Abstract
Context
The effect of platelet-rich plasma (PRP) on ovarian reserve markers in poor ovarian response (POR) is challenging.
Aim
This systematic review and meta-analysis was, therefore, designed to evaluate the effectiveness of intra-ovarian injection of autologous PRP on improving ovarian reserve markers and assisted reproductive technology (ART) outcomes in infertile women with POR.
Methods
A systematic search was conducted for the efficacy of intra-ovarian injection of autologous PRP on the improvement of ovarian reserve markers and ART outcomes in infertile women with POR. The methodological quality of the included studies was checked and eligible studies were included in the meta-analysis to find pooled results. Keywords were primary ovarian insufficiency, premature menopause, poor responder, poor ovarian response, diminished/decreased ovarian reserve, platelet-rich plasma, and intra-ovarian or a combination of them. The effect of PRP on fertility indices was evaluated using the standardized mean difference (SMD). The analysis was performed through STATA version 13.
Key results
13 studies containing 1289 patients were included. Mean age, body mass index (BMI) and duration of infertility was 37.63 ± 2.66 years, 24 ± 1.23 kg/m2 and 4.79 ± 1.64 years, respectively. Most of the studies measured the outcomes 2–3/3 months after intra-ovarian injection of autologous PRP. The antral follicular count (AFC) after treatment by PRP is higher with an SMD of 0.95 compared to before treatment. The day 3 follicle-stimulating hormone (FSH) after treatment by PRP is lower with an SMD of − 0.25 compared to before treatment. The day 3 estradiol (E2) after treatment by PRP is higher with an SMD of 0.17 compared to before treatment. The anti-Mullerian hormone (AMH) after treatment by PRP is higher with an SMD of 0.44 compared to before treatment. The total oocytes number after treatment by PRP is higher with an SMD of 0.73 compared to before treatment. The number of MII oocytes after treatment by PRP is higher with an SMD of 0.63 compared to before treatment. The number of cleavage-stage embryos after treatment by PRP is higher with an SMD of 1.31 compared to before treatment. The number of day 5 embryo after treatment by PRP is higher with an SMD of 1.28 compared to before treatment. Pooled estimation of a meta-analysis of prevalence studies reported a prevalence of 22% for clinical pregnancy, 5% for spontaneous pregnancy and 21% for ongoing pregnancy following PRP therapy.
Conclusion
Intra-ovarian injection of PRP improved ovarian reserve markers with increasing AFC, serum level of AMH and day 3 E2 and decreasing serum level of day 3 FSH. In addition, this treatment improved ART outcomes through the increasing of number total oocytes, number of MII oocytes, number of cleavage-stage embryos and number of day 5 embryos in POR women.
Implications
Although treatment of POR women remains challenging, the use of intra-ovarian injection of autologous PRP in POR patients prior to IVF/ICSI cycles is a sign of new hope for increasing the success of IVF/ICSI. However, further well-organized, randomized controlled trials should be conducted to substantiate this result and recommend intra-ovarian injection of PRP as part of routine treatment in women with POR.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ovarian responsiveness to controlled ovarian stimulation (COS) is a key determinant of clinical outcomes in assisted reproductive technology (ART) cycles [43]. Failure to respond appropriately to standard regimens and recruit sufficient follicles is referred to as poor ovarian response (POR). POR leads to inadequate mature oocyte retrieval, high rate of cycle cancellation, and low pregnancy rates in ART cycles [43]. The estimated rate of POR among patients facing ART is 9–24% [7]. Due to the variability of risk factors, there is no commonly established definition for POR. According to the BOLOGNA criteria published in 2011 by the European Society of Human Reproduction and Embryology (ESHRE), POR is identified by at least two of the three following criteria: (1) advanced maternal age (≥ 40 years) or any other risk factor for POR, (2) a previously characterized POR cycle (≤ 3 oocytes with a conventional stimulation protocol), (3) an abnormal ovarian reserve test (antral follicle count (AFC) < 5–7 follicles or anti-Mullerian hormone (AMH) < 0.5–1.1 ng/mL) [14]. However, Bologna criteria were critiqued in view of the lack of homogeneity in the population described and for not addressing important factors such as the effect of age on oocyte quality [11]. Newly, the terminology used to describe these patients evolved from POR to “low prognosis” with the introduction of the POSEIDON criteria, a more nuanced classification system that replicates the heterogeneity in the population [11].
