Abstract
Objectives
Exosomal Phospho-Tau-181(P-T181-tau), Total tau (T-tau), and amyloid-β peptide 42 (Aβ42) have been proved the capacity for the amnestic mild cognitive impairment (MCI) and the diagnosis of Alzheimer’s disease (AD). This study aimed to explore the cognitive function and the levels of P-T181-tau, T-tau, and Aβ42 in neuronal-derived exosomes (NDEs) extracted from plasma in normal cognitive adults over 45 years old with olfactory dysfunction.
Methods
A cross-sectional survey of 29 participants aged over 45 was conducted. Plasma exosomes were isolated, precipitated, and enriched by immuno-absorption with anti- L1 cell adhesion molecule (L1CAM) antibody. NDEs were characterized by CD81, and extracted NDE protein (P-T181-tau, T-tau, and Aβ42) biomarkers were quantified by enzyme-linked immunosorbent assay (ELISAs). Olfactory performance was assessed by Sniffin’ Sticks and cognitive performance was assessed by Montreal Cognitive Assessment (MoCA).
Results
There was no significant difference between adults with olfactory dysfunction and without olfactory dysfunction regarding the cognitive function as measured by MoCA and all the participants showed normal cognition. Adults with olfactory dysfunction showed a higher concentration of P-T181-tau in plasma NDEs than did adults without olfactory dysfunction (P = 0.034). Both the levels of P-T181-tau (r = − 0.553, P = 0.003) and T-tau (r = − 0.417, P = 0.034) negatively correlated with the odor identification scores. In addition, the level of T-tau negatively correlated with MoCA scores (r = − 0.597, P = 0.002). The levels of P-T181-tau (r = − 0.464, P = 0.022) and T-tau (r = − 0.438, P = 0.032) negatively correlated with the delayed recall scores.
Conclusions
This study demonstrated that cognition-related pathogenic proteins including P-T181-tau in plasma NDEs were significantly increased in adults over 45 years old with olfactory dysfunction before the occurrence of cognitive impairment. The impaired odor identification and the delayed recall function were highly associated with the increased levels of P-T181-tau and T-tau in plasma NDEs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It has been estimated that there will be 400 million elderly people in China by 2050[1]. Recent clinical and population-based studies indicated about 10–20% of adults aged ≥ 65 years presented with mild cognitive impairment (MCI) and the prevalence of MCI among Chinese community residents was 13.5–17.4% [2,3,4,5]. A community survey showed that about 6% of older adults with MCI turned to dementia every year and the rate of conversion from MCI to dementia was increasing, which has been an increasing social burden [6, 7]. Dementia is commonly attributed to Alzheimer’s disease (AD), with over 5 million people currently affected by AD. And about 13.8 million people are projected to be affected by the year 2050 [7]. Due to limited treatment, recent work has focused on the early detection of MCI through various biomarkers.
Olfactory dysfunction appears in the early course of AD and is a persistent symptom in the stage of MCI, especially odor identification (OI) impairment [8,9,10,11]. Previous studies have reported the clinical significance of olfactory dysfunction in predicting cognitive decline [12,13,14,15]. In a recent meta-analysis, the association between olfactory impairment and subsequent cognitive decline was tenacious in the pre-old population and the relative risk was up to 3.06 [16]. So, it is necessary to focus on middle-aged and pre-old populations with olfactory dysfunction, especially if there is no obvious cause of hyposmia. Olfactory dysfunction has been enrolled in the initial screening of cognition decline. However, the specificity (88%) and sensitivity (57%) of the initial screening of cognitive decline based on olfactory dysfunction alone remains to be improved [17,18,19,20,21].
Misfolding and aggregation of amyloid-β peptide (Aβ) and phosphorylated tau (p-tau) are molecular markers and early features of AD [22,23,24]. These aggregate-associated proteins are detectable in the cerebrospinal fluid (CSF) and blood of patients with AD or MCI [25]. NDEs in the plasma have been considered an important medium for transporting biomarkers [26,27,28,29]. The concordance has been confirmed between CSF biomarkers and NDEs biomarkers and both of them have the same capacity of diagnosis for AD [30]. Total tau (T-tau) proteins, phosphorylated tau (p-tau), and amyloid-β peptide 42 (Aβ42) in NDEs have been confirmed to be useful biomarkers for monitoring AD progression [31]. The levels of Phospho-Tau-S396 (P-S396-tau), Phospho-Tau-181(P-T181-tau), T-tau, and Aβ42 in NDEs strongly predicted cognitive decline in subjects with amnestic MCI [32, 33]. These biomarkers in NDEs even predicted the development of AD up to 10 years before clinical onset [34,35,36].
There is a dramatic increase in MCI prevalence in the community residents older than 60 years when compared to 7.6% of those aged between 55 and 59 years who were diagnosed with MCI [2]. It has been reported that the AD pathologic process began more than a decade before the occurrence of clinical symptoms and early supervision of high-risk groups (middle-aged and pre-old adults with olfactory dysfunction) is necessary [37, 38]. However, studies exploring the cognition-associated pathogenic protein in normal cognitive adults over 45 years old with poor olfactory performance are still lacking. This study aims to detect P-T181- tau, T-tau, and Aβ-42 protein levels in plasma NDEs in normal cognitive adults with olfactory dysfunction and to explore the association among olfaction, cognition, and biomarkers in plasma NDEs.
