Abstract
DWI has been described in some reports to be superior to FLAIR in early stage herpes simple virus encephalitis (HSE). Few data exist on detailed topographical MRI analysis in HSE. Our aim was to study DWI and FLAIR, and analyse topographically these sequences in non-neonatal HSE patients with MRI performed within 60 days. Eleven HSE patients were analysed retrospectively. For topographical analysis, we developed a radiological 50-point score (25 points for each hemisphere, with each point corresponding to a brain area). In patients with MRI performed within 2 weeks (n = 9), DWI detected 11 % more areas involved than FLAIR. Thalamic involvement was frequent (67 %) in the early phase on FLAIR, being the only brain substructure better visualized on FLAIR than on DWI. In areas involved on both sequences, DWI showed more extensive (especially cortical) abnormalities in 14 % of the areas. In patients with late MRI (n = 2), FLAIR was superior to DWI (with essentially white matter involvement). From the mesial temporal area, brain signal changes followed a centripetal (i.e. towards anterior, posterior, and superior parts of the brain) gradient. The cut-off score before involving the contralateral hemisphere was 8–9/25 in the initially involved hemisphere. DWI is slightly superior to FLAIR in acute–subacute HSE, except for the thalamus with FLAIR signal changes more frequently seen than earlier reported. Knowledge of typical topographical MRI involvement can help to differentiate from other conditions mimicking HSE.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Herpes simple virus encephalitis (HSE) is a life-threatening disease. Early diagnosis is essential since delayed diagnosis is associated with increased neurological deficit or death. Herpes simplex virus (HSV) primarily affects neurons, explaining the primary cortical distribution of HSE followed by secondary white matter involvement. In HSE, diffusion-weighted imaging (DWI) has been reported to show well (often even better, especially in early stage disease) and often earlier signal changes than on fluid-attenuated inversion recovery (FLAIR) sequences [1–10]. In these reported cases, low ADC values were often associated. Most reports comparing diffusion imaging with FLAIR/T2-weighted imaging in HSE, however, are based on DWI rather than on ADC imaging. Where diffusion abnormalities seem to subside after 2 weeks, FLAIR/T2 hyperintensities (together with gadolinium-enhancement) persist for several weeks.
In neonatal HSE, large series of patients have been analysed by MRI, showing diffuse, bilateral, and multilobar involvement [11]. In these neonatal HSE patients, thalamic involvement has been described in some patients [11].
In non-neonatal HSE, only a few studies analysed series of patients with DWI MRI [8–10]. In the largest study, by analysing DWI in viral encephalitis (including ten HSE patients), the majority of patients showed DWI as superior to conventional MRI in detecting signal abnormalities (although it was not specified in how much of the HSE patients this was the case) [9]. Most other reports describing DWI in non-neonatal HSE are case reports or very small series (including one series of three HSE patients and another study analyzing seven HSE patients) [9, 10]. In one relatively large study, comparing patients with human herpes virus 6 (n = 8) and HSV (n = 9) encephalitis, DWI was not found to be superior to FLAIR in HSE patients [12]. In non-neonatal HSE, thalamic involvement seems to be extremely rare.
Very few data exist on detailed anatomical MRI analysis of different brain substructures in HSE [12].
Our aim was to compare DWI and FLAIR sequences, and analyse the involvement of different brain substructures in non-neonatal HSE on both these MRI sequences performed within 60 days after symptom onset.
Methods
We searched, retrospectively, in the neurological database of our centre (CHU Nîmes, France) between January 2008 and December 2014 for non-neonatal HSE cases. Eleven HSE patients (3 women and 8 men) were found with MRI performed (including DWI and FLAIR sequences) within 60 days. Patient characteristics are shown in Table 1. Mean age of patients was 54 (range 8–84). MRI was performed after a mean of 15 days after symptom onset, with 9 patients who had first MRI within 2 weeks, whereas 2 patients had MRI at day 56). Different MRI scans and field strengths (0.3 T, n = 2; 1 T, n = 3, 1.5 T, n = 3; 3 T, n = 3) were used with slightly different TR and TE parameters. Slice thickness of 5 mm, without interslice gap, was identical for all patients.
