Abstract
Response to interferon-beta (IFN-beta) treatment is heterogeneous in multiple sclerosis (MS). We aimed to search for biomarkers predicting no evidence of disease activity (NEDA) status upon IFN-beta treatment in MS. 119 patients with relapsing–remitting MS (RRMS) initiating IFN-beta treatment were included in the study, and followed prospectively for 2 years. Neutralizing antibodies (NAb) were explored in serum samples obtained after 6 and 12 months of IFN-beta treatment. Soluble cytokines and blood lymphocytes were studied in basal samples by ELISA and flow cytometry, respectively. 9% of patients developed NAb. These antibodies were more frequent in patients receiving IFN-beta 1b than in those treated subcutaneous (p = 0.008) or intramuscular (p < 0.0001) IFN-beta 1a. No patient showing NAb remained NEDA during follow-up. Basal immunological variables are also associated with patient response. Percentages below 3% of CD19 + CD5 + cells (AUC 0.74, CI 0.63–0.84; OR 10.68, CI 3.55–32.15, p < 0.0001; Likelihood ratio 4.28) or above 2.6% of CD8 + perforin + T cells (AUC 0.79, CI 0.63–0.96; OR 6.11, CI 2.0–18.6, p = 0.0009; Likelihood ratio 5.47) increased the probability of achieving NEDA status during treatment. Basal blood immune cell subsets contribute to identify MS patients with a high probability of showing an optimal response to IFN-beta.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
IFN-beta is a safe and effective drug for the treatment of patients with relapsing–remitting multiple sclerosis (RRMS) [1,2,3,4]. However, response to this treatment is heterogeneous and the search of biomarkers allowing early identification of patients who will be optimal responders to IFN-beta is important to achieve a personalized therapy.
No evidence of disease activity (NEDA) is defined as the absence of new relapses, disability progression and magnetic resonance imaging (MRI) activity on a disease-modifying treatment [5]. Patients achieving NEDA in the first years of treatment have a high probability of remaining free of progression for long [6]. Thus, the finding of biomarkers predicting this status at the beginning of a treatment would be very useful for therapeutic decisions.
Several clinical markers as the appearance of relapses, new lesions on MRI, or disability worsening during the first years of treatment, could help to identify patients who will be optimal responders to IFN-beta at the long term [7]. However, the time needed for their evaluation ranges between 1 and 2 years, and this can imply some deterioration in non responders.
Biological biomarkers were also described [8, 9] but most of them are not being currently used, due in some cases to the lack of validation or to technical complexity. In fact, the presence of neutralizing antibodies (NAb) is the only biomarker used in clinical practice. They associate with a loss of bioavailability of the drug [10], appear between 6 and 12 months after treatment initiation and may persist for a long time.
We aimed to explore if the immunological profile present in blood before treatment initiation can identify optimal responders to IFN-beta. We studied effector and regulatory T and B lymphocytes and NK cells, since they play a role in the pathophysiology of MS [11] and are also related to the response to different MS treatments [8, 12,13,14].
Materials and methods
Patients
We included in the study two cohorts of patients diagnosed of RRMS according to modified McDonald criteria [15] that were initiating treatment with IFN-beta at Hospital Universitario Ramón y Cajal (Madrid, Spain) (119 patients, discovery cohort) and Hospital Universitari Vall d’Hebron (Barcelona, Spain) (49 patients, validation cohort). The study was approved by the Ethics Committees of both hospitals. Written informed consent was obtained from all patients before entry. Patients were not treated with immunosuppressive, immunomodulatory drugs or corticosteroids in the 6 months previous to IFN-beta therapy. Baseline clinical and demographic data of patients are shown in Table 1.
Patient follow-up
Patients were included consecutively in the study and followed for 2 years. Response to treatment was monitored by measuring the Expanded Disability Status Scale (EDSS) score, the number of new relapses, and the onset of new lesions on annual MRI scans. Relapses were defined as worsening of neurological impairment or appearance of new symptoms attributable to MS, lasting at least 24 h and preceded by stability of at least 1 month. Disability progression was defined as a worsening of ≥ 1 point in EDSS score persisting in two consecutive visits.
MRI scans of the brain were performed in a 1.5 T (Philips Gyroscan NT, Netherlands). Slice thickness of 5 mm were acquired to obtain contiguous axial sections that covered the entire brain. The following sequences were performed: T1 weighted imaging, T1 weighted imaging with gadolinium enhancement, axial FLAIR T2, axial T2-weighted imaging and axial proton density T2-weighted imaging.
