Abstract
Purpose
Although obstructive sleep apnea syndrome (OSAS) is known to be an important risk factor for cardiovascular diseases, the mechanism behind this association has not been fully elucidated. Transendothelial migration of monocytes mediated by adhesion molecules is a crucial step in the pathogenesis of atherosclerosis. We investigated the effect of hypoxic stress on plasma adiponectin and tumor necrosis factor-α (TNF-α) levels and whether adiponectin and TNF-α modulate adhesion molecules in patients with OSAS.
Methods
In 22 patients, plasma adiponectin and TNF-α levels and serum concentrations of soluble intercellular adhesion molecule-1 (sICAM-1) were determined early in the morning after polysomnography and after nasal continuous positive airway pressure (nCPAP) treatment.
Results
Plasma adiponectin levels were inversely correlated with the apnea–hypopnea index (AHI) (r = −0.582, p < 0.005) and % time in SpO2 <90 % (r = −0.539, p < 0.01) but not with the body mass index (BMI). TNF-α levels were positively correlated with the AHI (r = 0.462, p < 0.05) and BMI (r = 0.452, p < 0.05). Serum sICAM-1 levels were inversely correlated with plasma adiponectin levels (r = −0.476, p < 0.05) but not with TNF-α levels. Although plasma TNF-α levels decreased after overnight nCPAP treatment (p < 0.05), plasma adiponectin levels increased after long-term nCPAP (3 months) treatment (p < 0.02) in ten patients.
Conclusions
Our findings suggest that reduced adiponectin and elevated TNF-α levels in plasma are associated with OSAS-induced hypoxic stress. Decreased adiponectin levels are associated with sICAM-1 levels.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obesity is common in patients with obstructive sleep apnea syndrome (OSAS). A relationship between the severity of OSAS and body mass index (BMI) and, in particular, increased visceral fat, has been documented [1]. In recent years, it has been clarified that adipocytes express many genes that encode secretory proteins. Obese individuals have been shown to have low plasma levels of adiponectin, a secretory protein encoded by the adipose most abundant gene transcript-1 (apM1) [2, 3].
Although OSAS is associated with the development of atherosclerosis [4] and is an important risk factor for cardiovascular events [5], the mechanism behind this association has not been fully elucidated. Transendothelial migration of monocytes is considered a crucial step in the pathogenesis of atherosclerosis [6, 7]. Adhesion molecules, including intercellular adhesion molecule-1 (ICAM-1), mediate the initial binding of monocytes to the endothelium and their transendothelial migration [6]. Increased concentration of plasma sICAM-1 is a risk factor for cardiovascular events [8]. Adiponectin exerts anti-atherosclerotic effect by suppressing tumor necrosis factor (TNF)-α-dependent expression of adhesion molecules on vascular endothelial cells in vitro, and reduced plasma adiponectin levels are associated with coronary artery disease [9]. In contrast, TNF-α facilitates atherosclerosis by causing vascular endothelial cells to express adhesion molecules [10]. Several previous studies have suggested that OSAS is associated with an increase in the circulating levels of adhesion molecules [11–13]. However, it has not been investigated whether they are modulated by adiponectin and TNF-α.
In the present study, we hypothesized that hypoxic stress in patients with OSAS may affect plasma levels of adiponectin and TNF-α, resulting in elevated levels of circulating adhesion molecules and that this phenomenon may be responsible for cardiovascular events in patients with OSAS. To test this hypothesis, we determined plasma levels of adiponectin and TNF-α before and after nasal continuous positive airway pressure (nCPAP) treatment and investigated the relationship between these parameters and serum concentrations of soluble ICAM-1 (sICAM-1).
Materials and Methods
Twenty-two OSAS male patients with an apnea–hypopnea index (AHI) of 5 or above as assessed by overnight polysomnography (PSG) were recruited from the patients to be referred to our hospital for suspected sleep apnea. None of the patients had a history of cardiovascular, pulmonary, or metabolic diseases. PSG was performed using a computerized polysomnogram system (Alice 4; Respironics; Pittsburgh, PA, USA) for two consecutive nights. Apnea was defined as cessation of airflow or a decrease in airflow to less than 20 % of the baseline value for more than 10 s, and hypopnea was defined as a discernible decrease in airflow to less than 50 % of the baseline value associated with a fall in oxygen saturation of 4 % or more from baseline. Desaturation during sleep was assessed in terms of the time in relation to the total sleep time with SpO2 <90% (% time in SpO2 <90%) and lowest SpO2. All subjects successfully underwent CPAP titration during the second night under PSG.