To analyse different brain substructures, we developed a radiological 50 points score (25 points for each hemisphere). Each point corresponded to a brain area, taking into account preferentially areas typically involved in HSE. HSE typically involves temporal and frontal lobes. Therefore, relatively many points were attributed to these lobes (and thus each point corresponding to a relative small area in these lobes). In each hemisphere, on axial slices, we attributed 9 points for the temporal lobe, 9 for the frontal lobe, 3 for the parietal lobe, 2 for the occipital lobe, and 2 for the deep grey matter. Figure 1 shows the different brain areas taking into account by our score.
FLAIR imaging of patient nr 2 is shown. In this patient, HSE-related signal changes could be well visualized and scored, since MRI could be compared with an earlier MRI performed for neurological deficit related to vascular small vessel disease. Row 1 showing the areas scored for the temporal lobe (1–9). Row 2 showing the scored areas for the frontal lobe (1–9). Row 3 showing the areas scored for the parietal lobe (1–3), the occipital lobe (4 and 5), and the deep grey matter (6 and 7)
For the temporal lobe, we considered the following areas: On axial view at pontine level, the inferior part of the temporal pole (1 point). On axial view at the level of the uppermost part of the mesencephalon, the anterior part of the mesial temporal area (1 point), the posterior part of the mesial temporal area (1 point), the area between the limen insulae and the uncus (1 point), the anterior part of the lateral temporal lobe (1 point), and the posterior part of the lateral temporal lobe (1 point). On axial view at the subthalamic level: the limbic area (1 point). On axial view at insular level: the anterior part of the lateral temporal lobe (1 point) and the posterior part of the lateral temporal lobe (1 point).
For the frontal lobe, the following areas were considered: On axial view at the level of the uppermost part of the mesencephalon and the orbitofrontal cortex, the posterior part of the gyrus rectus (1 point), the anterior part of the gyrus rectus (1 point), the posterior part of the orbitofrontal area (1 point), and the anterior part of the orbitofrontal area (1 point). On axial view at the level of the anterior part of the corpus callosum, the anterior cingulate area (1 point), the polus frontalis (1 point), the inferior part of the lateral frontal area (1 point), and the insula (1 point). On axial views superior to the uppermost parts of the lateral ventricles: the superior part of the lateral frontal area and the mesial frontal area (1 point).
For the parietal lobe: the lowest part of the precuneus and the posterior cingulate area (1 point), the lateral parietal area (1 point), and the mesial parietal area (1 point).
For the occipital lobe: the lateral occipital area (1 point) and the mesial occipital area (1 point).
For the deep grey matter: the lentiform and caudate nucleus (1 point), and the thalamus (1 point).
We started from the hypothetical premise that in all HSE patients, MRI signal changes progress similarly over time. We looked to the distribution of HSE lesions in our patients and tried to propose an anatomical pattern of lesion progression in HSE.
Results
In 4/11 patients, DWI score was higher than FLAIR. In all these 4 patients, MRI was performed within 8 days. In 3/11 patients, FLAIR score was higher than DWI. In these 3 patients, MRI was performed 11 days or later after symptom onset. In the remaining 4/11 patients, FLAIR and DWI score were equal. In these 4 patients, MRI was performed between day 2 and 12.
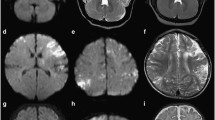
In the patients with MRI performed within 2 weeks, DWI detected overall more involved areas than FLAIR (146 against 132 areas, respectively, i.e. 14 areas more, corresponding to 11 %). In these patients, when considering each brain area, 19 areas (all cortical-subcortical zones) were pathological on DWI with normal FLAIR signal, and 5 areas (4 times the thalamus and 1 time the anterior part of the mesial temporal area) showed FLAIR signal changes with normal DWI signal. In areas involved on both sequences, DWI showed more extensive signal changes in 18 out of 127 areas (14 %). In all these 18 areas, DWI signal changes were especially more extensive in the cortex. In these patients with early (within 2 weeks) MRI, thalamic signal changes—especially in the mesial posterior part (Fig. 2)—were seen in 6 patients on FLAIR (patient nr 2, 3, 5, 7, 8) and also only present on DWI in 1 (patient nr 4) out of these 6 patients.
