Abstract
Background
Patients with chronic rhinosinusitis (CRS) have reported significantly cognitive and olfactory dysfunction. This study aimed to explore the relationship between cognitive function and olfaction-specific parameters in patients with CRS.
Methods
A cross-sectional survey method was used to investigate 98 participants, including 75 patients with CRS and 23 healthy controls. Cognitive function and psychophysical olfactory tests were performed. Olfactory cleft endoscopy scale and olfactory cleft computed tomography (CT) scores were obtained. Multivariate logistic regression was used to analyze the risk factors of Mild Cognitive Impairment (MCI) in patients with CRS.
Results
There are significant differences in age, Montreal Cognitive Assessment (MoCA) scores, number of MCI, Lund-Mackay olfactory cleft (LM-OC) score, and blood eosinophil count between CRS with and without olfactory dysfunction groups (all P < 0.05). Total MoCA scores were positively correlated with thresholds-discrimination-identification (TDI) score (r = 0.541, P < 0.001), olfactory threshold (OT) (r = 0.440, P < 0.001), olfactory discrimination (OD) (r = 0.541, P < 0.001), and olfactory identification (OI) (r = 0.382, P = 0.001) scores. Furthermore, total MoCA scores were negatively correlated with LM-OC scores (r = − 0.351, P = 0.002). After adjusting for patient demographics, only the OD score was an independent risk factor for MCI among patients with CRS (odds ratio = 0.792; P = 0.039). The OD scores less than 11.5 were the best predictor of MCI in patients with CRS.
Conclusion
Olfaction-specific clinical parameters were highly correlated with cognitive function in patients with CRS and the OD score was an independent risk factor for MCI in patients with CRS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic rhinosinusitis (CRS) is a common chronic sinonasal disease with a prevalence ranging from 10 to 12% [1]. Olfactory dysfunction is one of the main symptoms of patients with CRS, affecting up to 80% of patients with CRS [2]. CRS-associated olfactory dysfunction is caused by mucosal inflammation, which leads to olfactory epithelial damage or physical obstruction of the olfactory cleft. The nasal blockage leads to the inability to transmit odors to the olfactory cleft area. The inflammatory reaction in the vicinity of the olfactory cleft reduces the transmission of olfactory nerves, which eventually leads to a decrease in the volume of the olfactory bulb [3]. Moreover, sinonasal inflammation can cross the blood–brain barrier into the brain via the olfactory bulb and olfactory nerves, which affects olfactory transmission pathways and brain organization [4].
Symptomatology and manifestations of CRS has a huge impact on the quality of life (QOL) of patients, ranging from rhinology symptoms of nasal obstruction, nasal discharge, and headache to cognitive decline, including central behavioral fatigue, depression, reduced sleep, reduced attention, slowed thinking, and memory impairment [5,6,7]. The association between CRS and cognition has been demonstrated in previous studies. Patients with CRS were more likely to progress to dementia [8], and the prevalence of CRS was higher in patients with dementia than healthy controls [9]. Moreover, patients with CRS had worse cognitive function than control subjects without a history of sinusitis [5]. After medication or surgery on patients with CRS, significant improvements in cognitive function were found [10,11,12]. Olfactory dysfunction is positively related to increased medication use and decreased QOL [13, 14], which may increase the risk of major depression [15] and the economic burden of patients [16, 17]. Previous studies have shown a strong relationship between olfactory decline and cognitive impairment, and olfactory function can be used as a screening indicator for high-risk cognitive impairment before the development of mild cognitive impairment (MCI) or dementia [18, 19]. However, it is unclear whether the relationship of olfaction and cognition exists in patients with CRS and whether olfaction can predict the occurrence of MCI in patients with CRS have not been explored.
Based on the above findings, we assumed that olfactory decline may be associated with cognitive function and that examination of olfactory-specific parameters may help to screen for the risk of cognitive dysfunction in patients with CRS. In this study, we explore the association between olfactory function and cognitive function in patients with CRS and then examine the predictive significance of olfaction-specific parameters for cognitive dysfunction in patients with CRS.
Materials and methods
Patients with a diagnosis of CRS based on the European Position Paper on Rhinosinusitis and Nasal Polyps 2020 (EPOS20) and controls with the non-inflammatory disease from December 2021 to October 2022. All participants underwent a series of specialized otorhinolaryngological and cognitive examinations, which included sinus computed tomography (CT), nasal endoscopy, olfactory psychophysical test (Sniffin’ Stick test), outcome measure for CRS (the 22-item Sino-Nasal Outcome Test, SNOT-22) and neuropsychological testing (Montreal Cognitive Assessment, MoCA). Demographic characteristics were further collected. Exclusion criteria included: (1) any cancer, tumor, or chronic disease which has the potential to affect cognition and olfaction (2) uneducated or non-Mandarin Chinese speakers (3) autoimmune disease, ciliary dysfunction, cystic fibrosis, autoimmune disease, immunodeficiency (4) craniocerebral surgery, stroke, or brain trauma (5) antibiotics or any topical/systemic steroids medication in the last 4 weeks (6) patients who does not complete the whole test. Before treatment, 15 ml of peripheral venous blood was gathered from each subject in an EDTA anticoagulation tube. A complete peripheral blood cell count was performed by an automated analyzer (Beckman Coulter, Miami, Florida), and the blood eosinophil and basophil counts were calculated. All patients signed informed consent before participation. The flow diagram of the study design was shown in Fig. 1.