Despite technological and scientific advances in ART, the treatment of POR cases is still a questionable issue. Several methods have been developed to increase ovarian response in POR women. However, there is currently no effective treatment [3]. Platelet-rich plasma (PRP) is collected of a high concentration of autologous platelets extracted from the peripheral blood, which is applied to promote tissue repair and restoration, as well as treat various pathologies [12]. Platelets are anucleated blood cells with several secretory granules accountable for the release of cytokines, chemokines and several growth factors (GFs) [20]. The application of PRP and its effectiveness were established in the field of regenerative medicine, with extremely confident results [2, 17, 21]. In reproductive medicine, thin endometrium, POR and premature ovarian insufficiency (POI) are the main fields of research on PRP by intra-uterine infusion or intra-ovarian injection [25, 36]. Recently, intra-ovarian injection of PRP was conducted in POR women based on the theory that platelet-derived factors may promote ovarian angiogenesis and stimulate follicular development by regaining the ovarian microenvironment [22]. Emerging evidence shows the efficiency of intra-ovarian instillation of autologous PRP in POR cases. However, this treatment for POR women is still controversial [12, 22, 37]. Based on our knowledge, there is a lack of conclusive results and a comprehensive review regarding the effect of PRP on ART outcomes in POR cases. Therefore, this systematic review and meta-analysis were designed to evaluate the efficacy of intra-ovarian injection of autologous PRP on the improvement of ovarian reserve markers and ART outcomes in infertile women with POR.
Methods
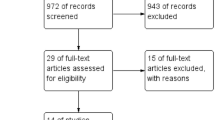
This systematic review and meta-analysis were conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses [27]. International databases such as PubMed, Web of Science (WOS) and Scopus were taken into account to search for English-language articles up to April 1, 2023. Inclusion criteria were studies that discussed the effects of intra-ovarian PRP injection in POR women. Exclusion criteria were studies discussing the effect of intra-ovarian PRP injection in women with premature ovarian insufficiency (POI), premature ovarian failure (POF), and diminished ovarian reserve (DOR), early menopause women, case reports, animal studies, and studies with incorrect values of the selected index (Fig. 1). Keywords were primary ovarian insufficiency, premature menopause, poor responder, poor ovarian response, diminished ovarian reserve, decreased ovarian reserve, poor ovarian reserve, early menopause, low ovarian reserve, cycle cancelation, platelet-rich plasma, OR cord blood platelet-rich plasma, CB-PRP, PRP, plasma rich in growth factor, plasma growth factor, PRGF, Autologous, allogeneic, intra-ovarian and intra-ovarian or a combination of them were used in the title/abstracts. After the collection of articles of interest (N = 421), references were imported to Endnote Software and duplicate titles were removed (N = 128). Then, after browsing titles, studies with irrelevant purposes were taken off and the remaining studies were assessed by two independent investigators. The selected studies were all performed procedures on humans and were published in English. Search strategy can be found in Supplementary File.
Data extraction
We had a checklist consisting of author name, year, sample size, type of study, country, age, type of PRP, follow-up, and effectiveness based on certain factors in Excel. The differences observed were corrected by a third investigator, independent of the other two. The quality of selected studies was checked by a quality assessment tool for before–after (pre–post) studies with no control group (Available at https://www.nhlbi.nih.gov/health-pro/guidelines/in-develop/cardiovascular-risk-reduction/tools/before-after.2014), in which approximately all were fair regarding “quality rating”.
Statistical analysis
The effect of PRP on fertility indices was assessed using standardized mean difference (SMD), also known as Cohen’s D. The SMD was calculated using means difference and standard deviations (SD) of before and after groups using SMD formula (SMD = mean difference in the intervention group – the mean difference in placebo group/pooled SD), as well as Pooled SD equals √ [(SD in intervention group) 2 + (SD in placebo group) 2/2]. In this study, we used Q‐test and I2 to assess heterogeneity among the studies. A random‐effects model was used for the continuous outcomes. In addition, random or fixed effects meta‐analysis was applied for estimating the main index that was pooled SMD at a 95% confidence interval. The forest plot was used to present the pooled SMD. Publication bias was assessed using Begg’s tests. The analysis was performed through STATA version 13.