Materials and methods
Adult patients over 45 years old were recruited from the Smell and Taste Center, Beijing Anzhen Hospital, Capital Medical University. All the participants underwent complete physical examination, nasal endoscopy, sinus computed tomography (CT), head magnetic resonance imaging (MRI), and Sniffin’ Sticks tests. Demographics including age, sex, body mass index (BMI), smoking, and drinking were collected. Inclusion criteria included participants with normal cognition assess by the Montreal Cognitive Assessment (MoCA). Exclusion criteria included (1) patients with olfactory dysfunction with known causes (e.g., trauma, chronic rhinosinusitis, upper airway infection, allergic rhinitis, exposure to toxins or medications, congenital disorders, and idiopathic olfactory dysfunction), (2) participants diagnosed with cognitive impairment, (3) participants with tumor, cancer or other chronic diseases that might influence the olfaction and cognition, and (4) participants without self-help skills. According to the performance in the Sniffin’ Sticks test, participants with olfactory dysfunction were enrolled in the experimental group, while participants with normal olfaction were enrolled in the control group. Finally, 18 patients with olfactory impairment (10 men and 8 women, mean 59.67 ± 10.64 years age) and 11 healthy controls without olfactory dysfunction (4 men and 7 women, mean 54.09 ± 10.10 years age) were included in the present study. This study was approved by the Ethics Committee at Beijing Anzhen Hospital (Beijing, China, No. 2019YFE0116000). The study design complied with the criteria of the Declaration of Helsinki for Medical Research involving Human Subjects. All participants provided written informed consent before participating in the study. The flow diagram of the study design was given in Fig. 1.
Montreal cognitive assessment (MoCA)
The cognitive status of participants was assessed by the Montreal Cognitive Assessment 7.0 (MoCA 7.0). MoCA was developed by Nasreddine [39] of Canada and it was utilized to detect MCI or AD. It can also be used to distinguish patients with cognitive dysfunction from patients with normal aging. The total score for the MoCA is 30 and it covers seven domains of cognition: visuospatial/executive functions (trail-making test: 1 point, copy tube: 1 point and clock drawing task: 3 points), naming (3 points), attention (forward digit span: 1 point, backward digit span: 1 point, vigilance: 1 point and serial 7 subtraction: 3 points), language (sentence repetition: 2 points, verbal fluency: 1 point), abstraction (2 points), delayed recall (5 points) and orientation (6 points) 0.1 point is added for adjustment when the education year is less than 12 years. The optimal cutoff scores for MCI screening were 19 for individuals with no more than 6 years of education, 22 for individuals with 7 to 12 years of education, and 24 for individuals with more than 12 years of education [40]. This cognitive assessment has been proved to be suitable for screening MCI in elderly Chinese people in previous studies [41,42,43,44,45].
Olfactory function test
Psychophysical testing of olfactory function was performed for each participant using Sniffin’ Sticks tests (Burghart, Gmbh, Wedel, Germany) which were consisted of odor threshold (OT), odor discrimination (OD), odor identification (OI), and the overall composite scores (TDI). Sniffn’ Sticks is the most common assessment for olfactory performance in the clinic. Its forced-choice method can eliminate the suspicion of malingering effectively. And it has been assessed in healthy Chinese adults and patients with olfactory dysfunction secondary to varied causes in our previous studies. It was suitable for application in the Chinese population to differentiate normosmia from hyposmia and anosmia [8, 46,47,48,49]. Standard administration was performed according to the manufacturer’s instructions. Felt-tip pens containing various odors were presented to the participants for testing. The pen’s tip was placed approximately 2 cm in front of both nostrils for bilateral stimulation. The test comprised three parts: OT, OD, and OI test. The overall composite scores were reported as TDI scores which ranged from 1 to 48, with higher scores indicating superior olfactory performance. A TDI score ≥ 31, 16–30, and < 15 indicated normosmia, hyposmia, and anosmia, respectively [8].
Collection of neuronal-derived exosomes from blood
We collected 10 mL of blood from each participant by vein puncture into sterile vacutainers under strict aseptic conditions. The blood samples were centrifuged for 30 min at 400 × g to separate the plasma. The plasma samples were aliquoted and immediately stored at − 80 °C until further analysis. NDEs were separated for consistency according to a published protocol [25, 34, 50,51,52]. In brief, 0.5-ml plasma was incubated with 0.15-ml thromboplastin-D (Thermo Fisher Scientific, MA) for 60 min. And 0.35-ml calcium and magnesium-free Dulbecco’s phosphate-buffered saline (DPBS, Thermo Fisher Scientific) with protease and phosphatase inhibitor cocktails (Thermo Fisher Scientific) were added. The mixed solution was centrifuged at 1500 g for 20 min. The supernatants were then mixed with ExoQuick exosome precipitation solution (EXOQ; System Biosciences, CA) and incubated for 1 h on ice. After centrifugation at 1500 g for 30 min, the pellets were resuspended in 250 μl DPBS (Santa Cruz, CA). Each sample was mixed with 100 μl 3% bovine serum albumin (BSA, Thermo Fisher Scientific) and then incubated for 1 h on ice with a mouse anti-human neural cell adhesion molecule (NCAM) antibody (2 pg/ml, Santa Cruz); the antibody was labeled with biotin using the EZ-Link sulfo-NHS-biotin system (Thermo Fisher Scientific). Then, 25 μl of Streptavidin Agarose Resin (Thermo Fisher Scientific) containing 50 μl of 3% BSA was added. After centrifugation at 200 g for 10 min at 4 ℃ and removal of the supernatant, each sample was resuspended in 50 μl 0.05 M glycine–HCl (pH = 3.0) by vortexing for 10 s and mixed with 0.45 ml DPBS containing 2 g/100 ml BSA, 0.10% Tween 20, and inhibitor cocktails. The separated neuronal-derived exosomes samples were stored at -80℃. The removed supernatants were collected and recentrifuged to obtain the non-immunoprecipitated exosomes, which was aimed to be a control to immunoprecipitated exosomes. Exosomes were resuspended in 0.25 ml of 0.05 M glycine–HCl (pH = 3.0) on ice and centrifuged at 200 g for 15 min. The pH of the supernatant was then adjusted to 7.0 with 1 M Tris–HCl (pH = 8.6).
