustained attention and information processing speed [paced auditory serial addition test at 3 (PASAT3″) and 2 (PASAT2″) seconds, symbol digit modalities test (SDMT)], and verbal fluency on semantic stimulus [word list generation (WLG)] [22]. Moreover, we administered the stroop color–word interference test (SCWIT) to better investigate executive functions. Neuropsychological scores were converted into Z scores using the available normative data of the Italian population [23]. Test failure was defined as a Z score ≤2. Patients were categorized as cognitively impaired (CI) when they failed at least two tests of the NPE, with at least one exploring hippocampal functions (verbal and/or visuo-spatial memory) [23]. Contrariwise RRMS patients who did not fail any test or just one, excluding verbal or visuo-spatial memory tests, were classified as cognitively preserved (CP).
MRI scanning protocol
MRI datasets were acquired on a 3-T GE Medical System (Milwaukee, WI, USA) scanner equipped with an 8-channel parallel head coil. The following images were acquired: (1) fast spin echo (FSE) dual-echo (DP-T2) [repetition time (TR) = 3,080, echo time (TE) 1/TE2 = 24/120 ms, axial slices = 88, matrix = 256 × 384, field of view (FOV) = 240 mm, thickness = 3 mm, interslice gap = 0 mm]; (2) T2-fluid-attenuated inversion recovery (T2-FLAIR) [TR = 9,000, TE = 1200, inversion time (TI) = 2500, axial slices = 44, matrix = 224 × 448, FOV = 240 mm, thickness = 3 mm, interslice gap = 0 mm]; (3) high-resolution 3D T1 (GE sequence IR-FSPGR, TR = 6988 ms, TI = 1100 ms, TE = 3.9 ms, flip angle = 10, voxel size = 1 × 1 × 1.2 mm3).
T2-LL measurement
Hyperintense T2 lesions were first identified on FSE dual-echo images by a single operator blinded to the patients’ cognitive status using the T2-FLAIR images as further reference. T2 lesions’ contours were transferred on electronic MRI data by means of a semiautomatic method implemented in Medical Image Processing, Analysis and Visualization application (MIPAV version 4.2.2, http://mipav.cit.nih.gov/). The same software was used to compute the global T2-LL for each subject.
Brain atrophy measurement
On high-resolution 3D T1-weighted images, normalized volumes of the whole brain were obtained by the SIENA (Structural Image Evaluation using Normalization of Atrophy) software, a fully automated and accurate method for measuring longitudinal and cross-sectional changes in brain volume [24] which is freely available as part of the FMRIB Software Library (FSL) (http://www.fmrib.ox.ac.uk/fsl). In the current study the cross-sectional version (SIENAX) was used. As a first step, this software performs brain segmentation from non-brain tissue. As a second step, the software creates a probabilistic brain mask making sure that certain structures such as eyes and optic nerves have not been included in the brain segmentation. Finally, MR imaging random field model-based segmentation [25] is used to segment the brain image into different tissue types giving a normalized volume of total brain, GM and WM.
To reduce the influence of WM lesions in the measurement of brain volumes, the lesion filling tool of the FSL (http://www.fmrib.ox.ac.uk/fsl) was used on 3D T1 datasets [26].
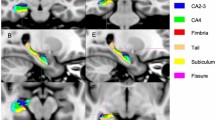
Hippocampus segmentation
3D-T1 images were re-oriented along the anterior–posterior commissure line, setting the anterior commissure as the origin of the spatial coordinates. Normalization was carried out through a 12-parameter affine transformation on a customized template [27]. A blinded tracer outlined the hippocampi, following a standardized and validated protocol [28] and using an interactive software program developed at the LONI [Laboratory of NeuroImaging, University of California at Los Angeles (http://www.loni.ucla.edu/Software/MultiTracer)] previously used in Alzheimer’s disease neuroimaging initiative (ADNI) project [28] and in MS patients [11]. Each hippocampus comprised approximately 30–40 consecutive slices and segmentation process took approximately 30 min per subject.
Since the HV is influenced by the head size, the absolute HV was multiplied by the volume correction factor determined by SIENAX. The normalized HV (NHV) was used for data analysis.
To test intra-rater reproducibility of hippocampal segmentation, our blinded tracer evaluated the scans of 25 randomly selected subjects twice, 3 weeks apart. Intra-class correlation coefficient was 0.95 for intra-rater assessment.
Data analysis
All data were analysed using the STATA version 12.0 software package (StataCorp, College Station, Texas, http://www.stata.com).
