Abstract
Blood biomarkers in degenerative ataxias are still largely missing. Here, we aimed to provide piloting proof-of-concept that serum Neurofilament light (NfL) could offer a promising peripheral blood biomarker in degenerative ataxias. Specifically, as a marker of neuronal damage, NfL might (1) help to differentiate multiple system atrophy of cerebellar type (MSA-C) from sporadic adult-onset ataxia (SAOA), and (2) show increases in repeat-expansion spinocerebellar ataxias (SCAs) which might be amenable to treatment in the future. To explore these two hypotheses, we measured serum NfL levels by single-molecule array (Simoa) technique in 115 subjects, comprising patients with MSA-C (n = 25), SAOA (n = 25), the most frequent repeat-expansion SCAs (SCA 1, 2, 3 and 6) (n = 20), and age-matched controls (n = 45). Compared to controls, NfL was significantly increased in MSA-C, with levels significantly higher than in SAOA (AUC = 0.74 (0.59–0.89), mean and 95% confidence interval, p = .004). NfL was also significantly increased in SCA patients as compared to controls (AUC = 0.91 (0.81–1.00), p < .001), including NfL increases in SCA1 and SCA3. These findings provide first proof-of-concept that NfL might provide a promising peripheral biomarker in degenerative ataxias, e.g. supporting the differentiation of MSA-C from SAOA, and indicating neuronal damage in repeat-expansion SCAs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Degenerative ataxias share the hallmark of progressive neurodegeneration of the cerebellum and the associated spinocerebellar tracts, frequently complicated by degeneration also of non-cerebellar neuronal systems. To date, reliable blood biomarkers for degenerative ataxias are still largely missing. However, biomarkers indicating neuronal damage are urgently warranted in degenerative ataxias. For example, such biomarkers may help differentiating multiple system atrophy of cerebellar type (MSA-C) from sporadic adult-onset ataxia (SAOA). While this differentiation can be facilitated by longitudinal clinical monitoring of the evolution of non-ataxia symptoms and the faster disease progression in MSA-C, differentiation on clinical grounds alone can sometimes be difficult [1]. A correct diagnosis, however, is important given the prognostic differences of MSA-C and SAOA in terms of mobility and survival [1]. Biomarkers indicating neuronal damage might also be helpful in those degenerative ataxias for which causal treatments are coming into reach, possibly serving as treatment-outcome parameters. For example, gene-specific treatment studies, like antisense oligonucleotides, are already coming into reach for repeat-expansion spinocerebellar ataxias (repeat SCAs) [2,3,4,5], necessitating the development of easily accessible peripheral biomarkers.
Blood levels of neurofilament light (NfL) have recently been demonstrated to serve as a cross-disease biomarker in several neurodegenerative diseases [6,7,8]. NfL allows capturing the degenerative damage of several parts of the central nervous system, being particularly sensitive to axonal destruction of long fibre tracts [9, 10]. Based on these observations, we here aimed to provide piloting proof-of-concept that serum NfL might also provide a promising candidate biomarker indicating the occurrence of neuronal damage in degenerative ataxias. To explore this hypothesis, we measured serum levels of NfL in patients with MSA-C, SAOA and the most frequent repeat SCAs, and age-matched healthy control cohorts, using an established single-molecule array (Simoa) assay [11,12,13].