ELISA quantification of exosome proteins
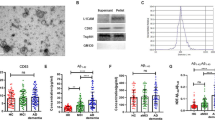
The L1 cell adhesion molecule (L1CAM) levels in non-immunoprecipitated exosomes were measured to confirm the neuronal-derived enrichment with an enzyme-linked immunosorbent assay (ELISA) (LifeSpan BioSciences) [30]. CD81, a specific membrane protein of exosomes, was quantified by ELISA kits (LifeSpan BioSciences). Exosome proteins were quantified by ELISA kits for human Aβ42 (Thermo Fisher Scientific, MA), human T-tau, and human P-T181-tau (INNOTEST). The concentrations of P-T181-tau, Aβ42, and T-tau were calculated according to the standard curves respective to each biomarker. The means of the values obtained from duplicate measurements were calculated for each biomarker and each sample.
Sample size calculation
This study was a case–control study. The olfactory dysfunction group was the experimental group, the normal olfactory group was the control group. The level of the T-tau was the main observation indicator in the present study. According to a recent study [30], it was estimated that the average T-tau level of the control group was 137 ± 46 pg/ml and the level of the experimental group was 195 ± 46 pg/ml. The difference between the two groups was 58 pg/ml. Set α = 0.05 (two-sided), β = 0.20, R = 0.6, the sample size of the experimental group and the control group calculated by PASS 15.0.5 software was 18 (N1) and 11 (N2), respectively.
Statistical analysis
Statistical analysis was performed with Statistical Product and Service Solutions 22.0 (SPSS 22.0, IBM Corporation, New York, NY, USA). Shapiro–Wilk test was used to test whether a continuous variable was normally distributed. Continuous variables were presented as mean ± standard deviation (SD) or median (with range or interquartile range) according to the data distribution. Two-tailed values of P < 0.05 were considered statistically significant. Comparison between groups was performed using an independent t‐test. Partial correlation analysis was used to calculate the level and direction of the correlation of two variables (r > 0 represents positive correlation, r < 0 represents negative correlation, the closer r value to 1, the stronger correlation is) [53]. Categorical variables were compared using the chi-square test.
Results
Patient demographics
The demographics and clinical characteristics of the enrolled participants were shown in Table 1. No significant differences were found between the adults with and without olfactory dysfunction regarding age, sex, education, smoking, drinking, BMI, MoCA scores, and MCI patients. In the present study, all the participants presented with normal cognition. Adults with olfactory dysfunction had a significantly lower OT (P < 0.001), OD (P < 0.001), OI (P < 0.001), and TDI score (P < 0.001). No cognitive decline was observed among these participants.
P-T181-tau, T-tau, and Aβ42 in plasma NDEs among normal cognitive adults with and without olfactory dysfunction
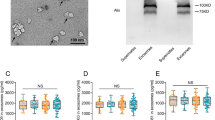
The mean of L1CAM in neural exosomes collected by immunoprecipitation method was 578.22 ± 382.62 pg/ml, and the mean of L1CAM in neural exosomes collected by a non-immunoprecipitation method was 165.60 ± 60.22 pg/ml. Compared with a non-immunoprecipitation method, the L1CAM in immunoprecipitated exosomes increased by about 3.50 ± 2.12 times. Based on these data, we confirmed that neuron-derived exosomes had been successfully collected. Next, we analyzed the level of P-T181-tau, T-tau, and Aβ42 in plasma NDEs (Table 2). Adults with olfactory dysfunction showed a higher concentration of P-T181-tau in plasma NDEs than did adults without olfactory dysfunction (P = 0.034). However, the levels of T-tau and Aβ42 in plasma NDEs were not significantly different between the two groups.
Correlation analysis
NDEs proteins and olfactory performance
A significant association between the levels of P-T181-tau, T-tau in plasma NDEs and OI was observed (Table 1). There is a significantly moderate negative correlation between P-T181-tau level and OI (Table 2, r = -0.417, P = 0.034), and a significantly strong negative correlation between T-tau level and OI (Table 2, r = − 0.553, P = 0.003). There is a significantly moderate negative correlation between T-tau level and TDI (Table 2, r = − 0.458, P = 0.019). However, no significant correlation was observed between Aβ42 and olfactory performance.
NDEs proteins and cognitive performance
A significantly strong negative association between the level of T-tau in plasma NDEs and the total scores of MoCA was observed (Table 3, r = − 0.597, P = 0.002). And there is a significantly moderate negative correlation between the P-T181-tau (Table 3, r =− 0.464, P = 0.022), T-tau level (Table 3, r = − 0.438, P = 0.032) in plasma NDEs level and the delayed recall scores of MoCA. There is a significant and negative correlation between the T-tau level and the Language scores in MoCA (Table 3, r = − 0.443, P = 0.030), and also a significant and negative correlation between the Aβ42 level and the naming scores in MoCA (Table 3, r = − 0.423, P = 0.039).
Discussion
This is the first study exploring the clinical significance of the NDEs biomarkers among normal cognitive adults over 45 years old with olfactory dysfunction.
