Abstract
Purpose of review
Chronic rhinosinusitis (CRS) is a prevalent and heterogeneous inflammatory disease that affects millions of people worldwide. Olfactory dysfunction (OD) is one of the most common symptoms of CRS patients. Increasing evidence suggests that the mechanisms underlying OD in different clinical subtypes and inflammatory endotypes of CRS may show discrepancy. Additionally, assessing the severity of OD in CRS patients, and selecting appropriate and effective treatment approaches have emerged as critical concerns in clinical practice. This article aims to provide new insights into the pathogenesis, diagnosis and treatment of OD in patients with CRS.
Recent findings
Recent studies have further highlighted the heterogeneity of CRS, categorizing it into Type 2 and non-Type 2 subtypes based on distinct inflammatory patterns. Furthermore, the diverse mechanisms of OD in patients with different subtypes of CRS have been revealed. Beyond the conventionally recognized conductive factors, inflammatory factors are increasingly being identified as crucial pathogenic contributors to OD in patients with CRS. Generally, the evaluation methods for OD mainly include three different categories, including self-reported assessments based on questionnaires or scales, psychophysical tests, and electrophysiological or imaging assessments. Despite considerable efforts on new approaches, the long-term and effective treatments of OD in CRS still remain elusive. Olfactory training (OT) can be recommended for patients with OD, although it requires further evaluation in CRS patients.
Summary
This article, based on recent progress, overviewed the epidemiological characteristics and pathophysiological mechanisms of OD in CRS patients, and summarized the methods of clinical assessment and treatment strategies of OD. We emphasized the diverse mechanisms of OD in different subtypes of CRS. Although drugs, biological products, and OT may be beneficial for improving olfactory function in CRS patients, further research is needed to confirm their long-term efficacy and develop more effective treatment approaches for OD in CRS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic rhinosinusitis (CRS) is one of the most common chronic inflammatory diseases worldwide [1••, 2]. CRS is a heterogeneous disorder that affects approximately 12% of individuals in the United States, and 11% in Europe [3,4,5,6]. In China the prevalence is about 8% [7].The clinical symptoms of CRS include nasal congestion, rhinorrhea and headache. Besides, olfactory dysfunction (OD) is one of the most common symptoms [1••, 3, 8••], which seriously affects the quality of patients' life (QOL) and consumes a large number of medical resources [9].
Increasing evidence suggests that the OD of CRS patients may be caused by various mechanisms, which may be related to conductive factors and inflammatory factors [8••]. With the deepening understanding of the intrinsic type of inflammation and associated biomarkers, the heterogeneity of CRS has been thoroughly recognized. The European Position Paper on Rhinosinusitis and Nasal Polyps (EPOS) 2020 have redefined the classification of CRS [1••], categorizing it into type 2 and non-type 2. Increasing studies have found that the mechanisms of OD in different subtypes of CRS may be distinct [1••]. In addition, the assessment of OD in CRS patients has become challenge in clinical practice, as well as the selection of appropriate and effective treatment methods. This article aims to review the research progress on the epidemiology, pathogenesis, assessment methods, and treatment strategies of OD in CRS patients.
Epidemiology
OD is a common clinical manifestation of CRS patients, which may also be one of the initial symptoms [1••, 3, 10•]. The severity of OD is closely related to the severity of disease [11]. Most studies indicate that approximately 60% to 80% of CRS patients experience varying degrees of OD [3, 12, 13]. However, several studies reported different data. A study from Japan found that only 38% of CRS patients suffer from OD [14]. Although the incidence of OD in CRS may be influenced by different olfactory assessments, the differences in OD prevalence among different geographical regions and ethnic backgrounds are still unclear (Fig. 1). More studies are needed to reveal the geographical and ethnic differences in the incidence of OD in CRS. OD significantly affects QOL [15,16,17], and both CRS and OD have been shown to be associated with depression, severely affecting the mental health of patients [18, 19]. In CRS, OD is independently correlated with the risks factors such as smoking, nasal polyps, and aspirin-exacerbated respiratory disease (AERD), as well as diabetes and age [13, 20, 21] (Table 1). Traditionally, the diagnosis of CRS is based on sinus CT and endoscopic examination to determine the presence of nasal polyps, and it is divided into CRS with nasal polyps (CRSwNP) and CRS without nasal polyps (CRSsNP) [3, 22]. Compared to CRSsNP patients, CRSwNP patients have a higher incidence of OD and more severe symptoms [12, 21, 23] (Table 1). In addition, according to the latest classification, a study of 811 CRS patients found that the incidence of OD in type 2 and non-type 2 CRSwNP was 86.1% and 71.0%, respectively [24]. However, for most CRSsNP patients, although subjective OD was reported, only 17% of patients exhibited OD determined through objective measurements [13].