Cognitive function assessment
Cognitive function was assessed by Montreal Cognitive Assessment 7.0 (MoCA 7.0). MoCA was invented by Prof. Nasreddine according to clinical experience and MMSE scoring criteria in 2004 [20]. It was widely used as an assessment tool for the rapid screening of MCI. The 30-point total MoCA score covers seven cognitive domains: visuospatial/executive (trail-making test: 1 point, copy tube: 1 point, clock drawing task: 3 points), attention (forward digit span: 1 point, backward digit span: 1 point, vigilance: 1 point and serial subtraction: 3 points), naming (3 points), delayed recall (5 points), language (verbal fluency: 1 point, sentence repetition: 2 points), abstraction (2 points), and orientation (6 points). It indicated as MCI if the total scores were < 26. Furthermore, the total scores will be added one point if the education year is less than 12 years [21]. The basic principle of the test is to be done in a quiet environment, with subjects with no inhibitions and staying awake. It takes approximately 10 min to complete the test. MoCA has been proven to be appropriate in the Chinese population in previous studies [22,23,24,25].
Olfactory function assessment
Olfaction function was examined by the Sniffin’ Sticks (Burghart Instruments, Germany). The assessment of olfactory function includes olfactory threshold (OT), olfactory discrimination (OD), olfactory identification (OI), and the sum composite scores (threshold, discrimination, and identification, TDI) [26]. Subjects were not allowed to smoke, eat, or drink anything but water for fifteen minutes before the test. The OT test used different concentrations of n-butanol, using a single-step, triple-forced selection, subjects selected the correct triplet pen and then replaced it with a lower concentration. The OD test used a triplet of pens, containing two of the same odors and one of a different odor, selecting different odors and counting one point. Sixteen odor pens were used in the OI test, and participants selected the odor that matched the options given. Each odor must be smelt only once, for 3-4 s, the interval between odor presentations was 20–30 s, and the pen should be put about 2 cm from the subject’s nostril. Identification and discrimination were scored between 0 and 16, and thresholds between 1 and 16. The results were calculated to a combined score of TDI ranging from 1 to 48. Higher scores indicated better olfactory function. Sniffin’ Sticks has been applied in the Chinese population to distinguish between normosmia, hyposmia, and anosmia [27]. In addition, in previous studies, we evaluated patients with healthy and patients with olfactory dysfunction caused by different etiologies in the Chinese population using Sniffer sticks [25, 28, 29]. In this study, we divided the subjects into three groups according to their TDI scores: normosmia (TDI > 30.75), hyposmia (16 < TDI < 30.75), and anosmia (TDI < 15) [30]. In the present study, patients with olfactory dysfunction included patients with hyposmia and anosmia.
Olfactory cleft-specific measures
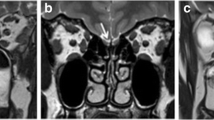
Sinonasal CT scan and endoscopy were performed in patients with CRS before surgery to determine the severity of the disease. The olfactory cleft is located between the olfactory filum (the anterior plane of the middle turbinate) and the pterygoid sinus. The lateral border of the olfactory cleft is the attachment of the middle and/or superior turbinates, The cribriform plate and 1 cm below the cribriform plate made up the top and bottom of the olfactory cleft. Classification of CRS olfactory cleft turbidity by olfactory cleft CT score, the score of olfactory cleft CT was used as a predictive factor of smell function in chronic rhinosinusitis with nasal polyposis [31], we scored sinus CT scans with the Lund-Mackay Olfactory Cleft Scale (LM-OC) [32]. The olfactory cleft score was the sum of the anterior and posterior olfactory cleft scores, 0 (no turbidity), 1 (25%), 2 (25–50%), 3 (50–75%), and 4 (75–100%) (score range, 0–8), respectively. Evaluation and classification of the pathology of the olfactory cleft with the Olfactory Cleft Endoscopy Scale (OCES) [33]. OCES quantifies the severity of the pathological changes of the olfactory cleft on a scale of 0–2, including discharge, nasal polyps, edema, crusting, and scarring (score range, 0–20). The higher the score on the above staging systems, the more severe the disease. Scoring by doctors with extensive experience in endoscopy.