The present study showed that plasma NDEs P-T181-tau levels in these normal cognitive adults with olfactory dysfunction were significantly increased as compared with that in normal cognitive adults without olfactory dysfunction. The P-T181-tau and T-tau were significantly and negatively correlated with OI scores. Previous studies demonstrated that neuroaxonal degeneration and tangle formation in patients with MCI were reflected by increased concentrations of T-tau and phospho-tau [54,55,56,57]. Early changes in Alzheimer's disease have been demonstrated in the projection pathway from the olfactory bulb to the secondary olfactory brain regions including the piriform and medial temporal cortex, entorhinal cortex, hippocampus, orbitofrontal cortex, and other marginal areas [11, 56]. OI task relied on both olfactory sensory/perceptual functioning and semantic memory [58]. Previous studies have also demonstrated that OI worsened with AD progression which may relate to both tau and tau pathology and neuroinflammation in medial temporal regions (hippocampus and the combined amygdala/parahippocampal gyrus), which were among the first brain area to show tau pathology corresponding to Braak stages I-III [55, 59,60,61,62,63,64,65]. The pathology of AD has been reported to occur 10–15 years or even earlier before the appearance of clinical symptoms [66]. It can be inferred that normal cognitive adults with olfactory dysfunction have already presented with abnormally increased cognition-associated pathogenic proteins. The increased cognition-associated pathogenic proteins may predict the preclinical AD or MCI progression in normal cognitive adults and further prospective studies are warranted to explore the predictive value of these cognition-associated pathogenic proteins in cognitive decline among normal cognitive adults with olfactory impairment.
There is also a significant association between cognitive performance and NDEs biomarkers, especially memory performance. The present study showed that the levels of P-T181-tau and T-tau were significantly and negatively correlated with the delayed recall scores, which indicated that the higher the level of P-T181-tau and T-tau, the worse the memory function. Previous studies have also demonstrated that poor delayed recall performance in middle-aged and pre-old adults was highly associated with the underlying AD pathology, especially in the MCI stage [67, 68]. During the stage of MCI and preclinical AD, tauopathy and tau-mediated neurodegeneration such as tau-induced synaptic loss could impair the memory-related brain areas and synaptic plasticity from these areas was important for information processing, learning, and memory encoding [69,70,71,72]. In tau pathological mouse models, the accumulation of P-Tau in the hippocampus reduced the number and complexity of axon ends of the GABAergic hippocampal pathway, resulting in loss of synapses and damage to hippocampal connections, finally resulting in memory impairment [73, 74]. Based on the above evidence, we speculated that the abnormally increased cognition-associated pathogenic proteins were related to the impaired memory performance in normal cognitive adults with olfactory dysfunction. The accumulation of tauopathy in memory-related regions such as the hippocampus occurred in the stage before MCI, during which the adults with olfactory dysfunction did not show cognitive impairment.
Limitation
The present study had several limitations. This is a case–control pilot study exploring the levels of cognition-associated pathogenic biomarkers in plasma NDEs in normal cognitive adults with olfactory dysfunction. The sample size was relatively small and the results should be further validated in a larger multicenter study. In addition, this is a cross-sectional study and long-term follow-up evaluations of cognition are needed to analyze the cognitive outcome among normal cognitive adults with olfactory dysfunction. Finally, the prediction of biomarkers in plasma NDEs concerning conversion from olfactory dysfunction to MCI or AD should be further verified.
The current challenge is to early identify individuals at high risk of cognitive decline and it is difficult to achieve the desired results by relying solely on olfactory assessment. The clinical relevance of this study is that significantly altered levels of P-T181-tau in plasma NDEs may assist in identifying patients with a high risk of developing cognitive decline among normal cognitive adults with olfactory dysfunction.
Conclusion
Our study firstly showed that normal cognitive adults with olfactory dysfunction presented with significantly increased P-T181-tau in plasma NDEs. Furthermore, altered cognition-associated pathogenic proteins such as P-T181-tau and T-tau in plasma NDEs were highly associated with severe impairment of OI and memory function. These findings demonstrated the potential of these biomarkers for exploring the cognition decline among middle-aged and pre-old adults with olfactory dysfunction.