Pathophysiological Mechanisms of OD in CRS Patients
The Olfactory System
The olfactory system is primarily composed of the olfactory epithelium (OE) and the central olfactory bulb (OB) (Fig. 2). OE is the surface layer of the olfactory mucosa (OM), which captures odor molecules through the ciliary olfactory receptors of olfactory sensory neurons (OSNs), thereby activating olfactory signals [25]. The main cellular components of the OE include OSNs, basal cells (globose basal cells and horizontal basal cells, GBCs & HBCs), olfactory sustentacular cells (OSCs), and Bowman gland cells, olfactory ensheathing cells (OECs) [26]. OSNs are the main sensory cells within the olfactory system. Mature OSNs have axons that pass through the cribriform plate to connect with the OB, as well as dendrites that infiltrate the epithelial surface to capture odor molecules in the nasal cavity through cilia [27]. Basal cells, especially HBCs, are responsible for differentiating into new OSNs or other type of cells to maintain cell renewal and regeneration in the olfactory system [28]. OSCs provide nutritional, metabolic, and other support for OSNs and the entire OE, and maintain the function of OSNs by endocytosing odor binding proteins/odor complexes [25, 29]. Bowman gland cells secrete mucus to keep the nasal cavity moist, providing a suitable microenvironment for OSNs, which promoting the generation and transmission of olfactory signals [22], and OECs ensheath, accompany and guide the axons of OSNs, and providing them with support, protection, and immune defense [30]. In addition, OB is the terminal nucleus of the olfactory nerve and the main central nervous system site for olfaction [31], after OSNs transmit olfactory information to OB, OB processes and integrates it and projects to the main olfactory centers, such as the limbic system (emotion) and hypothalamus (memory), and ultimately reaching the olfactory cortex, enabling people to acquire olfactory awareness [10•].
The illustration of the structure of the olfactory system and the inflammation induced damage leading to OD in CRS patients, including damage and apoptosis of OE cells, substitution of the olfactory epithelium by respiratory epithelium, squamous metaplasia, aggregation and infiltration of eosinophils, mucosal barrier disruption, OB atrophy. OE, olfactory epithelium; OB, olfactory bulb; OSNs, olfactory sensory neurons; OECs, olfactory ensheathing cells; GBCs, globose basal cells; HBCs, horizontal basal cells; OSCs, olfactory sustentacular cells; Eos, eosinophils; IFN-γ, interferon-gamma; IL, interleukin; TNF-α, tumor necrosis factor-alpha; IgE, immunoglobulin E; ECP, eosinophil cationic protein; MBP, major basic protein; EPX, eosinophil peroxidase; EDN, eosinophil-derived neurotoxins
Pathological Mechanisms
The traditional view is that the OD of CRS patients is mainly attributed to the conductive factors (conductive OD) [32]. The olfactory cleft (OC) is the region where OE is located. Pathological changes in the nasal cavity of CRS patients, including mucosal edema and the formation of nasal polyps [3], may obstruct the OC and restrict the transmission of odorants to the OE, thereby affecting olfactory function [32] (Fig. 1). Hence, the presence of nasal polyps has a significant impact on OD (Table 2). Nasal polyps in the olfactory region may disperse olfactory airflow and olfaction [33]. Therefore, polyps can substantially reduce olfactory function when they are located in the olfactory region or when their size leads to significant obstruction of the nasal cavity [33]. However, if the small polyps are only located in the middle meatus, its impact on olfactory function is relatively mild [33].
In addition to conductive factors, an increasing number of studies indicate that inflammatory factors (sensorineural OD) are important for the development of OD in CRS patients [13] (Fig. 1). OD is closely related to histopathological changes of OE in CRS patients [34]. Inflammation may cause damage of OE (Fig. 2). Inflammation may lead to morphological changes and a decrease in the number of OSNs, ultimately result in OD in CRS patients [31, 35]. Under inflammation, axonal degeneration, dendrite loss, ciliary damage, and apoptosis of OSNs are frequently observed [18, 36]. In addition, the inflammation, by damaging other cells within the olfactory system that are responsible for supporting, repairing, and protecting OSNs, can disrupt olfactory function [18, 31, 37]. For instance, recent study found that the number of undifferentiated HBCs significantly increased in OE due to chronic inflammation [37]. Chronic inflammation is able to direct an olfactory stem cell function switch from neuro regeneration to immune defense [37]. Another study also found that the number of immature OSNs in the OM of type 2 inflammatory mice decreased, while the number of mature sensory neurons was not affected. This may be due to the reduction of OSNs renewal, which may be related to the regenerative function of OSNs [38, 39]. Moreover, inflammation can directly damage OSCs. A significant impact is the disruption of nutrition and metabolism in OSNs, as well as impairments in the generation and transmission of olfactory signals [26, 37]. Inflammation can also lead to the impairment of Bowman gland cells and OECs. Damage to these cells impairs the moistening and self-cleaning functions of the nasal cavity and sinuses, compromising the integrity of the mucosal barrier. In turn, the destruction of the mucosal barrier affects the protective immune function of OSNs [22, 30, 40]. In addition, under the long-term impact of chronic inflammation, abnormal proliferation and differentiation of olfactory epithelial cells occur during tissue injury and repair (Fig. 2). This condition manifests as squamous metaplasia and substitution of the olfactory epithelium by respiratory epithelium [41]. The respiratory epithelium is characterized by goblet cell hyperplasia, which is often interspersed with OSCs within the OE [41, 42]. These changes may disrupt the normal structure and function of OE, potentially affecting olfactory function [38, 41, 42]. In addition, besides Bowman gland cells, goblet cells in the OE may also affect olfaction by alter the volume and composition of mucus in OC[18]. Excessive mucus may block the nasal cavity and olfactory channels, preventing odor molecules from reaching the olfactory receptors [43]. Changes in the chemical composition of mucus may also affect the local microenvironment, interfering with the binding of odor molecules to olfactory receptors on the cilia of OSNs [43, 44]. Additionally, impairment of olfactory mucosal barrier usually occurs in chronic inflammation induced CRS, especially in type 2 inflammatory response [38, 45, 46]. The breakdown of the barrier makes it easier for foreign and intrinsic antigens (pathogenic microorganisms, toxins, and inflammatory products, etc.) to enter the olfactory nerve pathway, further exacerbating OD [18, 45, 47]. Many inflammatory cytokines have been found to be associate with OD in CRS patients (Fig. 2).