In the patients where MRI was performed late (n = 2, patient nr 5 and 9, both on day 56), FLAIR was clearly superior to DWI with (38 involved areas against 15, respectively). In these patients, the white matter was more hyperintense than the cortex, and DWI hyperintensity (when present) was far less pronounced than in patients where MRI was performed early.
The lentiform nucleus and the caudate nucleus were never involved in our patients. The occipital lobe was involved in only one patient (patient nr 10).
In the seven patients with parietal lobe involvement, six had lateral parietal involvement (associated in three patients with precuneus involvement) and one patient had only precuneus involvement, all in absence of mesial parietal signal changes. The superior parts of the frontal lobes are involved (n = 3), however, the mesial part was clearly more severely involved than the lateral part.
When confounding all patients and sequences, the mesial temporal structures (the anterior and posterior part of the mesial temporal area, the area between the limen insulae and the uncus and the posterior limbic area), and the inferior–anterior part of the lateral temporal lobe were involved in all patients. The inferior part of the temporal pole and the posterior–superior part of the lateral temporal lobe were the last temporal lobe substructures involved, preceded by frontal insular involvement. Temporal and insular involvement was followed by parietal involvement (precuneus/posterior cingulate area and/or lateral parietal area) and/or involvement of the posterior parts of the gyrus rectus and the orbitofrontal area. This was followed by involvement of the mesial part of superior frontal lobe followed by involvement of the anterior parts of the gyrus rectus and the orbitofrontal area. The polus frontalis, the inferior part of the lateral frontal area, and the superior part of the lateral frontal area seem to be the last frontal lobe substructures involved. The thalamus started to show signal changes on FLAIR when the insula and the parietal lobe were also involved.
On FLAIR and DWI, involvement was strictly unilateral when the score was <8/50 and <9/50, respectively. Associated contralateral involvement was seen in all patients with FLAIR score >8/50 and with DWI score >9/50.
Discussion
Although still relatively small, we present the largest series of patients with non-neonatal HSE (n = 11) analyzed by FLAIR and DWI. Signal changes on FLAIR and DWI clearly differed in function of the time the MRI was performed.
In patients in whom MRI was performed within 2 weeks after symptom onset, DWI showed as many or more brain areas involved when compared to FLAIR. In patients with early MRI and equal number of involved brain areas on FLAIR and DWI, lesions were often better seen (especially in the brain cortex) on DWI than on FLAIR. Although almost never reported in non-neonatal HSE, two-third of our patients with early MRI had thalamic signal changes, almost exclusively present on FLAIR. In contrast to other brain areas, FLAIR seems to be superior to DWI with regard to the thalamus. Recently, thalamic pulvinar signal changes, especially on DWI, have been described in patients with seizures [13]. HSE is often associated with seizures. In our six patients with thalamic changes on FLAIR, only one showed associated DWI abnormalities and only two patients had clinical and/or electrophysiological (on electroencephalography) arguments in favour of epilepsy. Therefore, these thalamic signal changes seemed to be part of the radiological spectrum of possible MRI abnormalities seen in early stage HSE. These thalamic signal changes can be missed easily when not systematically analysed and because of the associated––often massive––cortico-subcortical abnormalities.
Because of the small number of patients included with late MRI (showing predominant subcortical FLAIR lesions), no firm conclusions can be made for these patients.
HSE typically starts unilaterally. Associated contralateral involvement is seen when lesions extend in the initially involved hemisphere. Based on our data, the cut-off score before involving the contralateral hemisphere seems to be 8–9/25 in the initially involved hemisphere.
Limitations of our study are the relatively small patient numbers, the retrospective character of our analyses, and the fact that different brain MRI scans and field strengths were used.