Methods
Subjects
We recruited a total of 115 subjects from the Department of Neurodegenerative Disorders, Hertie Institute for Clinical Brain Research, University Hospital Tübingen (sampling interval: 2014–2017). Our three patient cohorts comprised of a consecutive series of 25 MSA-C patients, 25 SAOA patients and 20 SCA patients. MSA-C patients fulfilled established criteria of probable MSA-C [14]. No neuropathological examination was performed in any of the MSA-C cases. In line with the current SAOA criteria operationalised for clinical studies [1], SAOA was defined as degenerative ataxia with age at onset ≥ 40 years, negative family history, negative genetic screening for SCA 1, 2, 3, 6, 7 and 17, absence of features indicative of MSA-C and absence of secondary ataxia causes. Such late-onset patients are also referred to as idiopathic late-onset cerebellar ataxia in the literature [15]. SCA patients consisted of genetically diagnosed patients with SCA1 (n = 6), SCA2 (n = 3), SCA3 (n = 8) and SCA6 (n = 3). We recruited two age-matched control cohorts to achieve adequate age-matching for our ataxia cohorts as the sporadic cohorts and the SCA cohort differed in age. These two control cohorts comprised a total of 45 healthy volunteers not having any history or clinical signs of neurodegenerative disease, as ascertained by neurologists with special expertise in neurodegenerative diseases. Control cohort #1 (n = 25) was age-matched to both MSA-C and SAOA patients. Control cohort #2 (n = 20) was age-matched to the SCA cohort. A sample size calculation was possible for comparing MSA-C and SAOA patients, building on the assumption that the effect size of the NfL difference in serum would correspond to the effect size previously observed in CSF [15] (estimated Cohen’s d = 1.54). This sample size calculation resulted in a minimum of 24 MSA-C and 24 SAOA patients (input parameters: α = 0.01, β = 0.01, equal size of both groups, use of two-tailed Wilcoxon–Mann–Whitney test) [16]. Since prior data on NfL levels in SCAs are not available, sample size calculation was not possible here, and our approach to studying the NfL increase in SCAs was a primarily exploratory approach, assuming that also for SCAs a cohort size of ≥ 20 subjects might allow capturing relevant differences as compared to controls.
Biomaterial
Serum samples were frozen at −80 °C within 1 h after collection, stored in the local biobank and analysed without any previous thaw-freeze cycle.
Measurements
Serum NfL levels were measured in duplicates with an ultra-sensitive single molecule array (Simoa) on the Simoa HD-1 Analyzer (Quanterix, Lexington, Massachusetts), as established previously [11]. Between-run precision was 6.4% (for a concentration of 8.3 pg/ml), 6.1% (for 20.1 pg/ml) and 2.9% (for 97.0 pg/ml), within-run precision was 6.6% (for samples with a mean concentration of 64 pg/ml), and all sample coefficients of variation from duplicate measurements [11] were below 12.5%.
Analysis
Data were reported as median and interquartile range, unless stated otherwise. We used robust non-parametric procedures to analyse group effects on NfL levels (Kruskal–Wallis and Mann–Whitney tests) and associations of NfL levels with disease severity and progression (Spearman’s correlation). Post-hoc tests were Bonferroni-corrected for multiple comparisons. All analyses were performed with IBM SPSS (Version 24).
Results
NfL for differentiating MSA-C from SAOA
To explore the hypothesis that serum NfL might provide a supportive diagnostic biomarker for differentiating MSA-C from SAOA, we compared the NfL serum levels between age-matched cohorts of MSA-C patients (n = 25), SAOA patients (n = 25) and healthy subjects (control cohort #1, n = 25) (Table 1). NfL levels differed significantly between groups (H(2) = 15.29, p < .001, Kruskal–Wallis test) (Fig. 1a). NfL levels were significantly higher in MSA-C [60.7 pg/ml (44.5–94.0), median (interquartile range)] than in SAOA [28.0 pg/ml (19.1–49.2)] (U = 165.00, z = 2.86, p = .012, r = 0.41), which suggested that serum NfL might help to differentiate these two forms of degenerative ataxia. Compared to controls [33.2 pg/ml (27.3–43.6)], NfL levels were significantly increased in MSA-C (U = 113.00, z = 3.87, p < .001, r = 0.55), but not in SAOA (U = 265.00, z = 0.92, n.s., r = 0.13, two-sided Mann–Whitney U tests, Bonferroni-corrected for multiple comparisons). The receiver operating characteristic (ROC) analysis indicated that the performance of NfL for differentiating MSA-C from SAOA was significant at a moderate degree (Fig. 1c), as quantified by the area under the curve [AUC = 0.74 (0.59–0.89), mean and 95% confidence interval, p = .004]. Though our study was not designed for these analyses, preliminary exploratory analyses suggested that serum NfL in MSA-C does not correlate with clinical disease severity (ϱ = − 0.08, p = .363), as determined by the scale for the assessment and rating of ataxia (SARA) score [17, 18], or with cross-sectional disease progression (ϱ = 0.26, p = .121, Spearman’s correlation coefficients, one-sided tests), as defined by the cross-sectional quotient of disease severity and disease duration.