Pre-inflammatory cytokines such as interleukin(IL)-6 and tumor necrosis factor-alpha(TNF-α), and inflammatory cytokines including IL-2, IL-4, IL-5, IL-10, IL-13, interferon-gamma(IFN-γ), IL-17, as well as chemokines including C–C motif chemokine ligand(CCL)2、CCL5、CCL11, which are related to OD[1••, 2, 18, 48,49,50,51,52,53](Table 2). Most of these cytokines and chemokines have potential neurotoxicity [54, 55], which may lead to damage and apoptosis of OSNs and cause temporary or permanent OD. However, a recent clustering analysis study based on the mucosal biomarkers collected from the OC of CRS patients showed that clusters dominated by type 2 inflammatory cytokines IL-5, IL-13, and immunoglobulin E(IgE) exhibited relatively low olfactory scores [56]. This result is consistent with another study clustering CRS cytokines, which also revealed a strong correlation between IL-5, IL-13 levels and olfactory function [57]. These data indicate that type 2 cytokines are more likely to lead to OD in CRS [38]. Additionally, TNF-α is a pleiotropic cytokine that has been universally associated with CRS [2], which inhibits the regeneration of OE by inhibiting the proliferation of basal progenitor cells and the production of immature OSNs [50]. The infiltration of eosinophils in the OM is one of the most common pathological changes in CRS [21, 58]. Compared with non-type 2 CRS and CRSsNP, both type 2 CRS and CRSwNP exhibit more significant eosinophil aggregation [38]. The aggregation of eosinophils is associated with the severity of inflammation in CRS and closely related to the OD [59]. A study on pathological examination and immunohistochemical analysis of OM in CRS patients found that OD patients had more severe erosion of the OM and higher density of eosinophil infiltration compared to those without OD [41]. More studies suggest that the extent of eosinophilic inflammation may directly and indirectly affect OD [60]. The direct impact is due to the degranulation proteins released by eosinophils, such as major basic protein (MBP), eosinophil peroxidase (EPX), eosinophil cationic protein (ECP) and eosinophil-derived neurotoxins (EDN), which can directly cause dysfunction and destruction of the cells in OE [60]. The indirect impact lies in the release of cytokines and chemokines by eosinophils, which can induce local inflammation, and cause damage to the OM [61, 62]. In addition, an eosinophilic biomarker Charcot Leyden crystal protein (CLC) was found in the upper turbinate of CRSwNP patients, supporting the possibility that local eosinophilic influx in OC may be related to OD [63]. Studies have found that type 2 CRS is significantly associated with comorbidities such as allergic rhinitis, asthma, and olfactory loss [64, 65]. Due to the high aggregation of eosinophils and the mediation of specific cytokines (such as IL-5 and IL-13) [66], patients with type 2 CRS exhibit significant structural and functional impairment of the OM [41, 45, 67]. This damage compromises the integrity of OSNs, undermines the supportive role of olfactory epithelial cells, impairs the defense function of the nasal mucosal barrier, and impedes the transmission of olfactory signals, ultimately leading to severe OD.
Moreover, studies have shown that the intrabulbar neural circuits of OB in patients with CRS is significantly disordered due to inflammation, manifesting as atrophy of the OB's superficial layer [31], reduction of OSNs transmission under inflammation of OC, and reduced volume of the OB [8••] (Fig. 2). Interestingly, it was observed that OB can recover from atrophy once chronic inflammation subsides. However, the regeneration of OSNs was found to be incomplete [31, 35]. Gudziol, et al. found that the volume of OB in CRS patients significantly increased after 3 months of treatment and was significantly correlated with an improvement of odor threshold, confirming that the size of OB volume correlates with olfactory function [68]. However, whether the reduction in OB volume is the cause or result of OD remains to be further explored.
In addition, the structure and integrity of olfactory-related regions in the cerebral cortex of CRS patients are also important [69]. The gray matter density of olfactory related areas in the brain of CRS patients with severe OD decreases, including the rectus gyrus, medial orbital frontal gyrus, thalamus, and insula [69]. The decrease in gray matter density in these regions may be related to the neural mechanisms of OD that affects olfactory processing and perception. Nevertheless, our understanding of CRS related brain structural changes is still limited and further research is needed.
Assessment of OD in CRS Patients
Questionnaires
A questionnaire is a simple and effective tool for evaluating olfactory function, using personal self-reporting to gain a deeper understanding of a patient’s level of OD and its impact on their QOL [70]. The Sino-Nasal Outcome Test-22 (SNOT-22) is a comprehensive questionnaire that evaluates nasal symptoms, olfactory function, emotional state, and sleep quality across 22 questions. Although the SNOT-22 questionnaire is one of the most widely used tools to describe sinonasal QOL in patients with CRS, it has only a single item dedicated to olfactory function [13]. Visual Analogue Scale (VAS) is an intuitive and accessible tool that enables participants to self-evaluate their olfactory function along a continuous scale [13]. The Questionnaire of Olfactory Disorders (QOD) is a widely used tool for evaluating the impact of OD on individual's QOL [70]. The Questionnaire of Olfactory Disorders-Negative Statements (QOD-NS) is a revised version of the QOD, which uses negative statements to reduce subjective biases in participant responses and improve the accuracy of evaluations. This adaptation has proven to have a strong correlation with CRS related OD [13]. However, some studies suggest that there may be a lack of direct correlation between changes in olfactory function and questionnaires [13], as the results may be easily influenced by the individual's psychological and emotional state [71].