Blood samples were collected at 6:30 AM before breakfast both after PSG and after nCPAP titration. Plasma samples were immediately separated and stored at –80 °C until analysis. Plasma adiponectin levels were determined by the Human Adiponectin RIA Kit (LINCO Research Inc., St. Charles, MO, USA), while plasma TNF-α levels were measured using the Human TNF-α ELISA Kit (Quantikine, R&D Systems Inc., Minneapolis, MN, USA). Serum sICAM-1 levels were determined by the Human sICAM-1 ELISA Kit (Chemicon International Inc., Temecula, CA, USA).
In ten patients who gave informed consent to participate in a follow-up study, plasma adiponectin levels after long-term nCPAP (3 months) treatment were determined. Compliance with the treatment was measured using the built-in data stores of the CPAP device (REMstar® Auto, Philips Respironics; Murrysville, PA, USA). The study was approved by the medical ethical committee of the Nara Medical University, and informed consent was obtained from all subjects.
Statistical Analysis
Values obtained were expressed as mean ± standard deviation (SD). Pearson’s correlation coefficients for polysomnographic data, adiponectin, TNF-α, and sICAM-1 were calculated. Plasma adiponectin levels were logarithmically transformed by calculating its natural logarithm (ln adiponectin) for use in a correlation analysis. A multivariate linear regression model was used to test the influence of AHI, BMI, and age on adiponectin, TNF-α, and sICAM-1 levels. Pre- and post-nCPAP treatment comparisons were evaluated using the paired t test. The level of statistical significance for each test was set at p value <0.05.
Results
Patient Characteristics
The mean BMI was 30.4 ± 9.6 kg/m2. During resting supine breathing, mean PaO2 and PaCO2 levels were 80.9 ± 13.8 and 44.1 ± 5.2 Torr, respectively (Table 1).
Polysomnographic Data
Polysomnographic data obtained before and after CPAP titration are shown in Table 2. Apnea and desaturation during sleep significantly improved following CPAP titration. The mean AHI decreased from 47.7 ± 25.4 to 7.3 ± 5.2, the lowest SpO2 from 74.9 ± 8.3 to 91.7 ± 5.2 % and % time in SpO2 <90 % from 20.3 ± 22.2 to 1 ± 2 %.
Relationship Between Plasma Adiponectin and TNF-α Levels and BMI
The mean values of plasma adiponectin and TNF-α levels were 14 ± 12.8 µg/ml and 2.37 ± 1.01 pg/ml, respectively. There was a significant correlation between the BMI and plasma TNF-α levels but not between the BMI and log-transformed plasma adiponectin levels (Table 3).
Relationship Between Plasma Adiponectin and TNF-α Levels and Polysomnographic Findings
The relationship between plasma adiponectin and TNF-α levels and polysomnographic findings is shown in Table 3. Plasma adiponectin levels were inversely correlated and TNF-α levels positively correlated with the AHI. Plasma adiponectin levels correlated significantly with % time in SpO2 <90% and the arousal index but not with the lowest SpO2. No significant correlation was observed between plasma TNF-α levels and % time in SpO2 <90 % and the lowest SpO2 (Table 3). Multiple linear regression analysis showed a significant relationship between AHI and adiponectin (p < 0.005) and TNF-α (p < 0.05) levels in both BMI and age adjusted model.
Relationship Between Serum sICAM-1 Levels and Nocturnal Hypoxemia and Plasma Levels of Adiponectin and TNF-α
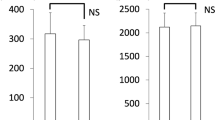
Mean serum sICAM-1 levels were 322 ± 157.8 ng/ml, and they correlated significantly with the AHI and % time in SpO2 <90 % (Fig. 1). Moreover, they correlated significantly with the lowest SpO2 (r = −0.645, p < 0.001) and arousal index (r = −0.458, p < 0.05). Multiple linear regression analysis showed a significant relationship between AHI and sICAM-1 levels (p < 0.01) in both BMI and age adjusted model. sICAM-1 levels were inversely correlated with plasma adiponectin but not with TNF-α levels (Fig. 2).