Statistical analysis
Data was analyzed with SPSS software (SPSS version 26.0, SPSS, Chicago, IL). and Graph Pad Prism (Graph Pad Prism version 9, San Diego, CA, USA). Continuous variable normal distribution test using Shapiro-Walk test. Continuous variables expressed as mean ± standard deviation (SD) or median (with extreme deviation or inter-quartile extreme deviation) indicate normal or non-normal distributions. Comparison of continuous variables using independent samples t-test or Mann–Whitney U-test (normally distributed or non-normally distributed). These frequency differences between the two groups were assessed by Chi-square (χ2) test. Spearman rank-order correlations were calculated to evaluate associations between olfaction-specific clinical parameters and cognition scores. Logistic regression analysis was used to assess risk factors associated with cognitive function in patients with CRS, effect sizes were expressed using regression coefficient β values and their 95% confidence intervals (CIs). The receiver operating characteristic (ROC) curve was used to find the optimal cutoff for each potential factor in patients with CRS with MCI. The area under the curve (AUC) was calculated for each potential factor, and the cutoff value was calculated by the maximum AUC. Bilateral P < 0.05 represents a statistically significant difference.
Result
A total of 98 participants were enrolled in this study. Both demographic information to be included and clinical characteristics are presented in Table 1. CRS patients were classified into two different cohorts depending on their olfactory function scores: CRS without olfactory dysfunction (N = 28, 37.33%) and CRS with olfactory dysfunction (N = 47, 62.67%). The mean age of the heathy controls was 45.04 ± 2.99 (mean ± SD). The median SNOT-22 scores in the control group were 11 (interquartile range: 4–27). The healthy control group had normal olfactory function with median TDI, OD and OI scores were 32.5 (31.5–37.5), 13 (12–14), and 12 (11–14), respectively. The mean OT scores was 8.95 ± 0.63. The median MoCA scores of heathy controls was 27(26–29), and the prevalence of MCI of heathy controls was 8.7%. Significant differences were observed in age, MoCA scores, number of MCI, blood eosinophil count, TDI score, OT score, OD score, OI score, and LM-OC scores between CRS with and without olfactory dysfunction groups (all P < 0.05).
Olfactory-specific parameters in CRS with and without olfactory dysfunction
Patients with CRS without olfactory dysfunction had higher olfactory scores (OT, OD, OI, and TDI scores) than patients with CRS with olfactory dysfunction (all P < 0.05). Meanwhile, LM-OC scores of patients with CRS without olfactory dysfunction were lower than patients with CRS with olfactory dysfunction (P = 0.011). There was no statistical difference in OCES scores was found between the two groups (P = 0.061) (Table 1).
Cognitive function among patients with CRS with and without olfactory dysfunction
The number of MCI was significantly higher in the CRS with olfactory dysfunction (N = 30, 63.83%) than in the CRS without olfactory dysfunction (N = 9, 32.14%) (P < 0.001). The patients with CRS accompanied by olfactory dysfunction showed statistically lower total MoCA (P < 0.001), delayed recall (P < 0.001), attention (P < 0.001), orientation (P = 0.007), visuospatial/executive (P = 0.015), and language (P = 0.033) scores than patients with CRS without olfactory dysfunction. However, no significant difference in naming and abstraction scores was observed between the two groups (P > 0.05) (Table 2).
Association of cognitive function with olfaction-specific clinical parameters in patients with CRS
We first analyzed the association between cognitive function and Sniffin’ Sticks test results. We found that total MoCA scores were positively correlated with TDI scores (r = 0.549, P < 0.001), OD (r = 0.541, P < 0.001), OT (r = 0.440, P < 0.001), OI (r = 0.382, P < 0.001) in patients with CRS. Delayed recall scores were positively correlated with TDI (r = 0.535, P < 0.001), OD (r = 0.527, P < 0.001), OT (r = 0.491, P < 0.001), OI (r = 0.386, P = 0.001) in patients with CRS. Attention scores were positively correlated with OD (r = 0.461, P < 0.001), TDI (r = 0.427, P < 0.001), OT (r = 0.319, P = 0.005), and OI scores (r = 0.265, P = 0.022). Orientation scores were positively correlated with OI (r = 0.395, P < 0.001), TDI (r = 0.369, P = 0.001), OD (r = 0.320, P = 0.005), and OT scores (r = 0.307, P = 0.007). Visuospatial/executive scores were positively correlated with TDI (r = 0.332, P = 0.004), OI (r = 0.290, P = 0.012), OD (r = 0.280, P = 0.015), OT scores (r = 0.248, P = 0.032). Language scores were positively correlated with OD (r = 0.291, P = 0.011) and TDI (r = 0.254, P = 0.028) scores. Both naming and abstraction scores presented no significant correlation with any result of the Sniffin’ Sticks test results. We next analyzed the association between cognitive function and the LM-OC score. The LM-OC scores were negatively correlated with attention (r = − 0.381, P = 0.001), total MoCA (r = − 0.351, P = 0.002), delayed recall (r = − 0.305, P = 0.009), language (r = − 0.284, P = 0.015), and visuospatial/executive (r = − 0.244, P = 0.038) scores. However, the LM-OC scores showed no significant correlation with orientation, naming, and abstraction scores (all P > 0.05) (Table 3).