Endoscopy Scale
Endoscopic scale is a reliable and intuitive assessment tool that can help clinicians quantify the severity of sinusitis symptoms and monitor the progression of the disease. Soler et al. proposed the Olfactory Cleft Endoscopy Scale (OCES), a scoring system that specifically focuses on the pathological status of OC [72]. As a tool specific to olfactory assessment, OCES provides additional information to traditional nasal endoscopy. Schlosser et al. further confirmed the correlation between OCES and the Sniffin' Sticks test as well as QOD-NS through a multicenter study [73]. This finding demonstrates the effectiveness of OCES in evaluating OD of CRS patients.
Psychophysical Tests
Psychophysical tests are designed to provide both qualitative and quantitative evaluations of olfactory function. The commonly used psychophysical tests include the Sniffin' Sticks test and the University of Pennsylvania Smell Identification Test (UPSIT), both of which are widely used to evaluate the OD of CRS patients [74, 75]. However, these tests may face challenges in cross-cultural applications, as odor identification tests rely on an individuals' previous experience with specific odors, which is often influenced by cultural backgrounds such as dietary habits, natural environment, and social customs [8••]. To overcome this limitation, researchers have developed olfactory recognition tests targeting different cultural groups. For example, in the United States, the most commonly used odor recognition test is UPSIT; in Spain, the Barcelona Smell Test-24 odors (BAST-24) is preferred; in Switzerland, the Smell Diskettes is widespread test; and in Japan, the Toyota & Takagi (T & T) Olfactometer has been extensively used [10•, 76]. Besides, Feng et al. developed a smell recognition test specifically for the Chinese population (CSIT) [77]. Although there are some differences in the programs of these tests, the major difference lies in the odor settings, which vary depend on the regions. Currently, through continuous improvements, UPSIT has become one of the most widely used clinical evaluation tools globally, and has been validated in numerous studies in different countries [8••, 78].
Electrophysiology
Electrophysiological testing plays a crucial role in evaluating olfactory function, objectively assessing olfactory function by recording of electrical signals caused by olfactory stimuli. The Electro-olfactogram (EOG) and Olfactory Event-Related potentials (OERP) are commonly used methods that provide objective information about the olfactory conduction pathway and the CNS's processing of olfactory information. By combining the use of EOG and OERP, olfactory function can be comprehensively evaluated, from the primary responses of the OE to the advanced processing of the brain. However, these methods are relatively expensive and require specialized equipment and technical personnel to operate, which will limit their clinical application [60].
Imaging
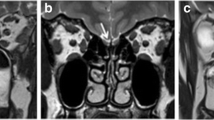
Imaging examinations provide important diagnostic information when evaluating the OD of CRS patients. These methods enable doctors to visualize the structures of the nasal cavity, sinuses, and brain, thereby determine the underlying causes and the severity of OD. Both computed tomography (CT) and magnetic resonance imaging (MRI) attempted to evaluate the olfactory function of CRS patients. CT is a routine clinical examination for CRS, used to evaluate the severity of sinonasal mucosal inflammation and help to determine the severity of CRS [13, 76]. Research has found a correlation between the degree of sinus opacity and the severity of OD [79]. In CRSwNP patients, the correlation between OC opacity and olfactory function is stronger compared to adjacent sinus opacity [80]. MRI provides superior visualization of soft tissues compared to CT [8••]. Especially when investigating intracranial structures and pathology related to OD, MRI is the preferred diagnostic method. It plays a crucial role in visualizing brain structures, particularly those related to the olfactory system, and can provide detailed imaging of OB, olfactory tract, olfactory sulcus, and central olfactory projection areas [8••]. There is evidence to suggest that the size of OB on MRI is associated with olfactory loss in CRS patients [13]. However, MRI typically requires more clinic time, and the cost-effectiveness should also be considered [8••].
Treatment of OD in CRS Patients
Medications
The conventional treatment for CRS with OD includes intranasal and systemic corticosteroids [1••, 8••]. Intranasal corticosteroids and saline irrigation are preferred as initial treatments for CRS patients, while systemic corticosteroids can be a useful addition to intranasal corticosteroids treatment in patients with partially controlled or uncontrolled disease [1••, 22]. Studies have shown that oral corticosteroids were more effective than intranasal corticosteroids in improving patients’ symptoms and olfactory function [81]. However, a meta-analysis revealed that although subjective improvement is observed with oral, topical, or combination steroid therapy, the improvement in objective olfactory outcomes was not significant [82]. Although medication may provide short-term relief, symptoms often recur rapidly once stopped. In addition to corticosteroids, other drugs such as phosphodiesterase inhibitors and intranasal calcium buffers have also been shown to improve OD [83, 84]. However, clinical evidence is still insufficient to support its application in the treatment of OD in CRS patients.
Surgery
When medical treatment fails and persistent symptoms occur, endoscopic sinus surgery (ESS) becomes the next treatment option for patients with CRS [1••, 4]. The surgery aims to remove pathological tissue from the sinuses, improve sinus ventilation and drainage, thereby reducing inflammation and improving OD. Meta-analyses shows that ESS has a positive impact on olfactory function in CRS patients, particularly those with severe nasal polyps [85, 86], and simultaneous nasal septoplasty can improve the likelihood of olfactory recovery [87]. However, the evaluation of olfactory improvement after ESS based on Sniffin's Sticks test has shown controversial results, which may be related to the severity of inflammation and damage to olfactory nerve epithelial cells [86]. Moreover, researchers have found that a considerable proportion of ESS do not alter olfactory outcomes and may even lead to olfactory imparirment. The study report revealed that the rate of postoperative anosmia may reach up to 19% [88]. Therefore, ESS is not recommended when OD is the only symptom, as its efficacy is difficult to predict [82].