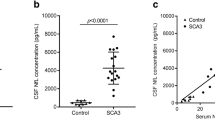
Serum neurofilament light (NfL) concentrations are increased in multiple system atrophy of cerebellar type (MSA-C) and in repeat-expansion spinocerebellar ataxias (SCAs). The boxplots illustrate serum NfL concentrations (pg/ml) in MSA-C, sporadic adult-onset ataxia (SAOA) and age-matched healthy controls (a), and serum NfL concentrations in SCAs and age-matched healthy controls (b). Central horizontal lines indicate median values, boxes illustrate the ranges between lower and upper quartiles, and error bars represent the full ranges of data. ***p < .001, *p < .05, n.s. p ≥ .05 (Bonferroni-corrected). Please note the logarithmic scale of the y-axis. Receiver operating characteristics (ROC) curves illustrate the sensitivity and the specificity of serum NfL for differentiating MSA-C patients from SAOA patients (c) and for differentiating SCA patients from healthy controls (d). The area under the curve (AUC) was used as a parameter to summarise the biomarker performance (MSA-C vs SAOA: AUC = 0.74 (0.59–0.89), SCAs vs controls: AUC = 0.91 (0.81–1.00), mean and 95% confidence interval of the mean)
NfL in repeat SCAs
To explore the hypothesis that NfL in serum might serve as a blood biomarker indicating neuronal damage in SCAs, we measured its levels in patients with the most frequent repeat SCAs (n = 20), namely SCA1, SCA2, SCA3 or SCA6, and a second, age-matched control cohort (control cohort #2, n = 20) (Table 1). NfL levels were significantly higher in repeat SCA patients [58.7 pg/ml (45.3–74.0)] than in controls [19.7 pg/ml (16.0–26.7)] (U = 35.00, z = 4.46, p < .001, r = 0.71) (Fig. 1b). NfL hereby differentiated patients and controls with high accuracy [AUC = 0.91 (0.81–1.00), p < .001] (Fig. 1d). Comparison of age-matched subsets of our repeat SCA and SAOA cohorts indicated that serum NfL was significantly higher in SCAs than in SAOA (z = 2.57, p = .009, Supplement 1). Though our exploratory study was not primarily intended to investigate genotype-specific effects of SCA types and, hence, not adequately powered for this purpose, we performed a preliminary exploratory analysis of potential NfL increases in SCA subsets and their a priori age-matched controls (Supplement 2). Despite the small subset sizes, we observed significantly increased NfL levels in SCA1 (p = .002, r = 0.83, n = 6) and SCA3 (p < .001, r = 0.84, n = 8, two-sided Mann–Whitney U tests). These findings provide first hints for increased NfL levels in repeat SCAs, which might be observed not only in an aggregation of different repeat SCAs, but also within specific SCA genotypes, like e.g. SCA1 and SCA3.