Multivariable logistic regression analysis for patients with CRS
To identify possible risk factors for MCI in patients with CRS, we further selected variables for multifactorial logistic regression analysis based on previous studies and clinical background. After adjusting for sex, BMI, age, smoking status, nasal polyps, drinking status, education level, and blood eosinophil count, the OD score was significantly associated with MCI in patients with CRS (odds ratio = 0.792; 95% confidence interval = 0.635–0.988; P = 0.039) (Table 4).
Predictors in CRS with MCI from ROC analysis
We further determined the cutoff values of each predictor by calculating the maximum AUC area of the ROC curve (Table 5 and Fig. 2). The accuracy of the OD score as a predictor for patients with CRS with cognitive impairment (AUC = 0.722, P = 0.001) was higher than the OT score (AUC = 0.652, P = 0.024), whereas the OI score is not available as a predictor for the cognition dysfunction in patients with CRS (P = 0.082). The OD score with a cutoff point below 11.5 had a higher prediction accuracy (sensitivity = 76.9%, specificity = 61.1%, Youden index = 0.38), and the OI score with a cutoff point below 6.125 had a worse prediction accuracy (sensitivity = 71.8%, specificity = 28.2%, and Youden index = 0.301).
Discussion
The high prevalence of CRS in patients with dementia and cognitive dysfunction in patients with CRS suggests a strong relationship between CRS and cognition [8, 9]. Olfactory dysfunction is not only the main symptom of CRS but also appears in the early stages of neurodegenerative diseases [34,35,36]. However, whether there is a common mechanism for olfactory function and cognitive function in patients with CRS remains unclear. This is the first study to reveal a tight association between olfaction-specific parameters and cognitive dysfunction in CRS patients. In addition, we determined the predictive role of olfactory discrimination on cognitive dysfunction in patients with CRS. This would help to select which patients are at high risk for cognitive dysfunction and to intervene early in patients.
In this study, we confirmed that patients with CRS had worse cognition scores and a higher prevalence of MCI compared to normal controls, which is consistent with previous findings [5]. Then, we classified patients with CRS into two groups based on olfactory assessment, CRS without olfactory dysfunction and CRS with olfactory dysfunction. In the present study, we found that the olfactory impairment caused by CRS was characterized by significantly impaired OD and OT scores. The OI scores are relatively preserved, but there was still a statistical difference between the two groups. CRS-related impaired OT scores are caused by the dysfunction of the peripheral olfactory system, resulting in obstruction and edema of the mucosa, preventing odors from passing through the olfactory cleft [37, 38]. Moreover, OD function is related to higher cognitive functions and responds to executive and memory function [38], which indicated that cognitive function may be impaired in CRS patients with olfactory dysfunction.
Our study also found that LM-OC scores were statistically different between the two groups, with higher scores indicating worse olfactory function. LM-OC scores reflect the degree of inflammation and edema in the olfactory cleft. This also suggests that the olfactory dysfunction in CRS is caused by conductive olfactory dysfunction, which is consistent with the previous results [8, 39]. Therefore, we included the LM-OC score as an objective indicator of olfactory function in the assessment of the cognitive function of patients with CRS.
As we expected, the prevalence of MCI was significantly higher in the patients with CRS with olfactory dysfunction than in the patients with CRS without olfactory dysfunction. By comparing the MoCA scores, we found that the patients with CRS with olfactory dysfunction had significantly higher scores than the patients with CRS without olfactory dysfunction. Furthermore, the MoCA scores and olfactory function scores showed a strong positive correlation, indicating that lower olfactory function scores indicated worse cognitive function. Same as previous studies on olfaction and cognition [40, 41], this trend that demonstrated the relationship between olfactory and cognitive function was also present in patients with CRS. Moreover, LM-OC scores showed negative correlation with cognitive function, further indicating that LM-OC scores might be an indicator of cognitive dysfunction. Both subjective CT examination and objective olfactory function examination showed an association between olfaction-specific parameters and cognitive function, which indicates that olfaction-specific parameters can be used as a non-invasive method to measure cognitive function in patients with CRS.
In order to explore the influence of olfaction on the cognitive subdomains of patients with CRS, we compared the cognitive subdomains of two groups. Patients with CRS with olfactory dysfunction had worse cognitive scores in delayed recall, attention, orientation, visuospatial/executive, and language scores. The assessment of olfactory function scores (OT, OD, and OI scores) in CRS patients was positively correlated with the total MoCA scores and each subdomain (delayed recall, attention, orientation, visuospatial/executive, and language scores of MoCA). However, we found no statistical difference between the CRS with and without olfactory dysfunction groups in naming and abstraction scores of MoCA, and olfaction-specific parameters did not correlate with naming and abstraction scores of MoCA. Naming scores are associated with the left and right globus pallidus; abstraction scores are associated with frontal lobe function [42, 43]. We speculate that CRS impairs specific brain regions or brain functional connections (Fc). Jafari et al.[44] analyzed resting-state functional magnetic resonance images of CRS patients and found a significant alteration in Fc in the frontoparietal network, which is an important control center for higher-order neural processing and shows greater activity in complex cognitive tasks. Pengfei et al. performed magnetic resonance imaging scans on CRS patients and found that the volume of olfaction-related gray matter was significantly reduced in CRS patients compared to healthy subjects [45], and the atrophy of gray matter is associated with OD and OI function [46].