The Shapiro–Wilk test and graphical methods were used to assess the normal distribution of data. In case of normal distribution, the data are presented as mean and standard deviation (SD); in case of deviation from the normal distribution, the data are presented as median and inter-quartile range (IQR).
A descriptive statistic for the collected data was performed. Between-group differences were tested by unpaired two-tailed Student t test for independent samples or the Mann–Whitney U test. HCs were compared to the whole group of MS patients and then separately to CI and CP patients. Finally CI and CP patients were compared each other. The significance level was set at 0.05.
Correlations between different MRI parameters and clinical/NPE data were analysed by Pearson correlation coefficient (r) or Spearman’s rank correlation coefficient (r 2), controlling for anxiety and depression.
Results
Our RRMS population included 26 CP and 20 CI patients. Clinical and demographic characteristics of all study participants are summarized in Table 1. Patients and HCs were matched for age, sex and education. All subjects were right-handed. Disease duration (p = 0.70), number of relapses in the previous two years (p = 0.37) and EDSS score (p = 0.33) were not statistically different between CI and CP patients. The proportion of patients failing each neuropsychological test in the whole MS group, CI and CP groups is summarized in Table 2.
As previously shown [29], in both HCs and MS patients, right NHV [HCs (mean = 4.103, SD = 0.364); (MS patients mean = 3.552, SD = 0.512)] was significantly higher than the left one [HCs (mean = 3.993, SD = 0.335); MS (mean = 3.421, SD = 0.488)] (HCs: p = 0.02; RRMS: p < 0.001). Therefore, statistical analyses were performed separately for right and left NHV. Right and left NHV were significantly lower in the whole group (p < 0.001), CI (p < 0.001) and CP (p ≤ 0.001) MS patients when compared to HCs. Right (p = 0.24) and left NHV (p = 0.17) were not significantly different between CP and CI patients (Table 3). GM volume (GMV) (p ≤ 0.001) and WM volume (WMV) (p ≤ 0.001) were significantly smaller in the whole group of MS patients compared to HCs (Table 3). These differences were still significant when comparing HCs to CI (p ≤ 0.001) or CP (GM: p = 0.002; WM: p = 0.006) separately. GMV was not statistically different between CI and CP patients (p = 0.25), while WMV was significantly smaller in CI as compared to CP patients (p = 0.01) (Table 3). LL was significantly higher in CI patients as compared to CP ones (p = 0.02) (Table 3).
In the whole RRMS group, clinical data (disease duration, EDSS) did not show any correlation with hippocampal atrophy.
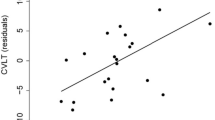
In the whole group of RRMS patients, the correlation analysis showed correlations between some cognitive tests’ performances and MRI measures of brain damage (T2-LL volume, hippocampal, WM and GM atrophy). Particularly GM atrophy was associated with visuo-spatial memory performances while atrophy of WM and left Hippocampus were correlated with verbal memory performances (Table 4). Finally, T2-LL volume was correlated with executive functions, visuo-spatial memory, processing speed and sustained attention performances (Table 4).
In the CI subgroup, significant correlations were found between verbal memory tests and atrophy of WM [LTS (r = 0.46; p = 0.05), CLTR (r = 0.55; p = 0.01), D-SRT (r = 0.45; p = 0.05)] and left Hippocampus [LTS (r = 0.46; p = 0.04), CLTR (r = 0.55; p = 0.01)].
Discussion
In the present study, we assessed MRI structural brain abnormalities, including hippocampal atrophy, in RRMS patients (with and without cognitive impairment) compared to HCs. We focused the attention on the hippocampus as it plays a crucial role in cognitive performances commonly impaired in MS patients (i.e., visual and verbal memory) [2, 22]. Therefore, it represents an interesting target of study to understand mechanisms underlying CD in MS.
As previously reported, our MS patients showed significant WM and GM atrophy relative to HCs [30, 31]. Furthermore, in line with a previous study using similar hippocampal segmentation software [11], we reported right and left hippocampal atrophy in MS patients with respect to HCs [8–11, 17]. Conversely, our CI RRMS patients showed exclusively WM atrophy and higher T2-LL when compared to CP patients. Therefore, we mainly confirm the results of a previous study showing the presence of hippocampal atrophy in MS patients and the absence of major hippocampal atrophy in CI with respect to CP patients [17]. We also confirm some studies investigating correlations between cognitive performances and MRI measures of brain damage, such as the relationship between T2-LL and processing speed/sustained attention performances and between left hippocampal atrophy and verbal memory [8, 30, 31]. The latter correlation reflects the structural and functional hippocampal organization [32]. However, previously reported correlations between cognitive performances and MRI parameters are not univocal, suggesting that cognitive dysfunctions in MS may depend on different factors such as T2-LL, lesion location (in WM and GM), global or regional GM/WM atrophy and normal appearing WM/GM damage.