Discussion
The increase of serum NfL in well-defined forms of degenerative ataxia—MSA-C and repeat SCAs—provides first preliminary evidence that serum NfL might be a promising biomarker in degenerative ataxias. This finding adds support to the notion that blood NfL serves as a cross-disease biomarker in several neurodegenerative diseases [6,7,8], extending this notion by demonstrating that blood NfL increases occur also in several degenerative diseases of the cerebellum and its associated tracts. Given the established relation between neuronal damage and blood NfL increases [6, 8, 12, 13], NfL seems to serve as an indicator for the occurrence of neuronal damage in degenerative ataxias. It might hereby be particularly sensitive to axonal destruction given its presence in long fibre tracts [9, 10], such as the spinocerebellar and corticospinal tracts affected in multisystemic degenerative ataxias. This might explain the observed increase of NfL particularly in multisystemic ataxias like MSA-C and repeat SCAs.
Specifically, our results suggest that serum NfL might serve as a supportive diagnostic biomarker for differentiating MSA-C from SAOA. Biomarkers for this differentiation are urgently needed as these two types of sporadic ataxias differ considerably in terms of prognosis and also in terms of their future molecular treatments, but often cannot be reliably differentiated on clinical grounds alone, particularly in early disease stages and at disease onset [1]. While previous research suggested that the differentiation between MSA-C and SAOA can be supported by NfL concentrations in cerebrospinal fluid (CSF) [15], our study indicates that also NfL in serum may be helpful for this purpose. The availability of a blood-based, easily accessible peripheral biomarker is of considerable practical relevance not only for the diagnostic work-up in clinical settings, but also for longitudinal sampling in future registry studies and clinical trials. The diagnostic accuracy of serum NfL observed in our study was good [AUC = 0.74 (0.59–0.89)], although less than previously reported for CSF NfL [15] [AUC = 0.93 (0.86–1.0)]. This might indicate the need for trading-off the benefits of an easily accessible peripheral fluid biomarker vs the better diagnostic performance of a CSF biomarker. To further confirm serum NfL as a biomarker supporting the differentiation of MSA-C from SAOA, prospective longitudinal studies are required, particularly in those patients with sporadic ataxia who do not yet meet the MSA-C criteria. Moreover, investigations of pathologically confirmed MSA cases are warranted to ascertain whether our findings in MSA do indeed reflect pathologically confirmed MSA.
NfL levels differentiate MSA not only from SAOA, as shown here, but also from parkinsonian disorders, as shown previously [7, 19, 20]. Recently, serum NfL levels in MSA, also measured by Simoa technique, have been demonstrated to be increased twofold as compared to Parkinson’s disease (PD), hereby excellently differentiating between MSA and PD [AUC = 0.87 (0.73–1.00)] [19]. This finding parallels the twofold NfL increase in MSA-C as compared to SAOA in our study. In contrast, the discriminatory utility of NfL levels for MSA might be less if compared against a background of other multisystemic neurodegenerative diseases with high brainstem or striatal involvement, such as progressive supranuclear palsy or corticobasal syndrome [7, 19].
Independent of the diagnostic value of NfL to differentiate MSA-C from SAOA, our results additionally suggest that NfL might also be useful in repeat SCAs to indicate the occurrence of neuronal damage. The observed increase of NfL levels in repeat SCAs is not an unspecific finding across all degenerative ataxias alike, as demonstrated by the observation that NfL levels were significantly lower in SAOA. This indicates that the pathogenic processes underlying repeat SCAs—rather than just any type of degenerative spinocerebellar disease—might be drivers of neuronal damage. If longitudinal studies confirm our findings, NfL might help to better stratify the onset of neurodegeneration in natural history studies of SCAs, possibly even in the preclinical phase, and to document the response to therapy in future gene-specific treatment studies, like e.g. antisense oligonucleotides [2]. Our results also provide the basis for sample size estimations for future larger NfL biomarker trials in SCAs. Based on the observed serum NfL increase in SCAs (effect size r = 0.71), a conservative sample size estimation demanding low α- and β-errors indicates that a confirmatory study would require only 15 SCA patients and 15 controls for confirmation of the NfL increase (assuming α = 0.01, β = 0.01, equal group size, use of two-tailed non-parametric test) [16]. Although our study was not designed to test specific SCA genotypes, the fact that significant differences with large effect sizes could be observed already in small SCA groups of SCA1 (r = 0.83) and SCA3 (r = 0.84) patients indicates that NfL might also serve as a biomarker for future clinical trials in these rare diseases. However, further longitudinal research is required to study NfL as a biomarker of disease severity and disease progression in SCAs, ideally including also investigations of presymptomatic SCA mutation carriers, associations with imaging parameters of the affected neuronal systems and genotype-specific analyses.