Relationship between serum concentrations of sICAM-1 and nocturnal hypoxic stress evaluated by the apnea–hypopnea index (AHI) and % time in SpO2 <90 % in patients with OSAS. Serum concentrations of sICAM-1 correlated significantly with the AHI (r = 0.747, p < 0.0001) and % time in SpO2 <90 % (r = 0.766, p < 0.0001)
Plasma Adiponectin and TNF-α Levels and Serum sICAM-1 Levels After nCPAP Treatment
Plasma adiponectin and TNF-α levels and serum sICAM-1 levels were determined after the overnight nCPAP treatment. There was no significant difference between plasma adiponectin levels before and after treatment (14.6 ± 14 vs. 12.2 ± 11.9 μg/ml) and serum sICAM-1 levels also were unchanged (296.8 ± 132.3 vs. 252.6 ± 140.2 ng/ml). On the other hand, plasma TNF-α levels significantly decreased (2.42 ± 1.05 vs. 1.95 ± 1.09 pg/ml, p < 0.05) as shown in Fig. 3.
Effects of nasal continuous positive airway pressure (nCPAP) on plasma levels of tumor necrosis factor-α (TNF-α) and adiponectin. Plasma TNF-α levels were significantly decreased after overnight nCPAP treatment (p < 0.05). Plasma adiponectin levels were significantly elevated after long-term (3 months) nCPAP treatment with no significant changes in the body mass index (p < 0.02)
In ten patients, plasma adiponectin levels were also determined after 3 months of nCPAP treatment. The mean values of baseline BMI and AHI were 31.3 ± 4.5 kg/m2 and 63.7 ± 14.9, respectively. The ten patients showed good compliance during 3 months of nCPAP treatment. Plasma adiponectin levels significantly increased (4.5 ± 2.3 vs. 6.8 ± 2.1 μg/ml, p < 0.02) with no significant changes in the BMI (Fig. 3).
Discussion
The present study showed a weak inverse correlation between plasma adiponectin levels and the BMI in patients with OSAS, similar to that observed in healthy individuals. However, the correlation between adiponectin levels and AHI was stronger in patients with OSAS. In addition, plasma adiponectin levels significantly increased after long-term nCPAP treatment, which suggests that OSAS-induced hypoxic stress may suppress the secretion of adiponectin. We also found that sICAM-1 levels correlated significantly with the AHI and % time in SpO2 <90 %. Furthermore, they were inversely correlated with plasma adiponectin levels but not with TNF-α levels.
Adiponectin exerts antiatherosclerotic effect by inhibiting the proliferation of the vascular smooth muscle cells and binding to collagen I, III, and V in the vascular intima which might be involved in the repair process of atherosclerotic change. Plasma adiponectin has been reported to suppress the expression of E-selectin, ICAM-1, and vascular cell adhesion molecule-1 on the vascular endothelial cells [9]. Therefore, a reduced adiponectin level may contribute to the pathogenesis of atherosclerosis and become a risk factor for cardiovascular events. Clinically, a reduced adiponectin level is associated with cardiovascular events [9, 14].
Circulating adiponectin levels in patients with OSAS have not been unequivocally determined so far. Lower adiponectin levels [15–18] and comparable or higher levels [19–22] compared with control subjects without OSAS and with similar BMI have been documented. Moreover, the relationship between nocturnal hypoxia evaluated by the AHI and adiponectin levels in patients with OSAS also is controversial [15, 16, 23]. We found that plasma adiponectin levels were significantly correlated with the severity of nocturnal hypoxia but not with the BMI. Furthermore, plasma adiponectin levels were significantly increased after 3 months of nCPAP treatment which is in line with several previous studies [24]. These data suggest that reduced adiponectin levels are associated with hypoxic stress during sleep in patients with OSAS. Indeed, recent studies have demonstrated that the mRNA degradation of adiponectin is accelerated under hypoxia compared with normoxia [17, 25]. Consequently, reduced adiponectin levels associated with hypoxic stress may explain, in part, the development of atherosclerosis in patients with OSAS.
TNF-α is an inflammatory cytokine, which is regulated by nuclear factor-κB (NF-κB), a master regulator of inflammatory gene expression. We have previously reported monocyte NF-κB activation in patients with OSAS [26]. TNF-α contributes to atherogenesis [6] and its circulating levels are correlated with cardiovascular risk [27]. In the present study, we found a significant positive correlation between plasma TNF-α levels and the AHI, which is in line with several previous studies [28, 29]. This may suggest that hypoxic stress can facilitate the production of TNF-α and that elevated TNF-α levels play an important role in the development of atherosclerosis in OSAS.