Biologics
At present, several biologics have shown significant therapeutic effects in the treatment of CRSwNP, such as dupilumab (anti-IL-4Rα), omalizumab (anti-IgE), mepolizumab (anti-IL-5) and benralizumab (anti-IL-5R) [89, 90]. These drugs reduce inflammation, improve nasal congestion and OD, while dupilumab shows significant improvement in OD [82, 89,90,91,92,93,94]. Mullol et al. showed that even in patients who have previously undergone sinus surgery or systemic corticosteroid therapy, dupilumab can rapidly and sustainably improve olfactory function [93]. However, OD may not necessarily be the first symptom that needs improvement [82]. Thus, olfactory function is not recommended as an early indicator of response to biologics. The early or late response in olfaction is related to the degree of inflammatory changes of the olfactory epithelium, which largely varies among CRS patients [82].
Olfactory Training (OT)
OT is a method that enhances olfactory ability through repeated exposure to different odors. Previous studies have confirmed that OT is beneficial for patients with post-traumatic OD (PTOD) and post-infectious OD (PIOD) [8••, 95], as it can promote the recovery of olfactory function by regulating the mechanism of brain structure. [96]. Hummel et al. found that after OT, patients with post-infectious and idiopathic loss of smell had higher EOG records in OE, indicating that OT not only affects central olfactory processing but also the recovery of OE [97]. However, the effectiveness of OT treatment for CRS related OD is still controversial. Recently, Park et al. found that a 12-week short-term OT program has a positive effect on the recovery of olfactory function in CRS patients after sinus surgery, especially in improving sensory-neural olfactory impairment [98]. However, the method mentioned in this study has not been widely validated. The advantage of OT lies in its simplicity, reasonable cost-effectiveness, and ease of self-management. As a potential treatment option, its clinical efficacy and long-term outcomes for CRS still need further exploration and verification [8••, 99].
Others
Recently, scientists are exploring various innovative therapies. Stem cell therapy is a new approach aimed at restoring olfactory function by transplanting stem cells to repair damaged olfactory tissue [8••, 100]. Additionally, OB stimulation therapy attempts to restore olfaction by directly stimulating the olfactory nerves [8••, 101]. There are also various pharmacological treatments, including vitamin A, platelet-rich plasma, omega-3 fatty acids, N-acetylcysteine, and gene therapy [8••, 92]. These treatment methods have not yet been applied to CRS related OD patients, and strict clinical trials need to be conducted to fully evaluate the safety and effectiveness of these therapies.
Conclusion
An increasing number of studies have revealed the diverse pathological mechanisms of OD in CRS patients. Inflammatory factors play a crucial role in the development of OD in patients with CRS. Usually, the deterioration of OD in CRS is associated with type 2 inflammation. Generally, the evaluation methods for OD mainly include three different categories, including self-reported assessments based on questionnaires or scales, psychophysical tests, and electrophysiological or imaging assessments. A series of treatment methods, including medication, surgical intervention, and OT, have shown certain benefits in improving olfactory function of CRS patients. In addition, emerging biologic therapies provide new treatment options for CRS patients with OD. Nevertheless, further studies need to elucidate the optimal indications and long-term outcomes of these treatment approaches.
Data Availability
No datasets were generated or analysed during the current study.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Fokkens, W.J., et al., European Position Paper on Rhinosinusitis and Nasal Polyps 2020. Rhinology, 2020. 58(Suppl S29): p. 1–464. This position paper of rhinosinusitis and nasal polyps proposed new classifications of CRS.
Lin YT, Yeh TH. Studies on clinical features, mechanisms, and management of olfactory dysfunction secondary to chronic rhinosinusitis. Front Allergy. 2022;3:835151.
Orlandi RR, et al. International consensus statement on allergy and rhinology: rhinosinusitis 2021. Int Forum Allergy Rhinol. 2021;11(3):213–739.
Bhattacharyya N, Gilani S. Prevalence of potential adult chronic rhinosinusitis symptoms in the united states. Otolaryngol Head Neck Surg. 2018;159(3):522–5.
Hirsch AG, et al. Nasal and sinus symptoms and chronic rhinosinusitis in a population-based sample. Allergy. 2017;72(2):274–81.
Yao Y, Zeng M, Liu Z. Revisiting Asian chronic rhinosinusitis in the era of type 2 biologics. Clin Exp Allergy. 2021;52(2):231–43.
Shi JB, et al. Epidemiology of chronic rhinosinusitis: results from a cross-sectional survey in seven Chinese cities. Allergy. 2015;70(5):533–9.
Whitcroft KL, et al. Position paper on olfactory dysfunction: 2023. Rhinology, 2023. This position paper of olfactory dysfunction updated the recommendations on the diagnosis and management of olfactory dysfunction.
Hastan D, et al. Chronic rhinosinusitis in Europe--an underestimated disease. A GA2LEN study. Allergy, 2011. 66(9): p. 1216–23.
Mullol J, et al, The sense of smell in chronic rhinosinusitis. J Allergy Clin Immunol, 2020. 145(3): p. 773–776. A comprehensive review of pathophysiology, diagnosis and management of olfactory dysfunction in chronic rhinosinusitis.
Alobid I, et al. Persistent asthma has an accumulative impact on the loss of smell in patients with nasal polyposis. Rhinology. 2011;49(5):519–24.
Kohli P, et al. The prevalence of olfactory dysfunction in chronic rhinosinusitis. Laryngoscope. 2017;127(2):309–20.