In addition, after adjusting for sex, age, BMI, smoking status, nasal polyps, drinking status, and education level, only OD is related to cognitive function. Furthermore, OD has a higher accuracy to predict MCI when compared to OT. When the maximum value of the Youden index was 0.38, the optimal cut-off point for OD was 11.5, with a specificity of 76.9% and a sensitivity of 61.1%. Previous studies have proposed that nasal polyps and pain were highly associated with decreased cognitive function in patients with CRS [47, 48]; This is the first study to explore the predictive significance of OD for MCI in patients with CRS. Several imaging studies have demonstrated that OD is regulated by the hippocampus [49]. The hippocampus was significantly activated during the process of OD [50]. In the functional olfactory cortical network, the hippocampus is crucial in olfactory learning and memory [51]. Furthermore, atrophy of the hippocampal is one of the features of neurodegenerative diseases, and significant hippocampal changes are observed in both Parkinson's disease (PD) and Alzheimer's disease (AD) patients with olfactory impairment. Specifically, reduced hippocampal activity was observed in patients with PD, and the volume of hippocampal and OD scores showed a strong relationship in patients with AD [52, 53]. Different from our results, previous studies have noted that OI scores as a predictor of cognition decline [54,55,56]. The reason may be explained is that OI impairment is more common in patients with corticobasal dementia, semantic dementia, and frontotemporal dementia [57]. OD has been proven to predict cognitive decline in healthy older individuals [58]. Our results indicate that the predictive effect of OD on cognitive decline is also applicable in patients with CRS.
Our study has some limitations. First, this was a cross-sectional study, the results need to be re-validated in longitudinal and multicenter studies. Second, the sample size was relatively small, the highly selective exclusion criteria exclude many patients. Finally, this study was conducted only on Chinese population. A larger sample size is needed in the future to investigate potential mechanisms of cognitive decline in patients with CRS to reduce the risk of cognitive impairment in patients with CRS. We will perform olfaction-specific clinical parameters and cognition tests in patients with CRS after surgery in our further study.
Conclusion
Our study showed a high association between olfaction-specific clinical parameters and cognitive function in patients with CRS. OD was an independent risk factor for MCI in patients with CRS.
Availability of data and materials
The data that support the fndings of this study are available from the corresponding author upon reasonable request.
References
Halawi AM, Smith SS, Chandra RK (2013) Chronic rhinosinusitis: Epidemiology and cost. Allergy Asthma Proc 34(4):328–334. https://doi.org/10.2500/aap.2013.34.3675
Kohli P, Naik AN, Harruff EE, Nguyen SA, Schlosser RJ, Soler ZM (2017) The prevalence of olfactory dysfunction in chronic rhinosinusitis. Laryngoscope 127(2):309–320. https://doi.org/10.1002/lary.26316
Hummel T, Whitcroft KL, Andrews P, Altundag A, Cinghi C, Costanzo RM, Damm M, Frasnelli J, Gudziol H, Gupta N, Haehner A, Holbrook E, Hong SC, Hornung D, Huttenbrink KB, Kamel R, Kobayashi M, Konstantinidis I, Landis BN, Leopold DA, Macchi A, Miwa T, Moesges R, Mullol J, Mueller CA, Ottaviano G, Passali GC, Philpott C, Pinto JM, Ramakrishnan VJ, Rombaux P, Roth Y, Schlosser RA, Shu B, Soler G, Stjarne P, Stuck BA, Vodicka J, Welge-Luessen A (2016) Position paper on olfactory dysfunction. Rhinology 56(1):1–30. https://doi.org/10.4193/Rhino16.248
Harrass S, Yi C, Chen H (2021) Chronic rhinosinusitis and alzheimer’s disease-a possible role for the nasal microbiome in causing neurodegeneration in the elderly. Int J Mol Sci. https://doi.org/10.3390/ijms222011207
Soler ZM, Eckert MA, Storck K, Schlosser RJ (2015) Cognitive function in chronic rhinosinusitis: A controlled clinical study. Int Forum Allergy Rhinol 5(11):1010–1017. https://doi.org/10.1002/alr.21581
Kollndorfer K, Kowalczyk K, Hoche E, Mueller CA, Pollak M, Trattnig S, Schopf V (2014) Recovery of olfactory function induces neuroplasticity effects in patients with smell loss. Neural Plast. https://doi.org/10.1155/2014/140419