Our results are in line with several recent studies that found that WM damage produces the major contribution to CD in MS, whereas GM damage adds only a small increment to the variance due to WM impairment [33–36]. Specifically, the slowed processing speed and particularly, the processing inefficiency were primarily associated with WM damage [37, 38].
Although our CI patients had been strictly selected for impairment of hippocampal domains, over 90 % of them showed also processing speed or attention deficits as generally reported in MS population; therefore, we hypothesize that the observed memory disorders may be due to an inadequate acquisition secondary to information processing insufficiency provoked by WM atrophy and higher T2-LL operating a disconnection among different cognitive networks [39, 40]. This hypothesis is also supported by several studies using advanced MRI techniques [i.e. functional MRI and diffusion weighted imaging (DTI)] showing that CD in MS mostly result from cortico-cortical or cortico-subcortical disconnection phenomena associated with abnormal functional connectivity (FC) [33, 40–45]. In CI patients, DTI studies found WM integrity changes, in relevant cognition areas, correlating with cognitive performances [33, 40–42]. FC changes at rest and during cognitive tasks were found in MS patients and to a major extent in CI patients [43–45]. Furthermore, FC changes may predate hippocampal atrophy as demonstrated either with functional MRI studies during memory task performances [17] or with resting-state functional MRI studies [16]. Interestingly, the fore-mentioned studies also found that damage of specific WM structures may have a significant impact on resting-state FC, while GM damage do not colocalize with resting-state FC changes.
Although we mainly confirmed previous findings, our study’s strength could be summarized in two main points: the sample selection and the detailed manual delineation of hippocampus following a standardized and validated segmentation protocol [11, 27, 28]. Indeed, to have a sample as homogeneous as possible, we recruited exclusively RRMS patients with similar clinical features and disability score. Differently from previous studies [8–11, 17], we divided MS patients in CP and CI, using a specific and validated battery (BRB) exploring all typical MS impaired domains and hippocampal functions (visuo-spatial and verbal memory). Moreover, in contrast to the majority of studies, we also acquired neuropsychological data from a control group [8–11].
With respect to the hippocampal manual segmentation, it is worthwhile to consider that several studies performing direct comparison between manual and automated hippocampal volume measurements showed that automated procedures give highly reproducible measurements but generate larger and less precise volumes than manual techniques [46, 47].
However, some limitations of the present study should be taken into account: (1) the sample size was small; (2) T2-LL and brain atrophy were not regionally assessed; (3) double inversion recovery pulse sequences, useful to detect hippocampal focal lesions, were not acquired; (3) CI patients were identified according to the criterion of “at least one abnormal memory test”; probably the inclusion of patients with only memory deficits, although difficult to select, would have provided more informative results on the role of hippocampal atrophy in this group of patients.
In conclusion, our study confirms and expands previous studies showing a significant GM and hippocampal atrophy in all MS patients, regardless of global cognitive status; it also emphasizes the role of WM atrophy and T2-LL in determining cognitive impairment, including memory disturbances, likely interrupting neuronal pathway involved in information processing.
Future studies, with a longitudinal design and a larger sample size, including patients with only memory deficits and preserved other cognitive domains, are warranted to better elucidate the role of hippocampal atrophy in determining CD in MS.
