Abstract
Patients with bilateral vestibular failure (BVF) exhibit imbalance when standing and walking that is linked to a higher fall risk. The purpose of this study was to identify risk factors for falls in BVF. We therefore systematically investigated the interrelationship of clinical and demographic characteristics, gait impairments, and the fall frequency of these patients. Clinical and demographic characteristics as well as quantitative measures of gait performance on a pressure-sensitive gait carpet were collected from 55 patients with different etiologies of BVF. Clinical and demographic data as well as spatiotemporal gait characteristics were used for ANOVA testing and a logistic regression model with categorized fall events as dependent variables. The impairment of peripheral vestibular function, duration of disease, and the overall gait status were not associated with the history of falls in patients with BVF. In contrast, the most predictive factors for falls in BVF were an increase in temporal gait variability, especially at slow walking speeds (p < 0.001; OR = 1.3), and the presence of a concomitant peripheral neuropathy (p < 0.045; OR = 3.6). BVF patients with a high risk of falling exhibit specific gait alterations in a speed-dependent manner. In particular, increased gait fluctuations during slow walking are most predictive for an increased fall risk. The presence of a concomitant peripheral neuropathy further critically impairs postural stability in these patients. Clinical assessment of both these aspects is therefore important to identify those patients at a particularly high fall risk and to initiate preventive procedures early.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bilateral vestibular failure (BVF) is a heterogeneous chronic illness with the cardinal symptoms of motion-dependent dizziness, oscillopsia during head movements, and unsteadiness of stance and gait [22]. The gait disorder is characterized by a broadened base of support, reduced stride length, and increased spatiotemporal gait fluctuations [15]. It thus corresponds to a sensory ataxic gait phenotype that undergoes context-specific exacerbation due to disturbed visual or proprioceptive feedback, e.g., when walking with eyes closed, in darkness, or on uneven ground [1]. Epidemiological fall risk studies and clinical experience indicate that the presence of a concomitant sensory deficit, in particular peripheral neuropathy, considerably affects gait performance and impairs patients’ ability to maintain dynamic stability [14]. Furthermore, gait unsteadiness of patients with BVF critically depends on walking speed. Increased gait fluctuations and unsteadiness are mainly observed during slow walking modes, whereas fast walking performance appears to be nearly normal [15].
The impaired gait performance of BVF patients also results in secondary effects of disease. For example limited daily mobility and reduced physical activity worsen social functioning [6]. Patients’ risk of falling is not only increased compared to healthy subjects, but also compared to patients with episodic forms of vertigo and dizziness [14]. Fall-related injuries together with the fall-induced fear of future falls can result in further deterioration of social functioning and quality of life. Thus, it would be desirable to establish clinical algorithms to assess the patient’s individual fall risk in order to improve the symptomatic therapeutic regimens for BVF.
The current study investigated the interrelationship of clinical and demographic characteristics, gait impairments, and falls in patients with BVF. Predictive factors for an increased risk of falls in patients with BVF will be useful for future clinical fall risk estimation and may help to reduce the incidence of falls in these patients.
Methods
Patients
Fifty-five patients with BVF were recruited from the outpatient clinic of the German Center for Vertigo and Balance Disorders (DSGZ) to participate in this study. All patients showed a clinically proven deficit (bilateral pathologic head impulse test and reduced or absent caloric responses (i.e., the sum of maximum slow-phase eye velocities during warm and cold caloric irrigation was below 10 °/s). Each patient underwent a complete neurological and physical examination prior to the experimental procedures. Exclusion criteria were other comorbidities affecting posture and locomotion abilities, such as CNS pathologies (tumor, stroke, neurodegenerative, and inflammatory diseases) and musculoskeletal disorders. Patients with sensory neuropathy (without weakness) were included into the study. All subjects gave their informed written consent prior to the experiments. The study protocol was approved by the local ethics committee.
Fall assessment
All patients participated in a standardized interview conducted by one of the authors (R.S. or C.S.). The following information was recorded: duration of symptoms, ambulatory status, functional status, medication, and falls in the preceding 6 months. Furthermore, their subjective level of stability was assessed by the Falls-Efficacy Scale-International (FES-I) and the Activity-specific Balance Confidence Scale (ABC) [5, 13].