Matsui T, Arai H, Nakajo M, Maruyama M, Ebihara S, Sasaki H, Nakajo M, Yoshida Y (2003) Role of chronic sinusitis in cognitive functioning in the elderly. J Am Geriatr Soc 51(12):1818–1819. https://doi.org/10.1046/j.1532-5415.2003.51572_5.x
Jung HJ, Lee JY, Choi YS, Choi HG, Wee JH (2021) Chronic rhinosinusitis and progression of cognitive impairment in dementia. Eur Ann Otorhinolaryngol Head Neck Dis 138(3):147–151. https://doi.org/10.1016/j.anorl.2020.05.017
Chung SD, Hung SH, Lin HC, Kang JH (2015) Dementia is associated with chronic rhinosinusitis: a population-based case-controlled study. Am J Rhinol Allergy 29(1):44–47. https://doi.org/10.2500/ajra.2015.29.4113
Rowan NR, Schlosser RJ, Storck KA, Ganjaei KG, Soler ZM (2019) The impact of medical therapy on cognitive dysfunction in chronic rhinosinusitis. Int Forum Allergy Rhinol 9(7):738–745. https://doi.org/10.1002/alr.22323
Yoo F, Schlosser RJ, Storck KA, Ganjaei KG, Rowan NR, Soler ZM (2019) Effects of endoscopic sinus surgery on objective and subjective measures of cognitive dysfunction in chronic rhinosinusitis. Int Forum Allergy Rhinol 9(10):1135–1143. https://doi.org/10.1002/alr.22406
Alt JA, Mace JC, Smith TL, Soler ZM (2016) Endoscopic sinus surgery improves cognitive dysfunction in patients with chronic rhinosinusitis. Int Forum Allergy Rhinol 6(12):1264–1272. https://doi.org/10.1002/alr.21820
Mattos JL, Schlosser RJ, Storck KA, Soler ZM (2017) Understanding the relationship between olfactory-specific quality of life, objective olfactory loss, and patient factors in chronic rhinosinusitis. Int Forum Allergy Rhinol 7(7):734–740. https://doi.org/10.1002/alr.21940
Schlosser RJ, Storck KA, Rudmik L, Smith TL, Mace JC, Mattos J, Soler ZM (2017) Association of olfactory dysfunction in chronic rhinosinusitis with economic productivity and medication usage. Int Forum Allergy Rhinol 7(1):50–55. https://doi.org/10.1002/alr.21841
Seiden AM, Duncan HJ (2001) The diagnosis of a conductive olfactory loss. Laryngoscope 111(1):9–14. https://doi.org/10.1097/00005537-200101000-00002
Rudmik L (2017) Economics of chronic rhinosinusitis. Curr Allergy Asthma Rep 17(4):20. https://doi.org/10.1007/s11882-017-0690-5
Soler ZM, Smith TL, Alt JA, Ramakrishnan VR, Mace JC, Schlosser RJ (2016) Olfactory-specific quality of life outcomes after endoscopic sinus surgery. Int Forum Allergy Rhinol 6(4):407–413. https://doi.org/10.1002/alr.21679
Attems J, Lintner F, Jellinger KA (2005) Olfactory involvement in aging and Alzheimer’s disease: an autopsy study. J Alzheimers Dis 7(2):149–157. https://doi.org/10.3233/jad-2005-7208. (discussion 173-180)
Lafaille-Magnan ME, Poirier J, Etienne P, Tremblay-Mercier J, Frenette J, Rosa-Neto P, Breitner JCS, Group P-AR (2017) Odor identification as a biomarker of preclinical ad in older adults at risk. Neurology 89(4):327–335. https://doi.org/10.1212/WNL.0000000000004159
Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H (2005) The montreal cognitive assessment, moca: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 53(4):695–699. https://doi.org/10.1111/j.1532-5415.2005.53221.x
Lu J, Li D, Li F, Zhou A, Wang F, Zuo X, Jia XF, Song H, Jia J (2011) Montreal cognitive assessment in detecting cognitive impairment in chinese elderly individuals: a population-based study. J Geriatr Psychiatry Neurol 24(4):184–190. https://doi.org/10.1177/0891988711422528
Wen HB, Zhang ZX, Niu FS, Li L (2008) the application of montreal cognitive assessment in urban chinese residents of beijing. Zhonghua Nei Ke Za Zhi 47(1):36–39
Yu K, Zhang S, Wang Q, Wang X, Qin Y, Wang J, Li C, Wu Y, Wang W, Lin H (2014) Development of a computerized tool for the chinese version of the montreal cognitive assessment for screening mild cognitive impairment. Int Psychogeriatr. https://doi.org/10.1017/S1041610214002269
Tang Z, Chen X, Zhang W, Sun X, Hou Q, Li Y, Feng X, Chen Y, Lv J, Ji L, Ding G, Li D (2021) Association between gamma-glutamyl transferase and mild cognitive impairment in chinese women. Front Aging Neurosci 13:630409. https://doi.org/10.3389/fnagi.2021.630409
Chen Z, Chang F, Yao L, Yuan F, Hong J, Wu D, Wei Y (2022) Clinical significance of the cognition-related pathogenic proteins in plasma neuronal-derived exosomes among normal cognitive adults over 45 years old with olfactory dysfunction. Eur Arch Otorhinolaryngol 279(7):3467–3476. https://doi.org/10.1007/s00405-021-07143-3