Pathogenetically, the NfL increase in MSA-C and repeat SCAs as compared to SAOA, respectively, might reflect the more rapid disease progression and/or the multisystemic, more widespread neurodegeneration in these two groups of degenerative ataxia. Indeed, the higher NfL concentration in MSA-C than in SAOA was paralleled by the higher cross-sectional disease progression in MSA-C (3.3 (2.9–5.1) SARA points per year, Table 1) than in SAOA [1.5 (0.8–2.7)]. Yet, in our small cross-sectional pilot study, NfL did not correlate with disease progression within the MSA-C cohort itself. Also, the increase of NfL in repeat SCAs, particularly in SCA1 and SCA3, might reflect the faster disease progression in these SCAs than e.g. in SAOA (disease progression in SCA1: 2.18 ± 0.17 SARA points per year (mean ± standard error); in SCA3: 1.61 ± 0.12, in SAOA: 1.1 ± 0.39, data from large longitudinal studies) [1, 21]. The marked NfL increases in MSA-C and SCAs as compared to SAOA, respectively, might reflect not only faster disease progression, but also damage of additional neuronal systems (e.g. the pyramidal tract) beyond the cerebellum and its fibre tracts. While our study was not designed to dissociate the effects of disease progression and multisystemic degeneration on NfL levels, future studies may address their differential effects by correlating NfL levels with imaging parameters of disease progression and involvement of multiple neuronal systems using a longitudinal study design. In addition, post-mortem investigations might help revealing which specific parts of the nervous system drive the NfL increase in multisystemic neurodegenerative ataxias. Specifically, correlative imaging and post-mortem studies might allow testing whether NfL levels indeed correlate with degeneration of long fibre tracts, or possibly also with atrophy of specific regions of the brainstem, striatum, or the cortex. Such studies might thus provide deeper insight into the underlying pathophysiology driving the increase of NfL levels in multisystemic degenerative ataxias.
References
Giordano I, Harmuth F, Jacobi H, Paap B, Vielhaber S, Machts J, Schols L, Synofzik M, Sturm M, Tallaksen C, Wedding IM, Boesch S, Eigentler A, van de Warrenburg B, van Gaalen J, Kamm C, Dudesek A, Kang JS, Timmann D, Silvestri G, Masciullo M, Klopstock T, Neuhofer C, Ganos C, Filla A, Bauer P, Tezenas du Montcel S, Klockgether T (2017) Clinical and genetic characteristics of sporadic adult-onset degenerative ataxia. Neurology 89:1043–1049
Scoles DR, Meera P, Schneider MD, Paul S, Dansithong W, Figueroa KP, Hung G, Rigo F, Bennett CF, Otis TS, Pulst SM (2017) Antisense oligonucleotide therapy for spinocerebellar ataxia type 2. Nature 544:362–366
Keiser MS, Kordasiewicz HB, McBride JL (2016) Gene suppression strategies for dominantly inherited neurodegenerative diseases: lessons from Huntington’s disease and spinocerebellar ataxia. Hum Mol Genet 25:R53–R64
Paulson HL, Shakkottai VG, Clark HB, Orr HT (2017) Polyglutamine spinocerebellar ataxias—from genes to potential treatments. Nat Rev Neurosci 18:613–626
Toonen LJA, Rigo F, van Attikum H, van Roon-Mom WMC (2017) Antisense oligonucleotide-mediated removal of the polyglutamine repeat in spinocerebellar ataxia type 3 mice. Mol Ther Nucleic Acids 8:232–242