Results
13 studies with 1289 patients were included in this meta-analysis (Fig. 1). The details of the included studies are presented in Table 1. Mean age, body mass index (BMI) and duration of infertility were 37.63 ± 2.66 years, 24 ± 1.23 kg/m2 and 4.79 ± 1.64 years, respectively (Table 2). Most of the studies measured the outcomes 2–3/3 months after intra-ovarian injection of autologous PRP (Fig. 2).
Figure 3 shows that antral follicular count (AFC) after treatment by PRP is higher with an SMD of 0.95 compared to before treatment.
Figure 4 shows that day 3 follicle-stimulating hormone (FSH) after treatment by PRP is lower with an SMD of − 0.25 compared to before treatment.
Figure 5 shows that day 3 estradiol (E2) after treatment by PRP is higher with an SMD of 0.17 compared to before treatment (N = 1134).
Figure 6 shows that anti-Mullerian hormone (AMH) after treatment by PRP is higher with an SMD of 0.44 compared to before treatment (N = 209).
Figure 7 shows that total oocyte number after treatment by PRP is higher with an SMD of 0.73 compared to before treatment (N = 1156).
Figure 8 shows that number of MII oocyte after treatment by PRP is higher with an SMD of 0.63 compared to before treatment (N = 1144).
Figure 9 shows that number of cleavage-stage embryo after treatment by PRP is higher with an SMD of 1.31 compared to before treatment (N = 818).
Figure 10 shows that number of day 5 embryo after treatment by PRP is higher with an SMD of 1.28 compared to before treatment (N = 808).
Pooled estimation of a meta-analysis of prevalence studies reported a prevalence of 22%, i.e., 22 out of every 100 women experience clinical pregnancy following PRP therapy (Fig. 11).
Pooled estimation of a meta-analysis of prevalence studies reported a prevalence of 5%, i.e., 5 out of every 100 women experience spontaneous pregnancy following PRP therapy (Fig. 12).
Pooled estimation of a meta-analysis of prevalence studies reported a prevalence of 21%, i.e., 21 out of every 100 women experience ongoing pregnancy following PRP therapy (Fig. 13).
Publication bias
The results of Begg’s test analysis also showed that publication bias did not affect the creation of negative results.
Discussion
In this systematic review and meta-analysis, 13 studies were included to evaluate the efficacy of the intra-ovarian injection of PRP in 1289 patients with poor ovarian response (POR). The forest plot analysis showed that intra-ovarian injection of PRP is effective in improving ovarian reserve markers with increasing AFC, serum level of AMH and day 3 E2 and decreasing serum level of day 3 FSH. In addition, forest plot analysis demonstrated that intra-ovarian injection of PRP is effective in improving ART outcomes with an increasing of number total oocytes, number of MII oocytes, number of cleavage-stage embryos and number of day 5 embryos. The spontaneous pregnancy rate, clinical pregnancy rate and ongoing pregnancy rate following intra-ovarian injection of PRP were 5% (5/100), 22% (22/100), and 21% (21/100), respectively. The highest SMD was obtained for the number of cleavage-stage embryos (1.31) and the lowest SMD was obtained for day 3 E2 (0.17).
In the present meta-analysis, studies discussing the effect of intra-ovarian infusion of PRP in women with diminished ovarian reserve (DOR), premature ovarian insufficiency (POI), early menopause and premature ovarian failure (POF) were excluded. It is important to note that POI and POF are the same condition, with POF being the old nomenclature. In addition, POR cases selected based on Bologna or Poseidon criteria were only included in this study. POR differs as a clinical diagnosis from DOR, POF and POI. POR refers to when an individual has a poor response to ovarian stimulation during ART cycles. However, DOR is a normal physiologic condition identified by abnormal but not postmenopausal ovarian reserve testing and regular menses. In addition, women identified with POI and POF have postmenopausal FSH levels without any menses for several months [32].