Gait assessment
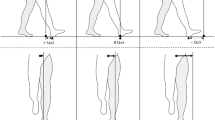
Gait analysis was performed using a 6.7 m-long pressure-sensitive carpet (GAITRite®, CIR System, Sparta, NJ, USA) with a sampling rate of 120 Hz. All patients had to walk over the carpet at three different speeds [preferred (PWS), slow (SWS), and maximally fast (MWS)]. Gait assessment was performed without additional ambulatory aids. Each walk was started 1.5 m in front of the mat and continued for 1.5 m beyond it to allow steady-state locomotion; each condition was tested four times. If necessary, the examiner walked beside the patient (approximately, 0.3 m behind) to prevent falls. Trials with near-fall events were discarded and repeated. Clinical gait assessment was based on the Functional Gait Assessment (FGA), a 10-item gait test developed for patients with balance deficits and vestibular disorders [17].
Data analysis
Patients were assigned according to their individual frequency of fall events within the preceding 6 months to the groups “non-faller” and “faller”. Walking velocity and six additional standard gait cycle parameters (i.e., cadence, stride time, stride length, base of support, swing phase percentage, and double support phase percentage) were analyzed for each walking speed condition. Furthermore, the variability magnitude of stride time, stride length, and base of support was analyzed by the coefficient of variation (i.e., CV(%) = (σ/μ) × 100 with standard deviation σ and mean μ).
The dependency of gait variability on walking speed was further estimated by second-order polynomial fits based on 12 gait trials (i.e., three speed conditions, each tested four times) following a previously described procedure [16]. Each fit function was normalized with respect to the individual´s PWS. Then the area under the curve (AUC) was calculated by \({\text{AUC}} = \int_{i}^{j} {{\text{CV}}_{\text{parameter}} (s){\text{d}}s/(j - i)}\) once for the whole speed range (with i = 0.25 × PWS and j = 1.75 × PWS) as well as for three separate speed sections (“slow”: with i = 0.25 × PWS and j = 0.75 × PWS; “medium”: with i = 0.75 × PWS and j = 1.25 × PWS; “fast”: with i = 1.25 × PWS and j = 1.75 × PWS). Those patients whose measured speed range did not reach 0.25 of PWS (slow walking) and 1.75 of PWS (maximally fast walking) were excluded from this analysis to avoid extrapolation of the regression fit.
Statistical analysis was performed using SPSS (Version 20). In a first step, a two-way ANOVA was used to determine significant differences between the non-faller and the faller groups with respect to demographic and clinical data, spatiotemporal gait parameters, and the AUC of gait variability with “walking speed” as a cofactor for the gait parameters. In a second step, a stepwise forward logistic regression model (controlled for age and gender) was used to identify those variables that are able to predict the individual fall status. The above-mentioned binominal categorized fall frequency was used as dependent variable. All significant parameters identified in the first-step ANOVA were used as covariates. Results were considered significant if p < 0.05.
Results
Patient characteristics
The BVF cohort had a mean age of 74 ± 12 years and a mean duration of symptoms of 31 ± 29 months. All patients had bilaterally pathologic head impulse tests clinically rated separately by two neuro-otological specialists. The mean sum of unilateral caloric nystagmus response was 3 °/s on the left and 4 °/s on the right side. The majority of the BVF cohort had an idiopathic form of BVF (48 patients, 88%). Four patients had a diagnosis of probable Menière´s disease (7%) and three patients ototoxic drug use (gentamicin; 5%); they were considered to have secondary forms of BVF (Table 1). Only 14 patients performed balance training in a physiotherapeutic setup 1–3 times a week. Twenty-four patients had clinical signs of a peripheral neuropathy (reduced vibrotactile thresholds, reduced ankle jerk reflexes) with sensory deficits of the legs, but without manifest motor dysfunction. Visual acuity with correction was on both eyes 0.8 in median, without correction left 0.35 and right 0.4 in median. Neither visual acuity nor other neuro-otological findings (Tables 1, 2) were significantly different between fallers and non-fallers.