Patients showing NEDA were considered as optimal responders (NEDA +). To have at least one relapse, increase of at least one point in the EDSS score confirmed in two consecutive visits, or presence of new T2 lesions or gadolinium-enhanced lesions were considered as suboptimal response (NEDA −).
Samples
Peripheral blood was collected before treatment initiation and at 6 and 12 months of treatment. For soluble factors and NAb detection, serum samples were obtained, aliquoted and stored at − 80 °C until used.
Lymphocyte subsets were analyzed in fresh whole blood or in isolated peripheral blood mononuclear cells (PBMC) as detailed below. PBMC were isolated from whole blood by Ficoll-Isopaque density gradient centrifugation (Life Technologies Ltd, UK) and stored in liquid nitrogen until used.
NAb detection
NAb were measured in serum samples obtained after 6 and 12 months of IFN-beta treatment by the cytopathic effect assay as described [16]. The titters were calculated according to Kawade’s formula [17], and expressed in tenfold reduction unit (TRU). Titers higher than 20 TRU/ml were considered as positive.
Study of serum cytokine levels
Serum cytokines were studied by ELISA (eBioscience, USA; R&D Systems, USA) following the manufacturer’s instructions. We studied serum levels of interleukin (IL)-1beta, IL-2, IL-4, IL-5, IL-6, IL-9, IL-10, IL-12, IL-13, IL-17A, IL-17F, IL-22, IFN-gamma and tumor necrosis factor (TNF)-alpha.
Monoclonal antibodies
The following monoclonal antibodies were used in the study: CD3-PerCP, CD3-APC-H7, CD4-FITC, CD4-APC, CD5-PE, CD5-APC, CD8-FITC, CD8-PerCP, CD8-PE-Cy7, CD16-FITC, CD16-PerCP, CD19-FITC, CD19-PerCP, CD19-PE-Cy7, CD19-APC, CD25-PE, CD27-FITC, CD38-FITC, CD45-PE, CD45-PerCP, CD45-APC, CD45-APC-H7, CD56-PerCP, CD56-APC, CD80-PE, CD86-PE, HLA-DR-PE, FoxP3-Alexa647, Perforin-FITC, IFN-gamma-FITC, TNF-alfa-PerCP (BD Biosciences), IL-10-PE (Biolegend, USA) and IL-17-APC (R&D Systems).
Analyses performed in whole blood
The following lymphocyte subsets were analyzed in whole peripheral blood samples: total CD19 + B cells, CD19 + CD5 + and CD19 + CD38 + B cells, total CD4 + and CD8 + T cells, CD56 + CD16 + natural killer (NK) cells, CD56bright cells, and CD3 + CD56 + NKT cells. Whole blood samples were labelled for 20 min at room temperature and erythrocytes lysed with FACS Lysing Solution (BD Biosciences). After washing, samples were resuspended in PBS and analyzed by flow cytometry.
Analyses performed in thawed PBMC
In the discovery cohort, thawed PBMC were used to study CD4 + CD25hiFoxP3 + T regulatory cells (Treg), CD8 + perforin + T cells, and intracellular cytokine production. In the validation cohort, all studies were performed in thawed samples. Analysis of Treg was made using the transcription factor buffer set (BD Biosciences) following the manufacturer’s instructions. To study perforin expression by cytotoxic T cells, surface antigens were labelled for 20 min at 4 °C in the dark, cells were permeabilized with Cytofix/Cytoperm (BD Biosciences), stained with an anti-perforin antibody, washed and analyzed by flow cytometry.
For intracytoplasmic cytokine detection, PBMC were resuspended in complete medium (CM), stimulated with 0.5 µg/ml of phorbol-12-myristate-13-acetate and 0.75 µg/ml ionomycin (Sigma-Aldrich, USA), in presence of 2 µg/ml Brefeldin A and 2.03 µM Monensin (BD Biosciences), and incubated 4 h at 37 °C in 5% CO2. For the analysis of IL-10 producing B cells, PBMC were incubated in CM with 3 µg/ml of ODN-CpG (InvivoGen, USA) for 20 h at 37 °C in 5% CO2 prior to stimulation. Then, surface markers were labelled for 20 min at 4 °C in the dark, cells washed, permeabilized with Cytofix/Cytoperm and incubated with monoclonal antibodies recognizing IFN-gamma, TNF-alpha, IL-10 and IL-17 for 20 min in the dark. After washing, stained cells were analyzed by flow cytometry.