Ahmed OG, Rowan NR. Olfactory Dysfunction and Chronic Rhinosinusitis. Immunol Allergy Clin North Am. 2020;40(2):223–32.
Yoshimura K, et al. Clinical epidemiological study of 553 patients with chronic rhinosinusitis in Japan. Allergol Int. 2011;60(4):491–6.
Soler ZM, et al. Olfactory-specific quality of life outcomes after endoscopic sinus surgery. Int Forum Allergy Rhinol. 2016;6(4):407–13.
Mattos JL, et al. Understanding the relationship between olfactory-specific quality of life, objective olfactory loss, and patient factors in chronic rhinosinusitis. Int Forum Allergy Rhinol. 2017;7(7):734–40.
Mattos JL. Mechanisms and treatment of olfactory dysfunction in chronic rhinosinusitis. Ann Allergy Asthma Immunol. 2020;124(4):307–8.
Song J, et al. Olfactory dysfunction in chronic rhinosinusitis: insights into the underlying mechanisms and treatments. Expert Rev Clin Immunol. 2023;19(8):993–1004.
Schlosser RJ, et al. Burden of illness: A systematic review of depression in chronic rhinosinusitis. Am J Rhinol Allergy. 2016;30(4):250–6.
Litvack JR, Mace JC, Smith TL. Olfactory function and disease severity in chronic rhinosinusitis. Am J Rhinol Allergy. 2009;23(2):139–44.
Schlosser RJ, et al. Factors driving olfactory loss in patients with chronic rhinosinusitis: a case control study. Int Forum Allergy Rhinol. 2020;10(1):7–14.
Bachert C, et al. Adult chronic rhinosinusitis. Nat Rev Dis Primers. 2020;6(1):86.
Qureshi HA, Lane AP. Olfaction Now and in the Future in CRSwNP. Am J Rhinol Allergy. 2023;37(2):168–74.
Macchi A, et al. Sense of smell in chronic rhinosinusitis: A multicentric study on 811 patients. Front Allergy. 2023;4:1083964.
Bryche B, Baly C, Meunier N. Modulation of olfactory signal detection in the olfactory epithelium: focus on the internal and external environment, and the emerging role of the immune system. Cell Tissue Res. 2021;384(3):589–605.
Liang F, Sustentacular Cell Enwrapment of Olfactory Receptor Neuronal Dendrites: An Update. Genes (Basel), 2020. 11(5).
Patel RM, Pinto JM. Olfaction: anatomy, physiology, and disease. Clin Anat. 2014;27(1):54–60.
Wu Q, et al. YAP signaling in horizontal basal cells promotes the regeneration of olfactory epithelium after injury. Stem Cell Reports. 2022;17(3):664–77.
Bilinska K, et al. Expression of the SARS-CoV-2 Entry Proteins, ACE2 and TMPRSS2, in Cells of the Olfactory Epithelium: Identification of Cell Types and Trends with Age. ACS Chem Neurosci. 2020;11(11):1555–62.
Leung JY, et al. Olfactory ensheathing cells are attracted to, and can endocytose, bacteria. Cell Mol Life Sci. 2008;65(17):2732–9.
LaFever BJ, Imamura F. Effects of nasal inflammation on the olfactory bulb. J Neuroinflammation. 2022;19(1):294.
Zhao K, et al. Conductive olfactory losses in chronic rhinosinusitis? A computational fluid dynamics study of 29 patients. Int Forum Allergy Rhinol. 2014;4(4):298–308.
Nishijima H, et al. Influence of the location of nasal polyps on olfactory airflow and olfaction. Int Forum Allergy Rhinol. 2018;8(6):695–706.
Hasegawa-Ishii S, Shimada A, Imamura F. Lipopolysaccharide-initiated persistent rhinitis causes gliosis and synaptic loss in the olfactory bulb. Sci Rep. 2017;7(1):11605.
Hasegawa-Ishii S, Shimada A, Imamura F. Neuroplastic changes in the olfactory bulb associated with nasal inflammation in mice. J Allergy Clin Immunol. 2019;143(3):978-989.e3.
Holbrook EH, Leopold DA, Schwob JE. Abnormalities of axon growth in human olfactory mucosa. Laryngoscope. 2005;115(12):2144–54.
Chen M, Reed RR, Lane AP. Chronic Inflammation Directs an Olfactory Stem Cell Functional Switch from Neuroregeneration to Immune Defense. Cell Stem Cell. 2019;25(4):501-513.e5.
Rouyar A, et al. Type 2/Th2-driven inflammation impairs olfactory sensory neurogenesis in mouse chronic rhinosinusitis model. Allergy. 2019;74(3):549–59.
Hauser LJ, et al. Role of tissue eosinophils in chronic rhinosinusitis-associated olfactory loss. Int Forum Allergy Rhinol. 2017;7(10):957–62.
Shirai T, et al. Functions of human olfactory mucus and age-dependent changes. Sci Rep. 2023;13(1):971.
Yee KK, et al. Neuropathology of the olfactory mucosa in chronic rhinosinusitis. Am J Rhinol Allergy. 2010;24(2):110–20.
Yee KK, et al. Analysis of the olfactory mucosa in chronic rhinosinusitis. Ann N Y Acad Sci. 2009;1170:590–5.
Tu Y, et al. Mucus composition abnormalities in sinonasal mucosa of chronic rhinosinusitis with and without nasal polyps. Inflammation. 2021;44(5):1937–48.
Dalton P. Olfaction and anosmia in rhinosinusitis. Curr Allergy Asthma Rep. 2004;4(3):230–6.