Abbreviations
- MCI:
-
Mild cognitive impairment
- AD:
-
Alzheimer’s disease
- Aβ:
-
Amyloid-β peptide
- p-tau:
-
Phosphorylated tau
- NDEs:
-
Neuronal-derived exosomes
- CSF:
-
Cerebrospinal fluid
- aMCI:
-
Amnestic mild cognitive impairment
- P-S396-tau:
-
Phospho-Tau-S396
- P-T181- tau:
-
Phospho-Tau-181
- T-tau:
-
Total tau
- L1CAM:
-
L1 cell adhesion molecule
- ELISA:
-
Enzyme-linked immunosorbent assay
- MoCA:
-
Montreal cognitive assessment
- OT:
-
Odor threshold
- OD:
-
Odor discrimination
- OI:
-
Odor identification
- NFTs:
-
Neurofibrillary tangles
- MTL:
-
Medial temporal lobe
References
Fang EF, Scheibye-Knudsen M, Jahn HJ, Li J, Ling L, Guo H, Zhu X, Preedy V, Lu H, Bohr VA, Chan WY, Liu Y, Ng TB (2015) A research agenda for aging in China in the 21st century. Ageing Res Rev 24(Pt B):197–205. https://doi.org/10.1016/j.arr.2015.08.003
Lu Y, Liu C, Yu D, Fawkes S, Ma J, Zhang M, Li C (2021) Prevalence of mild cognitive impairment in community-dwelling Chinese populations aged over 55 years: a meta-analysis and systematic review. BMC Geriatr 21(1):10. https://doi.org/10.1186/s12877-020-01948-3
Gauthier S, Reisberg B, Zaudig M, Petersen RC, Ritchie K, Broich K, Belleville S, Brodaty H, Bennett D, Chertkow H, Cummings JL, de Leon M, Feldman H, Ganguli M, Hampel H, Scheltens P, Tierney MC, Whitehouse P, Winblad B (2006) Mild cognitive impairment. Lancet (London, England) 367(9518):1262–1270. https://doi.org/10.1016/S0140-6736(06)68542-5
Deng Y, Zhao S, Cheng G, Yang J, Li B, Xu K, Xiao P, Li W, Rong S (2021) The prevalence of mild cognitive impairment among Chinese people: a meta-analysis. Neuroepidemiology 55:1–13. https://doi.org/10.1159/000512597
Petersen RC (2011) Clinical practice Mild cognitive impairment. N Engl J Med 364(23):2227–2234. https://doi.org/10.1056/NEJMcp0910237
Ding D, Zhao Q, Guo Q, Liang X, Luo J, Yu L, Zheng L, Hong Z (2016) Progression and predictors of mild cognitive impairment in Chinese elderly: a prospective follow-up in the Shanghai Aging Study. Alzheimer’s Dementia (Amsterdam, Netherlands) 4:28–36. https://doi.org/10.1016/j.dadm.2016.03.004
Hebert LE, Weuve J, Scherr PA, Evans DA (2013) Alzheimer disease in the United States (2010–2050) estimated using the 2010 census. Neurology 80(19):1778–1783. https://doi.org/10.1212/WNL.0b013e31828726f5
Su B, Bleier B, Wei Y, Wu D (2021) Clinical implications of psychophysical olfactory testing: assessment, diagnosis, and treatment outcome. Front Neurosci 15:646956. https://doi.org/10.3389/fnins.2021.646956
Bahar-Fuchs A, Moss S, Rowe C, Savage G (2010) Olfactory performance in AD, aMCI, and healthy ageing: a unirhinal approach. Chem Senses 35(9):855–862. https://doi.org/10.1093/chemse/bjq094
Chan A, Tam J, Murphy C, Chiu H, Lam L (2002) Utility of olfactory identification test for diagnosing Chinese patients with Alzheimer’s disease. J Clin Exp Neuropsychol 24(2):1380–3395. https://doi.org/10.1076/jcen.24.2.251.992
Devanand DP (2016) Olfactory identification deficits, cognitive decline, and dementia in older adults. Am J Geriatr Psychiatry 24(12):1545–7214. https://doi.org/10.1016/j.jagp.2016.08.010
Adams DR, Kern DW, Wroblewski KE, McClintock MK, Dale W, Pinto JM (2018) Olfactory dysfunction predicts subsequent dementia in older U.S. adults. J Am Geriatr Soc 2018:1532–5415. https://doi.org/10.1111/jgs.15048
Attems J, Walker L, Jellinger KA (2015) Olfaction and aging: a mini-review. Gerontology 61(6):485–490. https://doi.org/10.1159/000381619
Schubert CR, Carmichael LL, Murphy C, Klein BE, Klein R, Cruickshanks KJ (2008) Olfaction and the 5-year incidence of cognitive impairment in an epidemiological study of older adults. J Am Geriatr Soc 56(8):1517–1521. https://doi.org/10.1111/j.1532-5415.2008.01826.x
Windon MJ, Kim SJ, Oh ES, Lin SY (2019) Predictive value of olfactory impairment for cognitive decline among cognitively normal adults. Laryngoscope 130(4):840–847. https://doi.org/10.1002/lary.28166
Chen Z, Xie H, Yao L, Wei Y (2020) Olfactory impairment and the risk of cognitive decline and dementia in older adults: a meta-analysis. Braz J Otorhinolaryngol 87(1):94–102. https://doi.org/10.1016/j.bjorl.2020.07.009
Bathini P, Brai E, Auber LA (2019) Olfactory dysfunction in the pathophysiological continuum of dementia. Ageing Res Rev 55:100956
Servello A, Fioretti A, Gualdi G, Di Biasi C, Pittalis A, Sollaku S, Pavaci S, Tortorella F, Fusetti M, Valenti M, Masedu F, Cacciafesta M, Marigliano V, Ettorre E, Pagliarella M (2015) Olfactory dysfunction, olfactory bulb volume and Alzheimer’s disease: is there a correlation? A pilot study. J Alzheimers Dis 48(2):395–402. https://doi.org/10.3233/JAD-150232
Daulatzai MA (2015) Olfactory dysfunction: its early temporal relationship and neural correlates in the pathogenesis of Alzheimer’s disease. 122:1475–1497. https://doi.org/10.1007/s00702-015-1404-6
Seubert J, Laukka EJ, Rizzuto D, Hummel T, Fratiglioni L, Bäckman L, Larsson M (2017) Prevalence and correlates of olfactory dysfunction in old age: a population-based study. J Gerontol A Biol Sci Med Sci 72(8):1072–1079. https://doi.org/10.1093/gerona/glx054