Flow cytometry analysis
Data acquisition was performed with a FACSCanto II flow cytometer and analyzed with FACSDiva software V.7.0 (BD Biosciences). A gate including cells with intermediate to high CD45 and low to intermediate side scatter and excluding debris and apoptotic cells was used. A minimum of 30,000 events were collected for every analysis. Mean autofluorescence values were set using appropriate negative isotype controls.
Statistical analysis
Results were analyzed with the Prism 6.0 statistical package (GraphPad Software, USA). Mann–Whitney U tests were used for comparisons between groups; Fisher’s exact test to compare percentages and ROC-curve analyses to establish cut-off values. p values below 0.05 were considered significant.
Results
119 RRMS patients initiating treatment with IFN-beta were included consecutively in the study. 4 were lost during follow-up; 16 abandoned treatment for reasons not related to the efficacy of the drug, that is, desire for pregnancy, concomitant diseases, depression or poor tolerance to the drug, mild side effects. The remaining 99 patients were followed for 2 years and classified according to their clinical and MRI activity after follow-up in NEDA + or NEDA −.
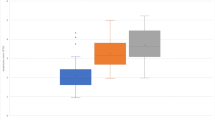
Nine patients presented NAb. Seven received IFN-beta 1b (Betaferon®) and two subcutaneous IFN-beta 1a 44 µg (Rebif®). No patient treated with intramuscular IFN-beta 1a (Avonex®) developed these antibodies. The proportion of patients showing NAb was significantly higher in patients treated with Betaferon. By contrast, no differences were found between patients treated with Avonex or Rebif (Fig. 1). All patients showing NAb were NEDA−. They were excluded from subsequent studies since treatment failure may be due to the lack of drug bioavailability produced by these antibodies.
Search for clinical and immunological biomarkers
34 patients (37.8%) achieved NEDA and the remaining 56 (62.2%) continued having disease activity during follow-up. No statistical differences were found in clinical and demographic characteristics of both groups of patients at treatment initiation, but they differed in clinical variables measured during treatment (Table 2).
No significant differences were observed in serum cytokines between both groups of patients (data not shown). However, we found differences between NEDA + and NEDA − in various lymphocyte subsets (Table 3). NEDA + patients showed lower percentages of B lymphocytes (p = 0.0003). These low values modestly associated with activated B cells CD19 + CD38 + (p = 0.02) and much clearly with the CD19 + CD5 + subpopulation (p = 0.0002) (Fig. 2a, c, d). NEDA + patients also showed a moderate decrease of Treg (p = 0.01) and a clear increase of CD8 + T lymphocytes producing perforin (p = 0.003, Fig. 2b, e, f). No differences were observed in any NK cell subset. When corrected p values by multiple comparison test (Bonferroni) data of total B cells (p = 0.006) and CD19 + CD5 + subset (p = 0.004) were the only ones that remained significant, although there was a trend for perforin + CD8 T cells (p = 0.06) (Table 3).
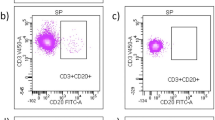
Percentages of CD19 + CD5 + B cells (a) and CD8 + perforin + T cells (b) shown by patients with no evidence (NEDA +) or ongoing (NEDA −) disease activity before interferon-beta treatment. Representative dot plots showing CD19 + CD5 + B cells from NEDA + (c) and NEDA − (d) patients and CD8 + perforin + T cells from NEDA + (e) and NEDA − patients (f)
Value of CD19 + CD5 + and CD8 + perforin + to predict response to IFN-beta
Using ROC curve analyses, we established cut-off values for percentages of CD19 + CD5 + B lymphocytes [area under the curve (AUC) 0.74, confidence interval (CI) 0.63–0.84, p = 0.0002, likelihood ratio 4.28] and CD8 + perforin + T cells. (AUC 0.79, CI 0.63–0.96, p = 0.003; Likelihood ratio 5.47). Having less than 3% of CD19 + CD5 + B lymphocytes; odds ratio (OR) 10.68, CI 3.55–32.15, p < 0.0001 or more than 2.6% of CD8 + perforin + T cells (OR 6.11, CI 2.0–18.6, p = 0.0009) associated with optimal responses to IFN-beta. The combination of both subsets had no added value for predicting treatment response (data not shown).