Several markers of ovarian reserve have been studied in the last few decades, but an ideal marker still has not been established. Serum levels of FSH and E2 on days 3–5 of the menstrual cycle have been commonly used in reproductive medicine to measure ovarian reserve [42]. However, these are indirect markers of ovarian reserve and their blood concentrations increase only when the ovarian reserve is harshly declined [42]. Emerging evidence indicated that AMH and AFC provide direct and precise measurements of ovarian reserve [15]. In POR women compared to fertile women, serum levels of AMH, E2 and AFC values strongly declined, whereas FSH levels moderately increased [34]. The present study showed that intra-ovarian injection of PRP increased the serum level of AMH and AFC, and decreased the serum level of day 3 FSH in POR women. Cakiroglu et al. in a clinical trial study in which 510 POR patients were included showed that PRP treatment led to an increase of AFC, serum AMH, number of mature oocytes, cleavage, and blastocyst stage embryos as well as a decrease in serum FSH [5]. They showed that the level of serum AMH and AFC as main markers of ovarian reserve increased with a statistically significant difference of p-value = < 0.01. In addition, Navali et al. showed that intra-ovarian injection of PRP in 35 POR women significantly increased the number of oocytes, the number of cleavage-stage embryos and E2 level after a 2-month follow-up [24]. However, Farimani et al. showed that although total oocyte number and MII oocyte significantly increased after intra-ovarian infusion, PRP, FSH, LH, AMH and estradiol did not significantly change [13]. Based on current evidence, it seems that intra-ovarian infusion of PRP could effectively promote folliculogenesis, restore ovarian functionality and subsequently ART outcomes. However, further well-organized, randomized controlled trials are required to validate this result.
The exact mechanism action of PRP in promoting folliculogenesis and ovarian function is not yet well understood. It is emphasized that PRP may play a vital role in ovarian niche restoration, mostly through the promotion of the physiological processes of angiogenesis, growth, apoptosis, inflammation control, proliferation and cell migration [23, 29]. It is supposed that platelet-derived factors may promote ovarian angiogenesis and stimulate follicular development by recovering the ovarian microenvironment [10]. It was shown that PRP augments ischemic neovascularization, presumably due to the stimulation of angiogenesis, vasculogenesis, and arteriogenesis [12]. The granules in platelets have important growth factors such as epidermal growth factor (EGF), hepatocyte growth factor (HGF), transforming growth factor-β (TGF-β), vascular endothelial growth factor (VEGF) and insulin-like growth factors 1 and 2 (IGF-1 and IGF-2) [20]. These factors have a vital role in healing and tissue regenerative, which is supposed to restore the ovarian hormonal profile and folliculogenesis after intra-ovarian injection [28]. Latest clinical studies have shown that ovarian fragmentation can activate follicles. The mechanism is that the mechanical signal of cutting triggers actin polymerization. This process leads to the disruption of the Hippo pathway and subsequently induces the upregulation of downstream and apoptosis inhibitors, thereby promoting follicle development [44]. Therefore, the Hippo pathway plays a crucial role in both the activation and development of follicles. Together, procedures of mechanical stimulation and/or pharmaceutical administration in order to disrupt the Hippo pathway activity in order to control follicle growth have potential for treating infertility. It would be worthy to evaluate the effect of intra-ovarian injection of PRP on Hippo pathway as part of possible mechanism of PRP action in ovary. Overall, although the effective mechanisms of PRP are not clear, it has been suggested that these findings may be due to the effect of platelet-derived growth factors which may improve the ovarian microenvironment, enhance ovarian vascular activation and stabilization or even result in de novo oocyte development from precursor stem cells [41].
POR represents a heterogeneous population. The young subpopulation has a better clinical prognosis in terms of clinical pregnancy rate [35]. Epidemiological studies showed that the clinical pregnancy rate in POR women undergoing ART was almost 18% [16]. In most studies, pregnancy characteristics of intra-ovarian PRP injection after ICSI such as implantation rate, clinical pregnancy rate, miscarriage rate, and live birth rate have not been assessed. Farimani et al. have examined pregnancy rates in women with POR diagnosed according to the POSEIDON criteria and have reported a 14.6% pregnancy rate among these women [13]. In the present study, the clinical pregnancy rate was 22% following intra-ovarian infusion of PRP. In line with these results, Cakiroglu et al. have reported a 20.5% pregnancy rate in POR patients who received an intra-ovarian infusion of PRP [5]. It is important to note that these individuals had no history of clinical pregnancy before using the intra-ovarian injection of PRP.