Wilke C, Preische O, Deuschle C, Roeben B, Apel A, Barro C, Maia L, Maetzler W, Kuhle J, Synofzik M (2016) Neurofilament light chain in FTD is elevated not only in cerebrospinal fluid, but also in serum. J Neurol Neurosurg Psychiatry 87:1270–1272
Bacioglu M, Maia LF, Preische O, Schelle J, Apel A, Kaeser SA, Schweighauser M, Eninger T, Lambert M, Pilotto A, Shimshek DR, Neumann U, Kahle PJ, Staufenbiel M, Neumann M, Maetzler W, Kuhle J, Jucker M (2016) Neurofilament light chain in blood and CSF as marker of disease progression in mouse models and in neurodegenerative diseases. Neuron 91:56–66
Zetterberg H (2016) Neurofilament light: a dynamic cross-disease fluid biomarker for neurodegeneration. Neuron 91:1–3
Menke RA, Gray E, Lu CH, Kuhle J, Talbot K, Malaspina A, Turner MR (2015) CSF neurofilament light chain reflects corticospinal tract degeneration in ALS. Ann Clin Transl Neurol 2:748–755
Skillback T, Farahmand B, Bartlett JW, Rosen C, Mattsson N, Nagga K, Kilander L, Religa D, Wimo A, Winblad B, Rosengren L, Schott JM, Blennow K, Eriksdotter M, Zetterberg H (2014) CSF neurofilament light differs in neurodegenerative diseases and predicts severity and survival. Neurology 83:1945–1953
Kuhle J, Barro C, Andreasson U, Derfuss T, Lindberg R, Sandelius A, Liman V, Norgren N, Blennow K, Zetterberg H (2016) Comparison of three analytical platforms for quantification of the neurofilament light chain in blood samples: ELISA, electrochemiluminescence immunoassay and Simoa. Clin Chem Lab Med 54:1655–1661
Disanto G, Barro C, Benkert P, Naegelin Y, Schadelin S, Giardiello A, Zecca C, Blennow K, Zetterberg H, Leppert D, Kappos L, Gobbi C, Kuhle J, Swiss Multiple Sclerosis Cohort Study G (2017) Serum neurofilament light: a biomarker of neuronal damage in multiple sclerosis. Ann Neurol 81:857–870
Gattringer T, Pinter D, Enzinger C, Seifert-Held T, Kneihsl M, Fandler S, Pichler A, Barro C, Grobke S, Voortman M, Pirpamer L, Hofer E, Ropele S, Schmidt R, Kuhle J, Fazekas F, Khalil M (2017) Serum neurofilament light is sensitive to active cerebral small vessel disease. Neurology 89:2108–2114
Gilman S, Wenning GK, Low PA, Brooks DJ, Mathias CJ, Trojanowski JQ, Wood NW, Colosimo C, Durr A, Fowler CJ, Kaufmann H, Klockgether T, Lees A, Poewe W, Quinn N, Revesz T, Robertson D, Sandroni P, Seppi K, Vidailhet M (2008) Second consensus statement on the diagnosis of multiple system atrophy. Neurology 71:670–676
Abdo WF, van de Warrenburg BP, Munneke M, van Geel WJ, Bloem BR, Kremer HP, Verbeek MM (2006) CSF analysis differentiates multiple-system atrophy from idiopathic late-onset cerebellar ataxia. Neurology 67:474–479
Faul F, Erdfelder E, Buchner A, Lang A-G (2009) Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behav Res Methods 41:1149–1160
Schmitz-Hubsch T, du Montcel ST, Baliko L, Berciano J, Boesch S, Depondt C, Giunti P, Globas C, Infante J, Kang JS, Kremer B, Mariotti C, Melegh B, Pandolfo M, Rakowicz M, Ribai P, Rola R, Schols L, Szymanski S, van de Warrenburg BP, Durr A, Klockgether T, Fancellu R (2006) Scale for the assessment and rating of ataxia: development of a new clinical scale. Neurology 66:1717–1720
Matsushima M, Yabe I, Oba K, Sakushima K, Mito Y, Takei A, Houzen H, Tsuzaka K, Yoshida K, Maruo Y, Sasaki H (2016) Comparison of different symptom assessment scales for multiple system atrophy. Cerebellum 15:190–200