References
Julian LJ (2011) Cognitive functioning in multiple sclerosis. Neurol Clin 29(2):507–525
Chiaravalloti ND, DeLuca J (2008) Cognitive impairment in multiple sclerosis. Lancet Neurol 7:1139–1151
Benedict RH, Weinstock-Guttman B, Fishman I et al (2004) Prediction of neuropsychological impairment in multiple sclerosis: comparison of conventional magnetic resonance imaging measures of atrophy and lesion burden. Arch Neurol 61:226–230
Zivadinov R, Sepcic J, Nasuelli D et al (2001) A longitudinal study of brain atrophy and cognitive disturbances in the early phase of relapsing-remitting multiple sclerosis. J Neurol Neurosurg Psychol 70:773–780
Benedict RH, Bruce JM, Dwyer MG et al (2006) Neocortical atrophy, third ventricular width, and cognitive dysfunction in multiple sclerosis. Arch Neurol 63:1301–1306
Pelletier J, Suchet L, Witjas T et al (2001) A longitudinal study of callosal atrophy and interhemispheric dysfunction in relapse-remitting multiple sclerosis. Arch Neurol 58:105–111
Roosendaal SD, Moraal B, Pouwels PJ et al (2009) Accumulation of cortical lesions in MS: relation with cognitive impairment. Mult Scler 15(6):708–714
Sicotte NL, Kern KC, Giesser BS et al (2008) Regional hippocampal atrophy in multiple sclerosis. Brain 131(pt 4):1134–1141
Kiy G, Lehmann P, Hahn HK et al (2011) Decreased hippocampal volume, indirectly measured, is associated with depressive symptoms and consolidation deficits in multiple sclerosis. Mult Scler 17(9):1088–1097
Anderson VM, Fisniku LK, Zi Khaleel et al (2010) Hippocampal atrophy in relapsing-remitting and primary progressive MS: a comparative study. Mult Scler 16(9):1083–1090
Longoni G, Rocca MA, Pagani E et al (2015) Deficits in memory and visuospatial learning correlate with regional hippocampal atrophy in MS. Brain Struct Funct 220(1):435–444. doi:10.1007/s00429-013-0665-9
Bird CM, Burgess N (2008) The hippocampus and memory: insights from spatial processing. Nat Rev Neurosci 9:182–194
Geurts JJ, Bö L, Roosendaal SD et al (2007) Extensive hippocampal demyelination in multiple sclerosis. J Neuropathol Exp Neurol 66(9):819–827
Roosendaal SD, Moraal B, Vrenken H et al (2008) In vivo MR imaging of hippocampal lesions in multiple sclerosis. J Magn Reson Imaging 27(4):726–731
Sumowski JF, Wylie GR, De Luca J, Chiaravalloti N (2010) Intellectual enrichment is linked to cerebral efficiency in multiple sclerosis: functional magnetic resonance imaging evidence for cognitive reserve. Brain 133:362–374
Roosendaal SD, Hulst HE, Vrenken H et al (2010) Structural and Functional Hippocampal Changes in Multiple Sclerosis Patients with Intact Memory Function. Radiology 255:595–604
Hulst HE, Schoonheim MM, Roosendaal SD et al (2012) Functional adaptive changes within the hippocampal memory system of patients with multiple sclerosis. Hum Brain Mapp 33(10):2268–2280
Polman CH, Reingold SC, Edan G et al (2005) Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald Criteria”. Ann Neurol 58:840–846
Spielberg CD, Gorsuch RL, Luskene RE (1996) STAI, State-Trait Anxiety Inventory, Forma Y. Florence, Organizzazioni Speciali
Solari A, Motta A, Mendozzi L et al (2004) Italian version of the Chicago multiscale depression inventory: translation, adaptation and testing in people with multiple sclerosis. Neurol Sci 24(6):375–383
Kurtzke JF (1983) Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 33:1444–1452
Rao SM, Leo GJ, Bernardin L et al (1991) Cognitive dysfunction in multiple sclerosis. I. Frequency, patterns, and prediction. Neurology 41(5):685–691
Amato MP, Portaccio E, Goretti B et al (2006) The Rao’s Brief Repeatable Battery and Stroop Test: normative values with age, education and gender corrections in an Italian population. Mult Scler 12:787–793
Smith SM, Zhang Y, Jenkinson M et al (2002) Accurate, robust and automated longitudinal and cross-sectional brain change analysis. NeuroImage 17:479–489
Zhang Y, Brady M, Smith S (2001) Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans Med Imaging 20:45–57
Battaglini M, Jenkinson M, De Stefano N (2012) Evaluating and reducing the impact of white matter lesions on brain volume measurements. Hum Brain Mapp 33:2062–2071
Pruessner JC, Li LM, Serles W et al (2000) Volumetry of hippocampus and amygdala with high-resolution MRI and three-dimensional analysis software: minimizing the discrepancies between laboratories. Cereb Cortex 10:433–442
Cavedo E, Redolfi A, Angeloni F et al (2014) The Italian Alzheimer’s disease neuroimaging initiative (I-ADNI): validation of structural MR imaging. J Alzheimer’s Dis 40(4):941–952
Pedraza O, Bowers D, Gilmore R (2004) Asymmetry of the hippocampus and amygdala in MRI volumetric measurements of normal adults. J Int Neuropsychol Soc 10(5):664–678