Ambulatory status and fall history
The Functional Gait Assessment (FGA) scores of the enrolled subjects showed a moderate impairment of gait function with a median of 12 (range 4; 26). The ABC scores were at mean 65%, thus indicating a moderate level of physical functioning of the patients. There were no significant differences between patients with falls and those without falls. The FES-I indicated a moderate level of fear of falling with a median of 34 points. Although a slight tendency to increased FES-I scores was found in patients with falls compared to those without falls (37 vs. 32 points), this difference did not reach significance. Twenty-one patients reported falls in the preceding 6 months. Thirteen patients (24%) had one fall, and eight (15%) reported frequent falls (Table 1). An overall of 32 falls were described by the patients. The fall status was independent of the FGA score, individual caloric responses, primary or secondary forms of BVF, and the duration of symptoms. The falls occurred mainly when the visual acuity was diminished by darkness or when walking on uneven ground (88%). Only two fall events took place during ambulation at home. Thirty-eight patients (69%) reported ambulation without aids, whereas 10 patients (18%) reported occasional use of aids. Seven patients (13%) reported a major use of aids, and three patients relied mainly on the personal assistance of their partners. The fall status was, however, also independent of the use of walking aids. Only four patients reported a fall that happened when using the aid, whereas the majority (80%) reported that falls usually occurred without the aid.
Gait characteristics of fallers vs. non-fallers
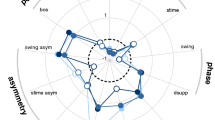
Comparisons of the common spatiotemporal gait parameters between fallers and non-fallers revealed significant differences, mainly during the condition of preferred walking speed. At that condition, walking speed was significantly lower in fallers (p < 0.05), stride length was reduced (p < 0.05), base of support was increased (p < 0.001), swing phases reduced (p < 0.05), and double support phases increased (p < 0.05). The CV values of stride time and stride length were increased in fallers compared to non-fallers, for slow walking (all p < 0.05), and for preferred walking speeds (CV of stride time p < 0.01; CV of stride length p < 0.05). No differences were found for the CV of base of support. Moreover, the spatiotemporal gait parameters did not differ between fallers and non-fallers for the condition of fast walking (Table 1 of the supplemental data). Comparisons of the AUC of gait variability parameters yielded significantly higher AUCs of stride time CV in all three speed sections for fallers (all p < 0.001, Table 3; Fig. 1). The AUC of stride length CV was found to be significantly higher in fallers only for the slow speed section (p < 0.05). Significant correlations with FES-I were found for walking speed (R 2 = −0.488, p < 0.05) and for CV of stride time (R 2 = 0.421, p < 0.05), both during preferred walking.
Fall status predictors
The binary logistic regression model showed that only two parameters were predictive for the individual fall status in the study cohort: peripheral neuropathy and the AUC of stride time. The presence of peripheral neuropathy was associated with an OR of 3.6 [1.0; 12.9] for the occurrence of falls. Moreover, the AUC of stride time CV during slow walking was associated with an OR of 1.3 [1.2; 1.4] for the occurrence of falls (Table 4; Fig. 2).
Discussion
The current study investigated for the first time the relationship between gait impairments, clinical and demographic characteristics, and the history of falls in patients with BVF. These patients experience oscillopsia and a persistent instability when standing and walking which is linked to a higher risk of falls [10]. A recent epidemiological study on different diagnoses of peripheral vertigo and dizziness found that patients with BVF are at the highest risk of falling [14]. Our findings show lower fall prevalence: In the present BVF cohort, 24% were occasional fallers and 15% were recurrent fallers. The outcome of the present study allows us to identify the relevant factors associated with an increased fall risk in BVF and may help to identify those patients at risk as well as initiate protective procedures for them at an early stage. We found that the impairment of peripheral vestibular function, the duration of disease, and the overall gait status (as measured by the FGA score) were not associated with the history of falls in the present BVF study cohort. In contrast, specific quantitative alterations in the patient’s walking pattern as well as the presence of a concomitant loss of proprioceptive feedback were found to be most predictive for the patients’ fall status. The majority of falls occurred during situations of disturbed visual (darkness) or proprioceptive (uneven ground) feedback control. This emphasizes the hypothesis that patients with bilateral vestibular hypofunction highly rely on vision and proprioception to stabilize gait. The prophylactic treatment of fall-prone patients with BVF should therefore include recommendations to ensure sufficient lighting and even floor conditions at home. For off-site ambulation, patients should be advised to choose light and even walkways and to use protective aids in complex walking situations.
An apparent feature of the gait disorder of patients with BVF is the high variability of their stepping pattern [12, 15]. Due to the loss of vestibular feedback information, they cannot adequately adjust their walking pattern to naturally occurring internal and external perturbations. This results in increased spatiotemporal stride-to-stride fluctuations. An increase in gait variability has been shown to reflect a loss of dynamic walking stability in several neurological gait disorders [3, 7–9]. This appears to be also true for patients with BVF, since those patients with a history of falls are especially characterized by elevated magnitudes of spatiotemporal gait variability compared to non-fallers. The observed differences in gait variability depended on the gait velocity and mainly occurred during slow to moderate walking modes. Such modes are thought to critically rely on adequate sensory feedback which allows the individual to maintain dynamic gait stability [2, 20, 21]. Patients with a history of falls further exhibited a slowing of walking speed with a broadened base of support and prolonged double support phases in comparison to non-fallers. These gait alterations were predominantly present during preferred locomotion speeds. They indicate a cautious mode of walking that most likely reflects a compensatory strategy to stabilize impaired walking performance [21].