Hansson O, Janelidze S, Hall S, Magdalinou N, Lees AJ, Andreasson U, Norgren N, Linder J, Forsgren L, Constantinescu R, Zetterberg H, Blennow K (2017) Blood-based NfL: a biomarker for differential diagnosis of parkinsonian disorder. Neurology 88:930–937
Sako W, Murakami N, Izumi Y, Kaji R (2015) Neurofilament light chain level in cerebrospinal fluid can differentiate Parkinson’s disease from atypical parkinsonism: evidence from a meta-analysis. J Neurol Sci 352:84–87
Jacobi H, Bauer P, Giunti P, Labrum R, Sweeney MG, Charles P, Durr A, Marelli C, Globas C, Linnemann C, Schols L, Rakowicz M, Rola R, Zdzienicka E, Schmitz-Hubsch T, Fancellu R, Mariotti C, Tomasello C, Baliko L, Melegh B, Filla A, Rinaldi C, van de Warrenburg BP, Verstappen CC, Szymanski S, Berciano J, Infante J, Timmann D, Boesch S, Hering S, Depondt C, Pandolfo M, Kang JS, Ratzka S, Schulz J, Tezenas du Montcel S, Klockgether T (2011) The natural history of spinocerebellar ataxia type 1, 2, 3, and 6: a 2-year follow-up study. Neurology 77:1035–1041
Acknowledgements
Biosamples were obtained from the Neuro-Biobank of the University of Tübingen, Germany, which is supported by the University of Tübingen, the Hertie Institute for Clinical Brain Research (HIH) and the German Center for Neurodegenerative Diseases (DZNE). MS was supported by the Else Kröner-Fresenius-Stiftung.
Author information
Authors and Affiliations
Contributions
CW: design and conceptualisation of the study, acquisition of data, analysis of the data, drafting and revision of the manuscript. FB: acquisition of data, revision of the manuscript. SH: acquisition of data, revision of the manuscript. KB: acquisition of data, revision of the manuscript. LS: acquisition of data, revision of the manuscript. JK: acquisition and analysis of data, design and conceptualisation of measurements, revision of the manuscript. MS: design and conceptualisation of the study, acquisition of data, drafting and revision of the manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
JK’s institution (University Hospital Basel) received in the last 3 years and used exclusively for research support: consulting fees from Novartis, Protagen AG; speaker fees from the Swiss MS Society, Biogen, Novartis, Roche, Genzyme; travel expenses from Merck Serono, Novartis; grants from ECTRIMS Research Fellowship Programme, University of Basel, Swiss MS Society, Swiss National Research Foundation, Bayer (Switzerland) AG, Genzyme, Novartis. MS received speaker’s honoraria and research support from Actelion Pharmaceuticals, unrelated to the current project and manuscript. The other authors declare no competing financial interests.
Ethical statements
The university’s ethics committee approved the study; the methods were carried out in accordance with the relevant guidelines and regulations.
Informed consent
All the subjects gave a written informed consent prior to participation.
Data availability
The datasets analysed in the current study are available from the corresponding author on reasonable request.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wilke, C., Bender, F., Hayer, S.N. et al. Serum neurofilament light is increased in multiple system atrophy of cerebellar type and in repeat-expansion spinocerebellar ataxias: a pilot study. J Neurol 265, 1618–1624 (2018). https://doi.org/10.1007/s00415-018-8893-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-018-8893-9