Previous studies have provided evidence that atherosclerosis is related to inflammatory processes involving adhesion molecules [6, 8, 9]. In particular, transendothelial migration of monocytes induced by adhesion molecules is a crucial step in the pathogenesis of atherosclerosis. The circulating levels of soluble adhesion molecules are elevated in patients with OSAS [11] and are reduced by nCPAP treatment [30, 31]. We found that sICAM-1 levels significantly correlated not only with the severity of nocturnal hypoxia but also with adiponectin levels in this patient group. TNF-α activates NF-κB by phosphorylation of IκB-α and facilitates the transcription of adhesion molecule genes in the vascular endothelial cells. On the other hand, adiponectin inhibits the activation of NF-κB by suppressing the phosphorylation of IκB-α and subsequent gene transcription [32]. Therefore, decreased adiponectin levels related to hypoxic stress during sleep appear to facilitate atherosclerosis by increased expression of adhesion molecules on the vascular endothelial cells in patients with OSAS. However, we did not observe any correlation between TNF-α and sICAM-1 levels in the present study, which suggests that sICAM-1 levels are modulated mainly by adiponectin. Furthermore, adiponectin may suppress the production of TNF-α. Indeed, it was demonstrated that adiponectin knockout mice showed high levels of TNF-α mRNA in the adipose tissue and high plasma TNF-α levels, which could be reversed by virus-mediated adiponectin expression [33].
The effect of nCPAP treatment on the circulating levels of adiponectin and sICAM-1 has not been fully elucidated. It was documented that reduced adiponectin levels during sleep were attenuated by overnight CPAP treatment in severe OSAS [17]. After 12 months of nCPAP treatment, a significant decrease in plasma sICAM-1 levels was reported [31]. In the present study, no significant effect of overnight nCPAP treatment on the circulating levels of adiponectin and sICAM-1 was observed, while plasma TNF-α levels significantly reduced after the treatment. Although the reason for this discrepancy is unclear, a possible explanation would be the marked difference between baseline plasma concentrations of adiponectin and TNF-α, remarkably short half-life (approximately 6–7 min) of TNF-α [34] and immediate variation in plasma TNF-α levels under hypoxic stress in patients with OSAS [35]. In addition, a previous study demonstrated that etanercept, a TNF-α antagonist, did not decrease adiponectin levels [36]. This suggests that adiponectin is produced and secreted independently of TNF-α. The effect of long-term nCPAP treatment on an increase in plasma adiponectin levels without significant changes in BMI was prominent. Further studies are needed to elucidate the mechanism underlying this phenomenon.
Our study has several limitations. First, because it did not include a control group, the baseline values of adiponectin, sICAM-1, and TNF-α are difficult to interpret. Second, we enrolled a small number of patients with OSAS, especially those undergoing nCPAP treatment. Therefore, a future study with a larger number of subjects is required to validate our findings.
In conclusion, our findings suggest that both reduced adiponectin and elevated TNF-α in plasma are associated with OSAS-induced hypoxic stress. Decreased adiponectin levels are associated with elevated sICAM-1 levels. The effect of nCPAP treatment on adiponectin levels seems to be of clinical importance for preventing cardiovascular events in patients with OSAS.