Lojkowska W, Sawicka B, Gugala M, Sienkiewicz-Jarosz H, Bochynska A, Scinska A, Korkosz A, Lojek E, Ryglewicz D (2011) Follow-up study of olfactory deficits, cognitive functions, and volume loss of medial temporal lobe structures in patients with mild cognitive impairment. Curr Alzheimer Res 8(6):689–698. https://doi.org/10.2174/156720511796717212
Bamberger C, Pankow S, Martínez-Bartolomé S, Ma M, Diedrich J, Rissman RA, Yates JR 3rd (2021) Protein footprinting via covalent protein painting reveals structural changes of the proteome in Alzheimer’s disease. J Proteome Res. https://doi.org/10.1021/acs.jproteome.0c00912
Abbasi J (2020) Alzheimer blood test using tau biomarker is in development. JAMA 323(14):1336. https://doi.org/10.1001/jama.2020.4542
Barthélemy NR, Horie K, Sato C, Bateman RJ (2020) Blood plasma phosphorylated-tau isoforms track CNS change in Alzheimer’s disease. J Exp Med 217(11):861. https://doi.org/10.1084/jem.20200861
Thompson AG, Gray E, Heman-Ackah SM, Mäger I, Talbot K, Andaloussi SE, Wood MJ, Turner MR (2016) Extracellular vesicles in neurodegenerative disease—pathogenesis to biomarkers. Nat Rev Neurol 12(6):346–357. https://doi.org/10.1038/nrneurol.2016.68
Chiasserini D, van Weering JRT, Piersma SR, Pham TV, Malekzadeh A, Teunissen CE, de Wit H, Jiménez CR (2014) Proteomic analysis of cerebrospinal fluid extracellular vesicles: a comprehensive dataset. J Proteomics 106:191–204. https://doi.org/10.1016/j.jprot.2014.04.028
Shi M, Liu C, Cook TJ, Bullock KM, Zhao Y, Ginghina C, Li Y, Aro P, Dator R, He C, Hipp MJ, Zabetian CP, Peskind ER, Hu S-C, Quinn JF, Galasko DR, Banks WA, Zhang J (2014) Plasma exosomal α-synuclein is likely CNS-derived and increased in Parkinson’s disease. Acta Neuropathol 128(5):639–650. https://doi.org/10.1007/s00401-014-1314-y
Cheng L, Sharples RA, Scicluna BJ, Hill AF (2014) Exosomes provide a protective and enriched source of miRNA for biomarker profiling compared to intracellular and cell-free blood. J Extracellular Vesicles 3:23743. https://doi.org/10.3402/jev.v3.23743
Fauré J, Lachenal G, Court M, Hirrlinger J, Chatellard-Causse C, Blot B, Grange J, Schoehn G, Goldberg Y, Boyer V, Kirchhoff F, Raposo G, Garin J, Sadoul R (2006) Exosomes are released by cultured cortical neurones. Mol Cell Neurosci 31(4):642–648. https://doi.org/10.1016/j.mcn.2005.12.003
Jia L, Qiu Q, Zhang H, Chu L, Du Y, Zhang J, Zhou C, Liang F, Shi S, Wang S, Qin W, Wang Q, Li F, Wang Q, Li Y, Shen L, Wei Y, Jia J (2019) Concordance between the assessment of Aβ42, T-tau, and P-T181-tau in peripheral blood neuronal-derived exosomes and cerebrospinal fluid. Alzheimers Dement 15(8):1071–1080. https://doi.org/10.1016/j.jalz.2019.05.002
Nam E, Lee Y-B, Moon C, Chang K-A (2020) Serum Tau proteins as potential biomarkers for the assessment of Alzheimer’s disease progression. Int J Mole Sci 21(14):5007. https://doi.org/10.3390/ijms21145007
Chen T-B, Lee Y-J, Lin S-Y, Chen J-P, Hu C-J, Wang P-N, Cheng IH (2019) Plasma Aβ42 and Total Tau predict cognitive decline in amnestic mild cognitive impairment. Sci Rep 9(1):13984. https://doi.org/10.1038/s41598-019-50315-9
Parnetti L, Chiasserini D, Eusebi P, Giannandrea D, Bellomo G, De Carlo C, Padiglioni C, Mastrocola S, Lisetti V, Calabresi P (2012) Performance of aβ1-40, aβ1-42, total tau, and phosphorylated tau as predictors of dementia in a cohort of patients with mild cognitive impairment. J Alzheimer’s Dis 29(1):229–238. https://doi.org/10.3233/JAD-2011-111349
Fiandaca MS, Kapogiannis D, Mapstone M, Boxer A, Eitan E, Schwartz JB, Abner EL, Petersen RC, Federoff HJ, Miller BL, Goetzl EJ (2015) Identification of preclinical Alzheimer’s disease by a profile of pathogenic proteins in neurally derived blood exosomes: a case-control study. Alzheimer’s Dementia 11(6):600-607. https://doi.org/10.1016/j.jalz.2014.06.008
Kapogiannis D, Mustapic M, Shardell MD, Berkowitz ST, Diehl TC, Spangler RD, Tran J, Lazaropoulos MP, Chawla S, Gulyani S, Eitan E, An Y, Huang C-W, Oh ES, Lyketsos CG, Resnick SM, Goetzl EJ, Ferrucci L (2019) Association of extracellular vesicle biomarkers with Alzheimer disease in the baltimore longitudinal study of aging. JAMA Neurol 76(11):1340–1351. https://doi.org/10.1001/jamaneurol.2019.2462
Winston CN, Goetzl EJ, Akers JC, Carter BS, Rockenstein EM, Galasko D, Masliah E, Rissman RA (2016) Prediction of conversion from mild cognitive impairment to dementia with neuronally derived blood exosome protein profile. Alzheimers Dement (Amst) 3:63–72. https://doi.org/10.1016/j.dadm.2016.04.001
Villemagne VL, Burnham S, Bourgeat P, Brown B, Ellis KA, Salvado O, Szoeke C, Macaulay SL, Martins R, Maruff P, Ames D, Rowe CC, Masters CL (2013) Amyloid β deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer’s disease: a prospective cohort study. Lancet Neurol 12(4):357–367. https://doi.org/10.1016/s1474-4422(13)70044-9
Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, Iwatsubo T, Jack CR Jr, Kaye J, Montine TJ, Park DC, Reiman EM, Rowe CC, Siemers E, Stern Y, Yaffe K, Carrillo MC, Thies B, Morrison-Bogorad M, Wagster MV, Phelps CH (2011) Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7(3):280–292. https://doi.org/10.1016/j.jalz.2011.03.003
Nasreddine Phillips Bédirian Charbonneau Whitehead Isabelle Collin Cummings Chertkow ZSNAVSVJLH (2005) The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 53(4):695–699. https://doi.org/10.1111/j.1532-5415.2005.53221.x
Lu J, Li D, Li F, Zhou A, Wang F, Zuo X, Jia XF, Song H, Jia J (2011) Montreal cognitive assessment in detecting cognitive impairment in Chinese elderly individuals: a population-based study. J Geriatr Psychiatry Neurol 24(4):184–190. https://doi.org/10.1177/0891988711422528
Wen HB, Zhang ZX, Niu FS, Li L (2008) The application of Montreal cognitive assessment in urban Chinese residents of Beijing. Zhonghua Nei Ke Za Zhi 47(1):36–39
Yu J, Li J, Huang X (2012) The Beijing version of the Montreal Cognitive Assessment as a brief screening tool for mild cognitive impairment: a community-based study. BMC Psychiatry 12:156. https://doi.org/10.1186/1471-244x-12-156
Yu K, Zhang S, Wang Q, Wang X, Qin Y, Wang J, Li C, Wu Y, Wang W, Lin H (2014) Development of a computerized tool for the chinese version of the montreal cognitive assessment for screening mild cognitive impairment. Int Psychogeriatr 27:213–219. https://doi.org/10.1017/s1041610214002269
Pendlebury ST, Cuthbertson FC, Welch SJV, Mehta Z, Rothwell PM (2010) Underestimation of cognitive impairment by mini-mental State examination versus the Montreal Cognitive Assessment in patients with transient ischemic attack and stroke: a population-based study. Stroke 41(6):1290–1293. https://doi.org/10.1161/STROKEAHA.110.579888
Tang Z, Chen X, Zhang W, Sun X, Hou Q, Li Y, Feng X, Chen Y, Lv J, Ji L, Ding G, Li D (2021) Association between gamma-glutamyl transferase and mild cognitive impairment in Chinese women. Front Aging Neurosci 13:630409. https://doi.org/10.3389/fnagi.2021.630409
Su B, Wu D, Wei Y (2021) Development of Chinese odor identification test. Ann Transl Med. 9(6):499. https://doi.org/10.21037/atm-21-913
Wu D, Li Y, Bleier BS, Wei Y (2020) Superior turbinate eosinophilia predicts olfactory decline in patients with chronic rhinosinusitis. Ann Allergy Asthma Immunol 125(3):304-310.e301. https://doi.org/10.1016/j.anai.2020.04.027
Huang T, Wei Y, Wu D (2021) Effects of olfactory training on posttraumatic olfactory dysfunction: a systematic review and meta-analysis. Int Forum Allergy Rhinol 11(7):1102-1112. https://doi.org/10.1002/alr.22758
Liu Y, Fang F, Zhan X, Yao L, Wei Y (2020) The impact of obstructive apnea sleep syndrome on chemical function. Sleep Breath 24(4):1549–1555. https://doi.org/10.1007/s11325-020-02022-3
De Toro J, Herschlik L, Waldner C, Mongini C (2015) Emerging roles of exosomes in normal and pathological conditions: new insights for diagnosis and therapeutic applications. Front Immunol 6:203. https://doi.org/10.3389/fimmu.2015.00203
Shaimardanova AA, Solovyeva VV, Chulpanova DS, James V, Kitaeva KV, Rizvanov AA (2020) Extracellular vesicles in the diagnosis and treatment of central nervous system diseases. Neural Regen Res 15(4):586–596. https://doi.org/10.4103/1673-5374.266908
Lakshmi S, Essa MM, Hartman RE, Guillemin GJ, Sivan S, Elumalai P (2020) Exosomes in Alzheimer’s disease: potential role as pathological mediators Biomarkers and Therapeutic Targets. Neurochem Res 45(11):2553–2559. https://doi.org/10.1007/s11064-020-03111-1
Cohen J (1988) Statistical power analysis for the behavioral sciences, 2nd edn. Hillsdale, New Jersey
Tsai C-L, Liang C-S, Lee J-T, Su M-W, Lin C-C, Chu H-T, Tsai C-K, Lin G-Y, Lin Y-K, Yang F-C (2019) Associations between plasma biomarkers and cognition in patients with Alzheimer’s disease and amnestic mild cognitive impairment: a cross-sectional and longitudinal study. J Clin Med 8(11):1893. https://doi.org/10.3390/jcm8111893
Pettigrew C, Soldan A, Sloane K, Cai Q, Wang J, Wang MC, Moghekar A, Miller MI, Albert M (2017) Progressive medial temporal lobe atrophy during preclinical Alzheimer’s disease. Neuroimage Clin 16:439–446. https://doi.org/10.1016/j.nicl.2017.08.022
Li S, Li W, Wu X, Li J, Yang J, Tu C, Ye X, Ling S (2019) Olfactory deficit is associated with mitral cell dysfunction in the olfactory bulb of P301S tau transgenic mice. Brain Res Bull 148:34–45. https://doi.org/10.1016/j.brainresbull.2019.03.006
Zetterberg H (2017) Review: Tau in biofluids—relation to pathology, imaging and clinical features. Neuropathol Appl Neurobiol 43(3):194–199. https://doi.org/10.1111/nan.12378
Devanand DP, Tabert MH, Cuasay K, Manly JJ, Schupf N, Brickman AM, Andrews H, Brown TR, DeCarli C, Mayeux R (2010) Olfactory identification deficits and MCI in a multi-ethnic elderly community sample. Neurobiol Aging 31(9):1593–1600. https://doi.org/10.1016/j.neurobiolaging.2008.09.008