Validation of these biomarkers in an independent cohort
We studied NAb, CD19 + CD5 + B cells and CD8 + perforin + T cells in an independent cohort of 49 patients with RRMS treated with IFN-beta. Four patients (8%) developed NAb. All showed a suboptimal response to the drug. We analyzed CD19 + CD5 + B lymphocytes and CD8 + perforin + T cells in the remaining 45 patients. NEDA + patients (13, 28.9% of the total group) showed higher levels of CD8 + T cells expressing perforin (p = 0.01, Fig. 3a), thus confirming results obtained in the discovery cohort. However, no significant differences in the percentage of CD19 + CD5 + B lymphocytes were observed (Fig. 3b). Of note, NEDA + and NEDA − patients showed low percentages of CD19 + CD5 + B cells similar to those with optimal responses in the discovery cohort (Fig. 3b). We investigated if this could be due to differences in sample processing, since the analysis was made in whole blood in the discovery cohort and in thawed PBMC in the validation one, and cryopreservation may alter B-cell percentages [18]. We studied a new series of five RRMS patients, analyzed CD19 + CD5 + B cells in whole blood and in cryopreserved cells, and noted that cryopreservation decreased percentages of CD19 + CD5 + B cells (Fig. 3c), thus preventing validation of this biomarker in frozen samples.
Differences in CD8 + perforin + T cells between patients with no evidence (NEDA +) or ongoing (NEDA −) disease activity were similar in the discovery and validation cohorts (a). However, the increase in CD19 + CD5 + cells observed in NEDA − patients of the discovery cohort was not confirmed in the validation one (b). We assessed if this could be due to the differences in cell processing in both cohorts and observed that the percentages of CD19 + CD5 + B cells diminished in thawed peripheral blood mononuclear cells (PBMC) compared with fresh blood (FB) (c)
Discussion
The increasing number of treatments actually available in MS and the heterogeneous response of patients to these drugs make necessary the search for biomarkers that allow early identification of optimal responders to every treatment.
Different blood biomarkers were proposed for predicting response to IFN-beta in MS. Circulating CD56 bright NK cells increased after treatment in optimal responders [8], but were not useful to identify them prior to treatment initiation. Serum IL-17F was also proposed as candidate biomarker [9] but could not be validated in later studies [19]. The presence of NAb is the only biomarker currently used to identify suboptimal responders [20]. Our study showed that no patient developing NAb remained NEDA after 2 years of follow-up, thus confirming the predictive value of these antibodies. The proportion of patients showing these antibodies was higher in patients treated with IFN-beta 1b, thus corroborating that IFN-beta 1a and 1b have different degrees of immunogenicity [21].
We studied in the remaining patients clinical and epidemiological variables, since optimal responders to treatment in MS can include benign patients that tend to have a milder disease course even if they do not receive immunomodulatory treatment. However, no pre-treatment differences were found between NEDA + and NEDA − patients, which suggest benign patients do not represent a substantial proportion of our responder cohort.
In the same way, no differences were observed in serum cytokine concentrations between optimal and suboptimal responders. By contrast, we noticed that NEDA − group had higher levels of Treg. This increase was modest (Indeed, significance was lost when Bonferroni correction was applied) and did not allow patient stratification.
One possible explanation for this phenomenon could be a higher subclinical inflammatory activity in NEDA − patients. Treg may attempt to counteract this inflammation with only partial success, since in MS these cells show a diminished regulatory function [22].
NEDA + patients showed lower percentages of circulating CD19 + CD5 + B cells and higher proportion of CD8 + perforin + T cells. Both lymphocyte subpopulations play an important role as effector mechanisms in MS physiopathology. CD19 + CD5 + B cells mediate T-independent B-cell responses, and thus can be activated directly by antigens without needing T-cell co-stimulation. High percentages of these cells associated with inflammatory activity in MS [23, 24]. IFN-beta would hardly modulate this T-independent B-cell response since it appears to act primarily on T cells [25]. CD8 + perforin + T cells are activated during Th1 immunological responses and play an important role in MS pathophysiology inducing axonal damage [26, 27]. These two mechanisms seem to be alternatively predominant in the blood of MS patients, that is, patients with high values of CD8 + perforin + T cells tend to have low levels of CD19 + CD5 + B cells. We hypothesize that IFN-beta may be more effective in patients with increased CD8 + perforin + T cell responses, which depend on IFN-gamma for their activation, than in those in which predominate T-cell independent B-cell responses.