Giorgio A, De Stefano N (2010) Cognition in Multiple Sclerosis: relevance of lesions, Brain atrophy and proton MR spectroscopy. Neurol Sci 31(Suppl 2):S245–S248
Rocca MA, Amato MP, De Stefano N et al (2015) Clinical and imaging assessment of cognitive dysfunction in multiple sclerosis. Lancet Neurol 14(3):302–317
Hou G, Yang X, Yuan TF (2013) Hippocampal asymmetry: differences in structures and functions. Neurochem Res 38(3):453–460
Hulst HE, Steenwijk MD, Versteeg A et al (2013) Cognitive impairment MS: impact of white matter integrity, gray matter volume, and lesions. Neurology 80(11):1025–1032
Papadopoulou A, Müller-Lenke N, Naegelin Y et al (2013) Contribution of cortical and white matter lesions to cognitive impairment in multiple sclerosis. Mult Scler 19:1290–1296
Llufriu S, Martinez-Heras E, Fortea J et al (2014) Cognitive functions in multiple sclerosis: impact of gray matter integrity. Mult Scler 20:424–432
Patti F, De Stefano M, Lavorgna L et al (2015) Lesion load may predict long-term cognitive dysfunction in multiple sclerosis patients. PLoS One 10(3):e0120754
Sanfilipo MP, Benedict RHB, Weinstock-Guttman B, Bakshi R (2006) Gray and white matter brain atrophy and neuropsychological impairment in multiple sclerosis. Neurology 66(5):685–692
Covey TJ1, Zivadinov R, Shucard JL, Shucard DW (2011) Information processing speed, neural efficiency, and working memory performance in multiple sclerosis: differential relationships with structural magnetic resonance imaging. J Clin Exp Neuropsychol 33(10):1129–1145
Guimara˜es J, Sa´ MJ (2012) Cognitive dysfunction in multiple sclerosis. Front Neurol 3:74
Dineen RA, Vilisaar J, Hlinka J et al (2009) Disconnection as a mechanism for cognitive dysfunction in multiple sclerosis. Brain 132:239–249
Mesaros S, Rocca MA, Kacar K et al (2012) Diffusion tensor MRI tractography and cognitive impairment in multiple sclerosis. Neurology 78:969–975
Bisecco A, Rocca MA, Pagani E et al (2015) Connectivity-based parcellation of the thalamus in multiple sclerosis and its implications for cognitive impairment: A multicenter study. Hum Brain Mapp. doi:10.1002/hbm.22809
Bonavita S, Gallo A, Sacco R et al (2011) Distributed changes in default-mode resting-state connectivity in multiple sclerosis. Mult Scler 17:411–422
Rocca MA, Valsasina P, Absinta M et al (2010) Default-mode network dysfunction and cognitive impairment in progressive MS. Neurology 20(74):1252–1259
Sumowski JF, Wylie GR, Leavitt VM et al (2013) Default network activity is a sensitive and specific biomarker of memory in multiple sclerosis. Mult Scler 19(2):199–208
Morey RA, Petty CM, Xu Y et al (2009) Rebuttal to Hasan and Pedraza in comments and controversies: “Improving the reliability of manual and automated methods for hippocampal and amygdala volume measurements”. Neuroimage 48(3):499–500
Cherbuin N, Anstey KJ, Réglade-Meslin C et al (2009) In vivo hippocampal measurement and memory: a comparison of manual tracing and automated segmentation in a large community-based sample. PLoS One 4(4):e5265
Acknowledgments
We gratefully acknowledge the patients and controls who took part in this study. We thank Dr. Valentina Panetta for providing statistical advice and the radiographer who acquired MRI images: Antonella Paccone (Neurological Institute for Diagnosis and Care “Hermitage Capodimonte”, Naples).
Conflict of interest
R. Sacco, M. Della Corte, S. Esposito, A. d’Ambrosio, R. Docimo, A. Bisecco, L. Lavorgna, D. Corbo, F. Esposito, M. Cirillo report no disclosures. A. Gallo and S. Bonavita received speakers honoraria from Biogen Idec, Novartis, and Merck-Serono. G. Tedeschi has received compensation for consulting services and/or speaking activities from Bayer Schering Pharma, Biogen Idec, Merck Serono, and Teva Pharmaceutical Industries; and receives research support from Biogen Idec, Merck Serono, and Fondazione Italiana Sclerosi Multipla.
Ethical standard
This study was approved by the Local Ethical Committees on human studies and written informed consent from each subject was obtained prior to their enrolment.
Author information
Authors and Affiliations
Corresponding author
Additional information
R. Sacco and A. Bisecco contributed equally to this study.
Rights and permissions
About this article
Cite this article
Sacco, R., Bisecco, A., Corbo, D. et al. Cognitive impairment and memory disorders in relapsing–remitting multiple sclerosis: the role of white matter, gray matter and hippocampus. J Neurol 262, 1691–1697 (2015). https://doi.org/10.1007/s00415-015-7763-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-015-7763-y