Klein J, Yan X, Johnson A, Tomljanovic Z, Zou J, Polly K, Honig LS, Brickman AM, Stern Y, Devanand DP, Lee S, Kreisl WC (2021) Olfactory impairment is related to Tau pathology and neuroinflammation in Alzheimer’s disease. J Alzheimers Dis 80(3):1051–1065. https://doi.org/10.3233/jad-201149
Braak H, Braak E (1991) Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 82(4):239–259. https://doi.org/10.1007/bf00308809
Kovacs T, Cairns NJ, Lantos PL (2001) Olfactory centres in Alzheimer’s disease: olfactory bulb is involved in early Braak’s stages. NeuroReport 12(2):285–288. https://doi.org/10.1097/00001756-200102120-00021
Klein J, Yan X, Johnson A, Tomljanovic Z, Zou J, Polly K, Honig LS, Brickman AM, Stern Y, Devanand DP, Lee S, Kreisl WC (2021) Olfactory impairment is related to Tau pathology and neuroinflammation in Alzheimer’s disease. J Alzheimer’s Dis 80(3):1051-1065. https://doi.org/10.3233/JAD-201149
Bouras C, Hof PR, Morrison JH (1993) Neurofibrillary tangle densities in the hippocampal formation in a non-demented population define subgroups of patients with differential early pathologic changes. Neurosci Lett 153(2):131–135. https://doi.org/10.1016/0304-3940(93)90305-5
Growdon ME, Schultz AP, Dagley AS, Amariglio RE, Hedden T, Rentz DM, Johnson KA, Sperling RA, Albers MW, Marshall GA (2015) Odor identification and Alzheimer disease biomarkers in clinically normal elderly. Neurology 84(21):2153–2160. https://doi.org/10.1212/wnl.0000000000001614
Wilson RS, Arnold SE, Schneider JA, Tang Y, Bennett DA (2007) The relationship between cerebral Alzheimer’s disease pathology and odour identification in old age. J Neurol Neurosurg Psychiatry 78(1):30–35. https://doi.org/10.1136/jnnp.2006.099721
Tarawneh R, Holtzman DM (2012) The clinical problem of symptomatic Alzheimer disease and mild cognitive impairment. Cold Spring Harb Perspect Med 2(5):a006148. https://doi.org/10.1101/cshperspect.a006148
Park S-J, Lee J-E, Lee K-S, Kim J-S (2018) Comparison of odor identification among amnestic and non-amnestic mild cognitive impairment, subjective cognitive decline, and early Alzheimer’s dementia. Neurol Sci 39(3):557–564. https://doi.org/10.1007/s10072-018-3261-1
Roberts RO, Christianson TJH, Kremers WK, Mielke MM, Machulda MM, Vassilaki M, Alhurani RE, Geda YE, Knopman DS, Petersen RC (2016) Association between olfactory dysfunction and amnestic mild cognitive impairment and Alzheimer disease dementia. JAMA Neurol 73(1):93–101. https://doi.org/10.1001/jamaneurol.2015.2952
Acebes A, Martin-Pena A, Chevalier V, Ferrus A (2011) Synapse loss in olfactory local interneurons modifies perception. J Neurosci 31(8):2734–2745. https://doi.org/10.1523/jneurosci.5046-10.2011
Liu X, Zeng K, Li M, Wang Q, Liu R, Zhang B, Wang J-Z, Shu X, Wang X (2017) Expression of P301L-hTau in mouse MEC induces hippocampus-dependent memory deficit. Sci Rep 7:4. https://doi.org/10.1038/s41598-017-04305-4
Terry RD, Masliah E, Salmon DP, Butters N, DeTeresa R, Hill R, Hansen LA, Katzman R (1991) Physical basis of cognitive alterations in Alzheimer’s disease: synapse loss is the major correlate of cognitive impairment. Ann Neurol 30(4):572–580. https://doi.org/10.1002/ana.410300410
Budnik V, Ruiz-Cañada C, Wendler F (2016) Extracellular vesicles round off communication in the nervous system. Nat Rev Neurosci 17(3):160–172. https://doi.org/10.1038/nrn.2015.29
Schinder AF, Morgenstern NA (2009) Adult neurogenesis is altered by GABAergic imbalance in models of Alzheimer’s disease. Cell Stem Cell 5(6):573–574. https://doi.org/10.1016/j.stem.2009.11.007
Sun B, Halabisky B, Zhou Y, Palop JJ, Yu G, Mucke L, Gan L (2009) Imbalance between GABAergic and Glutamatergic transmission impairs adult neurogenesis in an animal model of Alzheimer’s disease. Cell Stem Cell 5(6):624–633. https://doi.org/10.1016/j.stem.2009.10.003
Funding
This study is supported by grants from the Beijing Hospitals Authority Youth Program (QML20190617), Beijing Science and Technology Nova Program (Z201100006820086), Natural Science Foundation of China (82000954), and Beijing Hospitals Authority Clinical Medicine Development of Special Funding (XMLX202136), Beijing Hospitals Authority’ Mission Plan (SML20190601), Beijing Scholars Program (No. 051), and National Key R&D Program of China (2019YFE0116000).
Author information
Authors and Affiliations
Contributions
All authors have made substantial contributions to the conception, analysis, and interpretation of data in this article approved the submitted version and agreed both to be personally accountable for our contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which we are not personally involved, are appropriately investigated, resolved, and the resolution documented in the literature.
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chen, Z., Chang, F., Yao, L. et al. Clinical significance of the cognition-related pathogenic proteins in plasma neuronal-derived exosomes among normal cognitive adults over 45 years old with olfactory dysfunction. Eur Arch Otorhinolaryngol 279, 3467–3476 (2022). https://doi.org/10.1007/s00405-021-07143-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-021-07143-3