Lal D, et al. Mapping and comparing bacterial microbiota in the sinonasal cavity of healthy, allergic rhinitis, and chronic rhinosinusitis subjects. Int Forum Allergy Rhinol. 2017;7(6):561–9.
Wellford SA, and Moseman EA, Olfactory immunology: the missing piece in airway and CNS defence. Nat Rev Immunol, 2023.
Shaghayegh G, et al. Chronic Rhinosinusitis, S. aureus Biofilm and Secreted Products, Inflammatory Responses, and Disease Severity. Biomedicines, 2022. 10(6).
Schlosser RJ, et al. Mucous Cytokine Levels in Chronic Rhinosinusitis-Associated Olfactory Loss. JAMA Otolaryngol Head Neck Surg. 2016;142(8):731–7.
Soler ZM, et al. Correlation of mucus inflammatory proteins and olfaction in chronic rhinosinusitis. Int Forum Allergy Rhinol. 2020;10(3):343–55.
Turner JH, et al. Tumor necrosis factor alpha inhibits olfactory regeneration in a transgenic model of chronic rhinosinusitis-associated olfactory loss. Am J Rhinol Allergy. 2010;24(5):336–40.
Han X, et al. Type 1/type 2 inflammatory cytokines correlate with olfactory function in patients with chronic rhinosinusitis. Am J Otolaryngol. 2020;41(5):102587.
Wu J, et al. Olfactory and middle meatal cytokine levels correlate with olfactory function in chronic rhinosinusitis. Laryngoscope. 2018;128(9):E304-e310.
Chapurin N, et al. All chronic rhinosinusitis endotype clusters demonstrate improvement in patient-reported and clinical outcome measures after endoscopic sinus surgery. Int Forum Allergy Rhinol. 2024;14(4):765–74.
Vallieres L, et al. Reduced hippocampal neurogenesis in adult transgenic mice with chronic astrocytic production of interleukin-6. J Neurosci. 2002;22(2):486–92.
Kanekar S, Gandham M, Lucero MT. PACAP protects against TNFα-induced cell death in olfactory epithelium and olfactory placodal cell lines. Mol Cell Neurosci. 2010;45(4):345–54.
Soler ZM, et al. Endotyping chronic rhinosinusitis based on olfactory cleft mucus biomarkers. J Allergy Clin Immunol. 2021;147(5):1732-1741.e1.
Morse JC, et al. Patterns of olfactory dysfunction in chronic rhinosinusitis identified by hierarchical cluster analysis and machine learning algorithms. Int Forum Allergy Rhinol. 2019;9(3):255–64.
Mori E, et al. Risk factors for olfactory dysfunction in chronic rhinosinusitis. Auris Nasus Larynx. 2013;40(5):465–9.
Kuhar HN, et al. Inflammatory infiltrate and mucosal remodeling in chronic rhinosinusitis with and without polyps: structured histopathologic analysis. Int Forum Allergy Rhinol. 2017;7(7):679–89.
Rombaux P, et al. Olfaction in Chronic Rhinosinusitis. Curr Allergy Asthma Rep. 2016;16(5):41.
Bandeira-Melo C, and Weller PF, Mechanisms of eosinophil cytokine release. Mem Inst Oswaldo Cruz, 2005. 100 Suppl 1(Suppl 1): p. 73–81.
Miyata J, et al. Dysregulated fatty acid metabolism in nasal polyp-derived eosinophils from patients with chronic rhinosinusitis. Allergy. 2019;74(6):1113–24.
Lavin J, et al. Superior turbinate eosinophilia correlates with olfactory deficit in chronic rhinosinusitis patients. Laryngoscope. 2017;127(10):2210–8.
Shin SH, et al, Immunopathologic Role of Eosinophils in Eosinophilic Chronic Rhinosinusitis. Int J Mol Sci, 2022. 23(21).
Kanemitsu Y, et al. A novel pathophysiologic link between upper and lower airways in patients with chronic rhinosinusitis: Association of sputum periostin levels with upper airway inflammation and olfactory function. World Allergy Organ J. 2020;13(1):100094.
Schleimer RP. Immunopathogenesis of Chronic Rhinosinusitis and Nasal Polyposis. Annu Rev Pathol. 2017;12:331–57.
Czerny MS, et al. Histopathological and clinical analysis of chronic rhinosinusitis by subtype. Int Forum Allergy Rhinol. 2014;4(6):463–9.
Gudziol V, et al. Increasing olfactory bulb volume due to treatment of chronic rhinosinusitis–a longitudinal study. Brain. 2009;132(Pt 11):3096–101.
Han P, et al. Olfactory brain gray matter volume reduction in patients with chronic rhinosinusitis. Int Forum Allergy Rhinol. 2017;7(6):551–6.
Han P, et al, A systematic review of olfactory related questionnaires and scales. Rhinology journal, 2020. 0(0): p. 0–0.
De Sousa Machado A, et al. Visual Analog Scale and Olfactory Objective Tests in Hyposmia Patients: Is There a Link? Cureus, 2023.
Soler ZM, et al. The Olfactory Cleft Endoscopy Scale correlates with olfactory metrics in patients with chronic rhinosinusitis. Int Forum Allergy Rhinol. 2015;6(3):293–8.
Schlosser RJ, et al. The Olfactory Cleft Endoscopy Scale: a multi-institutional validation study in chronic rhinosinusitis. Rhinology. 2021;59(2):181–90.