Hummel T, Sekinger B, Wolf SR, Pauli E, Kobal G (1997) “Sniffin” sticks’: Olfactory performance assessed by the combined testing of odor identification, odor discrimination and olfactory threshold. Chem Senses 22(1):39–52. https://doi.org/10.1093/chemse/22.1.39
Yang L, Wei YX, Ren YY, Yu D, Sun YX, Yang BB (2013) clinical application of sniffin’ sticks olfactory psychophysical measurements. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 48(9):741–745
Su B, Bleier B, Wei Y, Wu D (2021) Clinical implications of psychophysical olfactory testing: assessment, diagnosis, and treatment outcome. Front Neurosci 15:646956. https://doi.org/10.3389/fnins.2021.646956
Liu Z, Hong J, Huang X, Wu D (2022) Olfactory cleft mucus galectin-10 predicts olfactory loss in chronic rhinosinusitis. Ann Allergy Asthma Immunol. https://doi.org/10.1016/j.anai.2022.07.014
Oleszkiewicz A, Schriever VA, Croy I, Hahner A, Hummel T (2019) Updated sniffin’ sticks normative data based on an extended sample of 9139 subjects. Eur Arch Otorhinolaryngol 276(3):719–728. https://doi.org/10.1007/s00405-018-5248-1
Vandenhende-Szymanski C, Hochet B, Chevalier D, Mortuaire G (2015) Olfactory cleft opacity and ct score are predictive factors of smell recovery after surgery in nasal polyposis. Rhinology 53(1):29–34. https://doi.org/10.4193/Rhino14.160
Chang H, Lee HJ, Mo JH, Lee CH, Kim JW (2009) Clinical implication of the olfactory cleft in patients with chronic rhinosinusitis and olfactory loss. Arch Otolaryngol Head Neck Surg 135(10):988–992. https://doi.org/10.1001/archoto.2009.140
Schlosser RJ, Smith TL, Mace JC, Alt JA, Beswick DM, Mattos JL, Ramakrishnan V, Massey C, Soler ZM (2021) The olfactory cleft endoscopy scale: a multi-institutional validation study in chronic rhinosinusitis. Rhinology 59(2):181–190. https://doi.org/10.4193/Rhin20.307
Bahar-Fuchs A, Moss S, Rowe C, Savage G (2010) Olfactory performance in ad, amci, and healthy ageing: a unirhinal approach. Chem Senses 35(9):855–862. https://doi.org/10.1093/chemse/bjq094
Chan A, Tam J, Murphy C, Chiu H, Lam L (2002) Utility of olfactory identification test for diagnosing chinese patients with alzheimer’s disease. J Clin Exp Neuropsychol 24(2):251–259. https://doi.org/10.1076/jcen.24.2.251.992
Devanand DP (2016) Olfactory identification deficits, cognitive decline, and dementia in older adults. Am J Geriatr Psychiatry 24(12):1151–1157. https://doi.org/10.1016/j.jagp.2016.08.010
Whitcroft KL, Cuevas M, Haehner A, Hummel T (2017) Patterns of olfactory impairment reflect underlying disease etiology. Laryngoscope 127(2):291–295. https://doi.org/10.1002/lary.26229
Hedner M, Larsson M, Arnold N, Zucco GM, Hummel T (2010) Cognitive factors in odor detection, odor discrimination, and odor identification tasks. J Clin Exp Neuropsychol 32(10):1062–1067. https://doi.org/10.1080/13803391003683070
Yuan F, Wu D, Wei Y (2022) Predictive significance of the questionnaire of olfactory disorders-negative statements for olfactory loss in patients with chronic rhinosinusitis. Eur Arch Otorhinolaryngol 279(11):5253–5262. https://doi.org/10.1007/s00405-022-07438-z
Doty RL (2017) Olfactory dysfunction in neurodegenerative diseases: is there a common pathological substrate? The Lancet Neurology 16(6):478–488. https://doi.org/10.1016/s1474-4422(17)30123-0
Mitchell AJ, Shiri-Feshki M (2009) Rate of progression of mild cognitive impairment to dementia—meta-analysis of 41 robust inception cohort studies. Acta Psychiatr Scand 119(4):252–265. https://doi.org/10.1111/j.1600-0447.2008.01326.x
Qin Z, Wu W, Liu D, Zheng C, Kang J, Zhou H, Meng X, Haacke EM, Wang L (2022) Quantitative susceptibility mapping of brain iron relating to cognitive impairment in hypertension. J Magn Reson Imaging 56(2):508–515. https://doi.org/10.1002/jmri.28043
Orprayoon N, Santibenchakul S, Hemrungrojn S, Phutrakool P, Kengsakul M, Jaisamrarn U, Chaikittisilpa S (2021) Effect of surgical menopause and frontal lobe cognitive function. Climacteric 24(4):389–393. https://doi.org/10.1080/13697137.2020.1867529
Jafari A, de Lima XL, Bernstein JD, Simonyan K, Bleier BS (2021) Association of sinonasal inflammation with functional brain connectivity. JAMA Otolaryngol Head Neck Surg 147(6):534–543. https://doi.org/10.1001/jamaoto.2021.0204