The logistic regression model also indicated that an increase of temporal gait variability levels during slow walking speed is the only gait characteristic predictive for an increased risk of falls in patients with BVF. Besides variability in magnitudes at specific walking speeds, the present study also analyzed speed-dependent integrals of gait variability (i.e., AUC of CV). As in an earlier study on fall risk estimation in patients with cerebellar ataxia [16], we also observed that a broad-spectrum measure is superior to commonly used discrete measures of gait variability for estimating the individual fall risk. The rationale behind this observation is that whereas preferred walking modes are very consistent in test–retest sequences due to biomechanical and energy consumption constraints [11], the examination of non-preferred slow or fast walking modes greatly depends on the examiner’s instruction as well as on the individual’s motivation. The speed-dependent integral compensates for such inconsistencies by covering a broader speed spectrum based on multiple repeated assessments of single walking conditions. Although this elaborate gait assessment is presumably restricted to specialized gait and balance centers, we nevertheless recommend including an examination of slow walking performance in the clinical assessment of patients with BVF. Furthermore, therapeutic interventions in these patients should focus on improving dynamic walking stability at slow to moderate walking modes. In this context, a new paradigm of imperceptible stochastic galvanic vestibular stimulation appears to be promising. It was recently demonstrated to effectively decrease spatiotemporal gait fluctuations in patients with BVF, particularly at slow walking speeds [18, 19]. However, its potential to reduce the fall risks in these patients has to be further investigated.
Another critical factor of increased fall risk in BVF is a concomitant loss of proprioceptive feedback. The logistic regression model indicates that the presence of peripheral neuropathy is the most important predictive factor for a high fall risk. To maintain postural stability, patients with BVF compensate for vestibular loss by increasingly relying on visual and proprioceptive feedback sources [4]. Any additional disturbance of proprioceptive feedback due to peripheral neuropathy therefore critically impairs balance control and increases the risk of falling. Schlick et al. observed that patients with multi-sensory deficits are at a higher risk of falling than patients with deficits in only one sensory modality [14]. These findings stress the importance of clinically assessing signs of neuropathy in patients with BVF to correctly estimate their fall risk. Moreover, treatment of risk factors for neuropathy should be included in the therapeutic regimen of patients with BVF. In contrast to a recent epidemiological study on fall risks in patients with vertigo and dizziness [14], we could actually not find a significant influence of age on the fall risks in our cohort. Thus, age has only minor effects on dynamic stability in BVF compared to the influence of the compensatory capacity of somatosensory feedback or compared to the overall extent of the gait disorder (measured by increased gait variability).
In summary, this study identified predictive factors for falls in BVF which may improve the early detection of patients at risk. The gait performance of patients with a history of falls considerably differed from that of non-fallers in a speed-dependent manner. This difference was most prominent during slow to moderate walking speeds. Accordingly, increased temporal gait variability during slow walking was significantly associated with a higher risk of falls. Furthermore, patients who have a concomitant peripheral neuropathy are at a particularly high risk of falling. The clinical assessment of both these aspects is therefore critically important to identify those patients at a particularly high fall risk and to initiate preventive procedures early.