Hummel T, et al. ‘Sniffin’ Sticks’: Olfactory Performance Assessed by the Combined Testing of Odour Identification, Odor Discrimination and Olfactory Threshold. Chem Senses. 1997;22(1):39–52.
Doty RL, et al. University of pennsylvania smell identification test: A rapid quantitative olfactory function test for the clinic. Laryngoscope. 2009;94(2):176–8.
Whitcroft KL, and Hummel T, Clinical Diagnosis and Current Management Strategies for Olfactory Dysfunction. JAMA Otolaryngology–Head & Neck Surgery, 2019. 145(9).
Feng G, et al. Development of the Chinese Smell Identification Test. Chem Senses. 2019;44(3):189–95.
Huang T, Wei Y, Wu D. Effects of olfactory training on posttraumatic olfactory dysfunction: a systematic review and meta-analysis. Int Forum Allergy Rhinol. 2021;11(7):1102–12.
Lee SE, et al. The relationship of sinus opacification, olfaction and dupilumab efficacy in patients with CRSwNP. Rhinol J, 2023. 0(0): p. 0–0.
Loftus C, et al. Olfactory cleft and sinus opacification differentially impact olfaction in chronic rhinosinusitis. Laryngoscope. 2019;130(10):2311–8.
Yang Q, Li M. Comparison of Therapeutic Effects and Olfactory Function of Oral Glucocorticoid and Intranasal Glucocorticoid on Chronic Rhinosinusitis Patients with Nasal Polyps. J Coll Physicians Surg Pak. 2021;30(6):699–702.
Tsetsos N, Markou K, Konstantinidis I. Effect of monoclonal antibodies on olfactory dysfunction caused by chronic rhinosinusitis with nasal polyps: a systematic review and meta-analysis. Int Forum of Allergy Rhinol. 2020;10(7):893–900.
Hosein W, and Henkin RI, Therapeutic diminution of Interleukin-10 with intranasal theophylline administration in hyposmic patients. American Journal of Otolaryngology, 2022. 43(2).
Whitcroft KL, et al. Intranasal sodium citrate solution improves olfaction in post-viral hyposmia. Rhinology journal. 2016;54(4):368–74.
Haxel BR, et al. Nasal polyp load determines the recovery of olfaction after surgery for chronic rhinosinusitis. Rhinol J, 2022. 0(0): p. 0–0.
Zhao R, Chen K, Tang Y. Olfactory changes after endoscopic sinus surgery for chronic rhinosinusitis: A meta-analysis. Clin Otolaryngol. 2021;46(1):41–51.
Mattos JL, et al. Olfactory Function After Surgical Treatment of CRS: A Comparison of CRS Patients to Healthy Controls. Am J Rhinol Allergy. 2021;35(3):391–8.
Musleh A, et al. Olfactory Change Pattern After Endoscopic Sinus Surgery in Chronic Rhinosinusitis Patients. Cureus. 2022;14(4):e24597.
Barroso B, et al. Smell improvement in chronic rhinosinusitis with nasal polyps with monoclonal antibodies: a systematic review. J Investig Allergol Clin Immunol, 2023: p. 0.
Cai S, et al. Comparison of Different Biologics for Treating Chronic Rhinosinusitis With Nasal Polyps: A Network Analysis. J Allergy Clin Immunol: In Practice, 2022. 10(7): p. 1876–1886.e7.
Kariyawasam HH, et al. Biologic treatment for severe chronic rhinosinusitis with nasal polyps: a systematic review and meta-analysis. Rhinology. 2023;61(2):98–107.
Jafari A, Holbrook EH. Therapies for Olfactory Dysfunction - an Update. Curr Allergy Asthma Rep. 2022;22(3):21–8.
Mullol J, et al. Olfactory Outcomes With Dupilumab in Chronic Rhinosinusitis With Nasal Polyps. J Allergy Clin Immunol Pract, 2022. 10(4): p. 1086–1095 e5.
Hellings PW, Verhoeven E, and Fokkens WJ, State-of-the-art overview on biological treatment for CRSwNP. Rhinol J, 2021. 0(0): p. 0–0.
Pekala K, Chandra RK, Turner JH. Efficacy of olfactory training in patients with olfactory loss: a systematic review and meta-analysis. Int Forum of Allergy Rhinol. 2015;6(3):299–307.
Al Aïn S, et al. Smell training improves olfactory function and alters brain structure. Neuroimage. 2019;189:45–54.
Hummel T, et al, Olfactory training changes electrophysiological responses at the level of the olfactory epithelium. Rhinol J, 2018. 0(0).
Park JY, et al. Olfactory training assists in olfactory recovery after sinonasal surgery. Laryngoscope Invest Otolaryngol. 2022;7(6):1733–9.
Pieniak M, et al. Olfactory training - Thirteen years of research reviewed. Neurosci Biobehav Rev. 2022;141:104853.
Kurtenbach S, et al. Cell-Based Therapy Restores Olfactory Function in an Inducible Model of Hyposmia. Stem Cell Reports. 2019;12(6):1354–65.
Holbrook EH, et al. Induction of smell through transethmoid electrical stimulation of the olfactory bulb. Int Forum of Allergy Rhinol. 2018;9(2):158–64.
Author information
Authors and Affiliations
Contributions
Ming Zeng (the corresponding author) produced and designed the manuscript and did the final revision. All authors contributed to review and analyze the relevant literature and drafting the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Conflict of Interest
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, D., Chen, Q., Zhang, X. et al. Olfactory Dysfunction in Chronic Rhinosinusitis. Curr Treat Options Allergy 11, 136–149 (2024). https://doi.org/10.1007/s40521-024-00363-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40521-024-00363-y