Han P, Whitcroft KL, Fischer J, Gerber J, Cuevas M, Andrews P, Hummel T (2017) Olfactory brain gray matter volume reduction in patients with chronic rhinosinusitis. Int Forum Allergy Rhinol 7(6):551–556. https://doi.org/10.1002/alr.21922
Bsteh GSR, Tuovinen N, Hegen H, Berek K, Wurth S, Auer M, Di Pauli F, Gizewski ER, Deisenhammer F, Berger T, Scherfler C (2020) Impairment of odor discrimination and identification is associated with disability progression and gray matter atrophy of the olfactory system in ms. Mult Scler 6(6):706–715. https://doi.org/10.1177/1352458519838205
Arslan F, Tasdemir S, Durmaz A, Tosun F (2018) The effect of nasal polyposis related nasal obstruction on cognitive functions. Cogn Neurodyn 12(4):385–390. https://doi.org/10.1007/s11571-018-9482-4
Tarasidis GS, DeConde AS, Mace JC, Ashby S, Smith TL, Orlandi RR, Alt JA (2015) Cognitive dysfunction associated with pain and quality of life in chronic rhinosinusitis. Int Forum Allergy Rhinol 5(11):1004–1009. https://doi.org/10.1002/alr.21578
Martin C, Beshel J, Kay LM (2007) An olfacto-hippocampal network is dynamically involved in odor-discrimination learning. J Neurophysiol 98(4):2196–2205. https://doi.org/10.1152/jn.00524.2007
Savic I, Gulyas B, Larsson M, Roland P (2000) Olfactory functions are mediated by parallel and hierarchical processing. Neuron 26(3):735–745. https://doi.org/10.1016/s0896-6273(00)81209-x
Fjaeldstad A, Fernandes HM, Van Hartevelt TJ, Gleesborg C, Moller A, Ovesen T, Kringelbach ML (2017) Brain fingerprints of olfaction: A novel structural method for assessing olfactory cortical networks in health and disease. Sci Rep 7:42534. https://doi.org/10.1038/srep42534
Lotsch J, Hummel T (2019) A machine-learned analysis suggests non-redundant diagnostic information in olfactory subtests. IBRO Rep 6:64–73. https://doi.org/10.1016/j.ibror.2019.01.002
Hummel T, Fliessbach K, Abele M, Okulla T, Reden J, Reichmann H, Wullner U, Haehner A (2010) Olfactory fmri in patients with parkinson’s disease. Front Integr Neurosci 4:125. https://doi.org/10.3389/fnint.2010.00125
Stephenson R, Houghton D, Sundarararjan S, Doty RL, Stern M, Xie SX, Siderowf A (2010) Odor identification deficits are associated with increased risk of neuropsychiatric complications in patients with parkinson’s disease. Mov Disord 25(13):2099–2104. https://doi.org/10.1002/mds.23234
Suzuki Y, Yamamoto S, Umegaki H, Onishi J, Mogi N, Fujishiro H, Iguchi A (2004) Smell identification test as an indicator for cognitive impairment in alzheimer’s disease. Int J Geriatr Psychiatry 19(8):727–733. https://doi.org/10.1002/gps.1161
Wilson RS, Arnold SE, Tang Y, Bennett DA (2006) Odor identification and decline in different cognitive domains in old age. Neuroepidemiology 26(2):61–67. https://doi.org/10.1159/000090250
Luzzi S, Snowden JS, Neary D, Coccia M, Provinciali L, Lambon Ralph MA (2007) Distinct patterns of olfactory impairment in alzheimer’s disease, semantic dementia, frontotemporal dementia, and corticobasal degeneration. Neuropsychologia 45(8):1823–1831. https://doi.org/10.1016/j.neuropsychologia.2006.12.008
Sohrabi HR, Bates KA, Weinborn MG, Johnston AN, Bahramian A, Taddei K, Laws SM, Rodrigues M, Morici M, Howard M, Martins G, Mackay-Sim A, Gandy SE, Martins RN (2012) Olfactory discrimination predicts cognitive decline among community-dwelling older adults. Transl Psychiatry 2(5):e118. https://doi.org/10.1038/tp.2012.43
Funding
Natural Science Foundation of China (82000954), Beijing Science and Technology Nova Program (Z201100006820086), Beijing Hospitals Authority Youth Program (QML20190617), and Beijing Hospitals Authority Clinical Medicine Development of Special Funding (XMLX202136).
Author information
Authors and Affiliations
Contributions
All authors have made substantial contributions to the conception, analysis, and interpretation of data in this article, approved the submitted version, and agreed to be personally accountable for our contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which we are not personally involved, are appropriately investigated, resolved, and the resolution documented in the literature.
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chang, F., Hong, J., Yuan, F. et al. Association between cognition and olfaction-specific parameters in patients with chronic rhinosinusitis. Eur Arch Otorhinolaryngol 280, 3249–3258 (2023). https://doi.org/10.1007/s00405-023-07853-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-023-07853-w