We attempted to validate these results in frozen PBMC from an independent patient cohort. We obtained equivalent results for CD8 + perforin + T cells but could not validate CD19 + CD5 + B cell results since B-cell percentages are altered by freezing [18], thus showing the importance of cell processing in biomarker validation.
Although our results should be confirmed in larger series, these data strongly suggest that the percentage of circulating CD19 + CD5 + B cells and CD8 + perforin + T cells identify patients with a high probability of showing an optimal response to IFN-beta. Both biomarkers are relatively easy to perform and could contribute to a personalized treatment of MS patients in clinical practice.
Change history
24 November 2017
The author claims that his name is incorrectly listed on PubMed. It seems that the first and last name has been mixed up.
References
The IFNB Multiple Sclerosis Study Group (1993) Interferon beta-1b is effective in relapsing-remitting multiple sclerosis. I. Clinical results of a multicenter, randomized, double-blind, placebo-controlled trial. Neurology 43:655–661
Jacobs LD, Cookfair DL, Rudick RA et al (1996) Intramuscular interferon beta-1a for disease progression in relapsing multiple sclerosis. The Multiple Sclerosis Collaborative Research Group (MSCRG). Ann Neurol 39:285–294
PRISMS (Prevention of Relapses and Disability by Interferon beta-1a Subcutaneously in Multiple Sclerosis) (1998) Study group randomised double-blind placebo-controlled study of interferon beta-1a in relapsing/remitting multiple sclerosis. Lancet 352:1498–1504
Einarson TR, Bereza BG, Machado M (2017) Comparative effectiveness of interferons in relapsing–remitting multiple sclerosis: a meta-analysis of real-world studies. Curr Med Res Opin 33:579–593
Giovannoni G, Turner B, Gnanapavan S, Offiah C, Schmierer K, Marta M (2015) Is it time to target no evident disease activity (NEDA) in multiple sclerosis? Mult Scler Relat Disord 4:329–333
Rotstein DL, Healy BC, Malik MT, Chitnis T, Weiner HL (2015) Evaluation of no evidence of disease activity in a 7-year longitudinal multiple sclerosis cohort. JAMA Neurol 72:152–158
Río J, Castilló J, Rovira A et al (2009) Measures in the first year of therapy predict the response to interferon beta in MS. Mult Scler 15:848–853
Martínez-Rodríguez JE, López-Botet M, Munteis E, Rio J, Roquer J, Montalban X, Comabella M (2011) Natural killer cell phenotype and clinical response to interferon-beta therapy in multiple sclerosis. Clin Immunol 141:348–356
Hartung HP, Steinman L, Goodin DS et al (2013) Interleukin 17F level and interferon beta response in patients with multiple sclerosis. JAMA Neurol 70:1017–1021
Pachner AR, Dail D, Pak E, Narayan K (2005) The importance of measuring IFNbeta bioactivity: monitoring in MS patients and the effect of anti-IFNbeta antibodies. J Neuroimmunol 166:180–188
Ransohoff RM, Hafler DA, Lucchinetti CE (2015) Multiple sclerosis—a quiet revolution. Nat Rev Neurol 11:134–142
De Mercanti S, Rolla S, Cucci A et al (2016) Alemtuzumab long-term immunologic effect: Treg suppressor function increases up to 24 months. Neurol Neuroimmunol Neuroinflamm 3:e194
Blumenfeld S, Staun-Ram E, Miller A (2016) Fingolimod therapy modulates circulating B cell composition, increases B regulatory subsets and production of IL-10 and TGFβ in patients with multiple sclerosis. J Autoimmun 70:40–51
Bielekova B, Catalfamo M, Reichert-Scrivner S et al (2006) Regulatory CD56 (bright) natural killer cells mediate immunomodulatory effects of IL-2R alpha-targeted therapy (daclizumab) in multiple sclerosis. Proc Natl Acad Sci USA 103:5941–5946
Polman CH, Reingold SC, Banwell B et al (2011) Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 69:292–302
García-Montojo M, Domínguez-Mozo MI, De las Heras V, Bartolome M, Garcia-Martinez A, Arroyo R, Alvarez-Lafuente R (2010) Neutralizing antibodies, MxA expression and MMP-9/TIMP-1 ratio as markers of bioavailability of interferon beta treatment in multiple sclerosis patients. A two years follow-up study. Eur J Neurol 17:470–478