References
Brandt T, Huppert T, Hufner K, Zingler VC, Dieterich M, Strupp M (2010) Long-term course and relapses of vestibular and balance disorders. Restor Neurol Neurosci 28:69–82
Brandt T, Strupp M, Benson J (1999) You are better off running than walking with acute vestibulopathy. Lancet 354:746
Callisaya ML, Blizzard L, Schmidt MD, Martin KL, McGinley JL, Sanders LM, Srikanth VK (2011) Gait, gait variability and the risk of multiple incident falls in older people: a population-based study. Age Ageing 40:481–487
Deshpande N, Patla AE (2005) Postural responses and spatial orientation to neck proprioceptive and vestibular inputs during locomotion in young and older adults. Exp Brain Res 167:468–474
Greenberg SA (2012) Analysis of measurement tools of fear of falling for high-risk, community-dwelling older adults. Clin Nurs Res 21:113–130
Guinand N, Boselie F, Guyot JP, Kingma H (2012) Quality of life of patients with bilateral vestibulopathy. Ann Otol Rhinol Laryngol 121:471–477
Hausdorff JM (2005) Gait variability: methods, modeling and meaning. J Neuroeng Rehabil 2:19
Hausdorff JM, Cudkowicz ME, Firtion R, Wei JY, Goldberger AL (1998) Gait variability and basal ganglia disorders: stride-to-stride variations of gait cycle timing in Parkinson’s disease and Huntington’s disease. Mov Disord 13:428–437
Hausdorff JM, Rios DA, Edelberg HK (2001) Gait variability and fall risk in community-living older adults: a 1-year prospective study. Arch Phys Med Rehabil 82:1050–1056
Herdman SJ, Blatt P, Schubert MC, Tusa RJ (2000) Falls in patients with vestibular deficits. Am J Otol 21:847–851
Holt KJ, Jeng SF, Rr RR, Hamill J (1995) Energetic cost and stability during human walking at the preferred stride velocity. J Mot Behav 27:164–178
Perring S, Summers T (2007) Laboratory-free measurement of gait rhythmicity in the assessment of the degree of impairment and the effectiveness of rehabilitation in patients with vertigo resulting from vestibular hypofunction. Physiol Meas 28:697–705
Powell LE, Myers AM (1995) The activities-specific balance confidence (ABC) scale. J Gerontol Ser A 50:M28–M34
Schlick C, Schniepp R, Loidl V, Wuehr M, Hesselbarth K, Jahn K (2016) Falls and fear of falling in vertigo and balance disorders: a controlled cross-sectional study. J Vestib Res 25:241–251
Schniepp R, Wuehr M, Neuhaeusser M, Kamenova M, Dimitriadis K, Klopstock T, Strupp M, Brandt T, Jahn K (2012) Locomotion speed determines gait variability in cerebellar ataxia and vestibular failure. Mov Disord 27:125–131
Schniepp R, Wuehr M, Schlick C, Huth S, Pradhan C, Dieterich M, Brandt T, Jahn K (2014) Increased gait variability is associated with the history of falls in patients with cerebellar ataxia. J Neurol 261:213–223
Wrisley DM, Marchetti GF, Kuharsky DK, Whitney SL (2004) Reliability, internal consistency, and validity of data obtained with the functional gait assessment. Phys Ther 84:906–918
Wuehr M, Nusser E, Decker J, Krafczyk S, Straube A, Brandt T, Jahn K, Schniepp R (2016) Noisy vestibular stimulation improves dynamic walking stability in bilateral vestibulopathy. Neurology 86:2196–2202
Wuehr M, Nusser E, Krafczyk S, Straube A, Brandt T, Jahn K, Schniepp R (2016) Noise-enhanced vestibular input improves dynamic walking stability in healthy subjects. Brain Stimul 9:109–116
Wuehr M, Schniepp R, Pradhan C, Ilmberger J, Strupp M, Brandt T, Jahn K (2013) Differential effects of absent visual feedback control on gait variability during different locomotion speeds. Exp Brain Res 224:287–294
Wuehr M, Schniepp R, Schlick C, Huth S, Pradhan C, Dieterich M, Brandt T, Jahn K (2014) Sensory loss and walking speed related factors for gait alterations in patients with peripheral neuropathy. Gait Posture 39:852–858
Zingler VC, Weintz E, Jahn K, Huppert D, Cnyrim C, Brandt T, Strupp M (2009) Causative factors, epidemiology, and follow-up of bilateral vestibulopathy. Ann N Y Acad Sci 1164:505–508
Acknowledgements
The authors thank Judy Benson for copy-editing the manuscript. The work was supported by the German Research Foundation (Deutsche Forschungsgemeinschaft, DFG JA 1087/1-1), the German Hertie Foundation, and the Federal Ministry for Education and Science (BMBF, Nr. 80121000-49) of Germany.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical standard statement
The study protocol was approved by the local Ethics Committee. It was conducted in accordance to the ethical standards laid down in the declaration of Helsinki and its later amendments.
Conflicts of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Schniepp, R., Schlick, C., Schenkel, F. et al. Clinical and neurophysiological risk factors for falls in patients with bilateral vestibulopathy. J Neurol 264, 277–283 (2017). https://doi.org/10.1007/s00415-016-8342-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-016-8342-6