References
Shinohara E, Kihara S, Yamashita S, Yamane M, Nishida M, Arai T, Kotani K, Nakamura T, Takemura K, Matsuzawa Y (1997) Visceral fat accumulation as an important risk factor for obstructive sleep apnoea syndrome in obese subjects. J Intern Med 241:11–18
Maeda K, Okubo K, Shimomura I, Funahashi T, Matsuzawa Y, Matsubara K (1996) cDNA cloning and expression of a novel adipose specific collagen-like factor, apM1 (adipose most abundant gene transcript 1). Biochem Biophys Res Commun 221:286–289
Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J, Hotta K, Shimomura I, Nakamura T, Miyaoka K, Kuriyama H, Nishida M, Yamashita S, Okubo K, Matsubara K, Muraguchi M, Ohmoto Y, Funahashi T, Matsuzawa Y (1999) Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun 257:79–83
Suzuki T, Nakano H, Maekawa J, Okamoto Y, Ohnishi Y, Yamauchi M, Kimura H (2004) Obstructive sleep apnea and carotid artery intima-media thickness. Sleep 27:129–133
Marin JM, Carrizo SJ, Vicente E, Agusti AGN (2005) Long-term cardiovascular outcomes in men with obstructive sleep apnoea–hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet 365:1046–1053
Rossa R (1999) Atherosclerosis: an inflammatory disease. N Engl J Med 340:115–126
Tamaki S, Yamauchi M, Fukuoka A, Makinodan K, Koyama N, Tomoda K, Yoshikawa M, Kimura H (2009) Nocturnal hypoxic stress activates invasive ability of monocytes in patients with obstructive sleep apnea syndrome. Respirology 14:689–694
Ridker PM, Hennekens CH, Roitman-Johnson B, Stampfer MJ, Allen J (1998) Plasma concentration of soluble intercellular adhesion molecule-1 and risks of future myocardial infarction in apparently healthy men. Lancet 351:88–92
Ouchi N, Kihara S, Arita Y, Maeda K, Kuriyama H, Okamoto Y, Hotta K, Nishida M, Takahashi M, Nakamura T, Yamashita S, Funahashi T, Matsuzawa Y (1999) Novel modulator for endothelial adhesion molecules: adipocyte-derived plasma protein adiponectin. Circulation 100:2473–2476
Albelda SM, Smith CW, Ward PA (1994) Adhesion molecules and inflammatory injury. FASEB J 8:504–512
Ohga E, Nagase T, Tomita T, Teramoto S, Matsuse T, Katayama H, Ouchi Y (1999) Increased levels of circulating ICAM-1, VCAM-1, and l-selectin in obstructive sleep apnea syndrome. J Appl Physiol 87:10–14
El-Solh AA, Mador MJ, Sikka P, Dhillon RS, Amsterdam D, Grant BJ (2002) Adhesion molecules in patients with coronary artery disease and moderate-to-severe obstructive sleep apnea. Chest 121:1541–1547
Ursavaş A, Karadağ M, Rodoplu E, Yilmaztepe A, Oral HB, Gözü RO (2007) Circulating ICAM-1 and VCAM-1 levels in patients with obstructive sleep apnea syndrome. Respiration 74:525–532
Dekker JM, Funahashi T, Nijpels G, Pilz S, Stehouwer CDA, Snijder MB, Bouter LM, Matsuzawa Y, Shimomura I, Heine RJ (2008) Prognostic value of adiponectin for cardiovascular disease and mortality. J Clin Endocrin Metab 93:1489–1496
Zhang XL, Yin KS, Wang H, Su S (2006) Serum adiponectin levels in adult male patients with obstructive sleep apnea syndrome. Respiration 73:73–77
Masserini B, Morpurgo PS, Donadio F, Bardessari C, Bossi R, Beck-Peccoz P, Orsi E (2006) Reduced levels of adiponectin in sleep apnea syndrome. J Endcrinol Invest 29:700–705
Nakagawa Y, Kishida K, Kihara S, Sonoda M, Hirata A, Yasui A, Nishizawa H, Nakamura T, Yoshida R, Shimomura I, Funahashi T (2008) Nocturnal reduction in circulating adiponectin concentrations related to hypoxic stress in severe obstructive sleep apnea–hypopnea syndrome. Am J Physiol Endocrinol Metab 294:E778–E784
Kim J, Lee CH, Park CS, Kim BG, Kim SW, Cho JH (2010) Plasma levels of MCP-1 and adiponectin in obstructive sleep apnea syndrome. Arch Otolaryngol Head Neck Surg 136:896–899
Sharma SK, Kumpawat S, Goel A, Banga A, Ramakrishnan L, Chaturvedi P (2007) Obesity, and not obstructive sleep apnea, is responsible for metabolic abnormalities in a cohort with sleep-disordered breathing. Sleep Med 8:12–17
Wolk R, Svatikova A, Nelson CA, Gami AS, Govender K, Winnicki M, Somers VK (2005) Plasma levels of adiponectin, a novel adipocyte-derived hormone, in sleep apnea. Obes Res 13:186–190