Kawade Y (1986) Quantitation of neutralization of interferon by antibody. Methods Enzymol 119:558–573
Reimann KA, Chernoff M, Wilkening CL, Nickerson CE, Landay AL (2000) Preservation of lymphocyte immunophenotype and proliferative responses in cryopreserved peripheral blood mononuclear cells from human immunodeficiency virus type 1-infected donors: implications for multicenter clinical trials. The ACTG immunology advanced technology laboratories. Clin Diag Lab Immunol 7:352–359
Bushnell SE, Zhao Z, Stebbins CC et al (2012) Serum IL-17F does not predict poor response to IM IFNbeta-1a in relapsing–remitting MS. Neurology 79:531–537
Bertolotto A, Sala A, Malucchi S, Marnetto F, Caldano M, Di Sapio A, Capobianco M, Gilli F (2004) Biological activity of interferon betas in patients with multiple sclerosis is affected by treatment regimen and neutralising antibodies. J Neurol Neurosurg Psychiatry 75:1294–1299
Bertolotto A, Malucchi S, Sala A et al (2002) Differential effects of three interferon betas on neutralising antibodies in patients with multiple sclerosis: a follow up study in an independent laboratory. J Neurol Neurosurg Psychiatry 73:148–153
Frisullo G, Nociti V, Iorio R et al (2009) Regulatory T cells fail to suppress CD4T + -bet + T cells in relapsing multiple sclerosis patients. Immunology 127:418–428
Seidi OA, Semra YK, Sharief MK (2002) Expression of CD5 on B lymphocytes correlates with disease activity in patients with multiple sclerosis. J Neuroimmunol 133:205–210
Villar LM, Espiño M, Roldán E, Marín N, Costa-Frossard L, Muriel A, Alvarez-Cermeño JC (2011) Increased peripheral blood CD5 + B cells predict earlier conversion to MS in high-risk clinically isolated syndromes. Mult Scler 17:690–694
Praksova P, Stourac P, Bednarik J, Vlckova E, Mikulkova Z, Michalek J (2012) Immunoregulatory T cells in multiple sclerosis and the effect of interferon beta and glatiramer acetate treatment on T cell subpopulations. J Neurol Sci 319:18–23
Skulina C, Schmidt S, Dornmair K et al (2004) Multiple sclerosis: brain-infiltrating CD8 + T cells persist as clonal expansions in the cerebrospinal fluid and blood. Proc Natl Acad Sci USA 101:2428–2433
Salou M, García A, Michel L et al (2015) Expanded CD8 T-cell sharing between periphery and CNS in multiple sclerosis. Ann Clin Transl Neurol 2:609–622
Acknowledgements
This work was supported by Grants PI15/00513, RD16/0015/0001, RD16/0015/0004 and RD16/0015/0013 from the Fondo para la Investigación Sanitaria, Instituto de Salud Carlos III, Ministerio de Economía y Competitividad, Spain and FEDER.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical statement
The study protocol was approved by the Ethics Committee of Hospital Universitario Ramón y Cajal (Madrid, Spain) and of Hospital Universitari Vall d’Hebron (Barcelona, Spain). The study was carried out according to the International Conference on Harmonization Guidelines for Good Clinical Practice and the Declaration of Helsinki.
Informed consent
Every patient provided written informed consent before entering the study.
Conflicts of interest
LMV, LCF, SSM, JCA-C, JR and XM received payment for lecturing or travel expenses or research Grants or consultancy from Merck-Serono, Biogen, Sanofi-Genzyme, Roche, Bayer and Novartis. The remaining authors declare no conflicts of interest.
Additional information
José C. Álvarez-Cermeño and Luisa M. Villar were principal co-investigators.
A correction to this article is available online at https://doi.org/10.1007/s00415-017-8679-5.
Rights and permissions
About this article
Cite this article
Alenda, R., Costa-Frossard, L., Alvarez-Lafuente, R. et al. Blood lymphocyte subsets identify optimal responders to IFN-beta in MS. J Neurol 265, 24–31 (2018). https://doi.org/10.1007/s00415-017-8625-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-017-8625-6