The premise that lesion progression in all HSE patients is similar is only hypothetical. Theoretically to analyze lesion progression, repeated MRI scans should be performed in each patient in the early phase (i.e. before starting antiviral treatment) of HSE. In daily practice, antiviral treatment is started promptly when HSE is suspected (most often based on clinical presentation and CSF data, thus often before MRI is performed). Therefore, lesion progression can only be studied in an indirect way. Although the diagnosis of HSE is mainly based on the clinical presentation (with rapidly progressive symptoms) and CSF analysis, some other disorders (also often manifesting with confusion/cognitive deficit––although classically more slowly progressive––and seizures) including limbic encephalitis, gliomatosis cerebri, lymphoma may mimic HSE on MRI. Knowledge of typical lesion distribution and progression (although less typical cases have been described earlier) can help to differentiate HSE from other disorders sometimes mimicking HSE on MRI.
References
McCabe K, Tyler K, Tanabe J (2003) Diffusion-weighted MRI abnormalities as a clue to the diagnosis of herpes simplex encephalitis. Neurology 61:1015–1016
Obeid M, Franklin J, Shrestha S, Johnson L, Quattromani F, Hurst D (2007) Diffusion-weighted imaging findings on MRI as the sole radiographic findings in a child with proven herpes simplex encephalitis. Pediatr Radiol 37:1159–1162
Nouranifar RK, Ali M, Nath J (2003) The earliest manifestation of focal encephalitis on diffusion-weighted MRI. Clin Imaging 27:316–320
Dhawan A, Kecskes Z, Jyoti R, Kent AL (2006) Early diffusion-weighted magnetic resonance imaging findings in neonatal herpes encephalitis. J Paediatr Child Health 42:824–826
Kubota T, Ito M, Maruyama K, Kato Y, Miyajima Y, Ogawa A, Kuno K, Okumura A, Watanabe K (2007) Serial diffusion-weighted imaging of neonatal herpes encephalitis: a case report. Brain Dev 29:171–173
Akyldz BN, Gümüş H, Kumandaş S, Coşkun A, Karakukuçu M, Yklmaz A (2008) Diffusion-weighted magnetic resonance is better than polymerase chain reaction for early diagnosis of herpes simplex encephalitis: a case report. Pediatr Emerg Care 24:377–379
Chung SP, You JS, Lee HS (2007) Use of the diffusion-weighted magnetic resonance imaging for early diagnosis of herpes simplex encephalitis in the ED: a case report. Am J Emerg Med 25:986.e5–e6
Küker W, Nägele T, Schmidt F, Heckl S, Herrlinger U (2004) Diffusion-weighted MRI in herpes simplex encephalitis: a report of three cases. Neuroradiology 46:122–125
Kiroğlu Y, Calli C, Yunten N, Kitis O, Kocaman A, Karabulut N, Isaev H, Yagci B (2006) Diffusion-weighted MR imaging of viral encephalitis. Neuroradiology 48:875–880
Sawlani V (2009) Diffusion-weighted imaging and apparent diffusion coefficient evaluation of herpes simplex encephalitis and Japanese encephalitis. J Neurol Sci 287:221–226
Bajaj M, Mody S, Natarajan G (2014) Clinical and neuroimaging findings in neonatal herpes simplex virus infection. J Pediatr 165:404–407.e1
Noguchi T, Yoshiura T, Hiwatashi A, Togao O, Yamashita K, Nagao E, Uchino A, Hasuo K, Atsumi K, Matsuura T, Kuroiwa T, Mihara F, Honda H, Kudo S (2010) CT and MRI findings of human herpesvirus 6-associated encephalopathy: comparison with findings of herpes simplex virus encephalitis. AJR Am J Roentgenol 194:754–760
Ohe Y, Hayashi T, Deguchi I, Fukuoka T, Horiuchi Y, Maruyama H, Kato Y, Nagoya H, Uchino A, Tanahashi N (2014) MRI abnormality of the pulvinar in patients with status epilepticus. J Neuroradiol 41:220–226
Conflicts of interest
None.
Ethical standard
Our study has been approved by the local ethics committee and performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. All persons gave their informed consent prior to their inclusion in the study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Renard, D., Nerrant, E. & Lechiche, C. DWI and FLAIR imaging in herpes simplex encephalitis: a comparative and topographical analysis. J Neurol 262, 2101–2105 (2015). https://doi.org/10.1007/s00415-015-7818-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-015-7818-0