Tokuda F, Sando Y, Matsui H, Koike H, Yokoyama T (2008) Serum levels of adiponectin and leptin, in patients with obstructive sleep apnea. Intern Med 47:1843–1849
Sánchez-de-la-Torre M, Mediano O, Barceló A, Piérola J, de la Peña M, Esquinas C, Miro A, Durán-Cantolla J, Agustí AG, Capote F, Marin JM, Montserrat JM, García-Río F, Barbé F (2012) The influence of obesity and obstructive sleep apnea on metabolic hormones. Sleep Breath 16:649–656
Makino S, Handa H, Suzukawa K, Fujiwara M, Nakamura M, Muraoka S, Takasago I, Tanaka Y, Hashimoto K, Sugimoto T (2006) Obstructive sleep apnoea syndrome, plasma adiponectin levels, and insulin resistance. Clin Endocrinol 64:12–19
Harsch IA, Wallaschofski H, Koebnik C, PourSchahin S, Hahn EG, Ficker JH, Lohmann T (2004) Adiponectin in patients with obstructive sleep apnea syndrome: course and physiological relevance. Respiration 71:580–586
Hosogai N, Fukuhara A, Oshima K, Miyata Y, Tanaka S, Segawa K, Furukawa S, Tochino Y, Komuro R, Matsuda M, Shimomura I (2007) Adipose tissue hypoxia in obesity and its impact on adipocytokine dysregulation. Diabetes 56:901–911
Yamauchi M, Tamaki S, Tomoda K, Yoshikawa M, Fukuoka A, Makinodan K, Koyama N, Suzuki T, Kimura H (2006) Evidence of activation of nuclear factor kappaB in obstructive sleep apnea. Sleep Breath 10:189–193
Ridker PM, Rifai N, Pfeffer M, Sacks F, Lepage S, Braunwald E (2000) Elevation of tumor necrosis factor-alpha and increased risk of recurrent coronary events after myocardial infarction. Circulation 101:2149–2153
Minoguchi K, Tazaki T, Yokoe T, Minoguchi H, Watanabe Y, Yamamoto M, Adachi M (2004) Elevated production of tumor necrosis factor-alpha by monocytes in patients with obstructive sleep apnea syndrome. Chest 126:1473–1479
Ryan S, Taylor CT, McNichols WT (2005) Selective activation of inflammatory pathways by intermittent hypoxia in obstructive sleep apnea syndrome. Circulation 112:2660–2667
Chin K, Nakamura T, Shimizu K, Mishima M, Nakamura T, Miyasaka M, Ohi M (2000) Effects of nasal continuous positive airway pressure on soluble cell adhesion molecules in patients with obstructive sleep apnea syndrome. Am J Med 109:562–567
Zamarron C, Riveiro A, Gude F (2011) Circulating levels of vascular endothelial markers in obstructive sleep apnoea syndrome. Effects of nasal continuous positive airway pressure. Arch Med Sci 6:1023–1028
Ouchi N, Kihara S, Arita Y, Okamoto Y, Maeda K, Kuriyama H, Hotta K, Nishida M, Takahashi M, Muraguchi M, Ohmoto Y, Nakamura T, Yamashita S, Funahashi T, Matsuzawa Y (2000) Adiponectin, an adipocyte-derived plasma protein, inhibits endothelial NF-κB signaling through a cAMP-dependent pathway. Circulation 102:1296–1301
Maeda N, Shimonura I, Kishida K, Kishida K, Nishizawa H, Matsuda M, Nagaretani H, Furuyama N, Kondo H, Takahashi M, Arita Y, Komuro R, Ouchi N, Kihara S, Tochino Y, Okutomi K, Horie M, Takeda S, Aoyama T, Funahashi T, Matsuzawa Y (2002) Diet-induced insulin resistance in mice lacking adiponectin/ACRP30. Nat Med 8:731–737
Bemelmans MH, van Tits LJ, Buurman WA (1996) Tumor necrosis factor: function, release and clearance. Crit Rev Immunol 16:1–11
Arberti A, Sarchielli P, Gallinella E, Floridi A, Floridi A, Mazzotta G, Gallai V (2003) Plasma cytokine levels in patients with obstructive sleep apnea syndrome: a preliminary study. J Sleep Res 12:305–311
Vgontzas AN, Zoumakis E, Lin HM, Bixler EO, Trakada G, Chrousos GP (2004) Marked decrease in sleepiness in patients with sleep apnea by etanercept, a tumor necrosis factor-α antagonist. J Clin Endocrinol Metab 89:4409–4413
Acknowledgments
This study was partly supported by a grant to the Respiratory Failure Research Group from the Ministry of Health, Labour and Welfare, Japan.
Conflict of interest
None of the authors have financial conflicts of interest to declare as it relates to the contents of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yoshikawa, M., Yamauchi, M., Fujita, Y. et al. The Impact of Obstructive Sleep Apnea and Nasal CPAP on Circulating Adiponectin Levels. Lung 192, 289–295 (2014). https://doi.org/10.1007/s00408-013-9550-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00408-013-9550-9