Spontaneous pregnancy is unusual in women with a history of POR. However, POR women are not menopausal, so they still have chance for spontaneous pregnancy. The chance of spontaneous conception is higher in younger women and is intensely associated with the duration of infertility [4, 39]. The spontaneous pregnancy rate is reported 17% among women who formerly achieved pregnancy through ART and 2.2% to 14.2% among women with the extreme form of POR [4, 39]. The present study reported 5% spontaneous pregnancy in POR women treated with PRP. Aflatoonian et al. showed that although a significant difference was not detected in the hormonal profile, 8/17 (47%) of POR patients reached spontaneous pregnancy after ovarian PRP injection [1]. In addition, Cakiroglu et al. demonstrated that after intra-ovarian infusion of PRP, 22 women from 510 (4.3%) conceived spontaneously [5]. Furthermore, Navali et al. indicated that 3 of 30 POR women (10%) within 4 months after PRP treatment reached spontaneous pregnancy. The mechanisms by which ovarian activation approaches induce spontaneous pregnancy are worthy of further study.
In several studies, a range of one to several intra-ovarian PRP injections with different time intervals have been conducted. Currently, no agreement exists upon a standard number of injections or frequency of intra-ovarian PRP injections. Three months of treatment with PRP was chosen based on the idea that follicle development takes 90 to 120 days from the time of primordial follicle recruitment to the final stages of antral development [38]. Different preparation methods and concentrations of PRP could result in different products, which can in turn have different effects. Herein in the included studies, PRP preparation was conducted with different commercial kits in which autologous blood from the peripheral vein was collected and centrifuged two times. After first centrifuge, the plasma layer and buffy coat were transferred to another tube and centrifuged to achieve 2–3 mL PRP of 3 to 5 times higher than basal blood samples. Finally, 2–4 mL PRP injection was performed under sedation anesthesia into each ovary transvaginally under ultrasound guidance. The main difference between commercial kits is likely the final concentration of injected PRP which is not mentioned clearly in included studies. Therefore, well-organized, randomized controlled trials are required to determine the effective concentration of PRP for intra-ovarian injection.
Most commercial PRP kits do not activate PRP. Using PRP without activation may result in a more normal physiologic activation [8]. However, likely, a proportion of platelet-derived factors are not released after injection, and this leads to decreased treatment efficiency [33]. To avoid this impediment, biological activators have been used to stimulate the platelets to release their granular content [12]. Indeed, activated PRP is the final product of PRP, without leukocytes and only covers a specific number of GFs and cytokines. Three studies out of 13 included studies used activated PRP. Parvanov et al. indicated that intra-ovarian injection of activated PRP led to insignificantly lower FSH levels, significantly higher AFC, AMH and number of retrieved oocytes, as well as insignificantly higher fertilization rate [31]. Based on the obtained SMD, activated PRP showed almost the same efficacy as PRP without activation. Collectively, since there is no unified technique to extract PRP from the peripheral blood, this study cannot provide a reasonable explanation for the relationship between the type of PRP and PRP dosage with the IVF/ICSI outcomes in POR women.
In all 13 studies, there were no significant adverse events in participants during the follow-up after the PRP injection. Although the safety of PRP in several clinical studies has been shown, there is a lack of evidence regarding the safety of intra-ovarian injection of PRP [9]. Since PRP is prepared from autologous peripheral blood, undoubtedly there are negligible risks for disease transmission, cancers, immunogenic reactions, and pregnancy complications. In addition, autologous PRP necessitates no culture and is characterized by ease of preparation and high safety [6, 25]. It is important to note that although intra-ovarian PRP injection is a minor surgical procedure, potential complications such as major bleeding, infection or abscess, and injury to surrounding structures estimated to occur. Several weaknesses should be considered in the interpretation of the acquired results in this systematic review and meta-analysis. First is the lack of internal control for intra-ovarian injection of PRP. Internal control is required to exclude the effect of mechanical stimulation by needle puncturing on ovarian reserve markers and ART outcomes. Recently, Olesen et al. showed that needle puncturing of human ovarian cortex affects angiogenic genes and improved follicle morphology [26]. Second, the possible dose–response effect of PRP was not assessed in any of the included studies Platelet concentration and the content of growth factors must be identified to recognize molecular mechanisms behind PRP. Third, there is methodological heterogeneity across the included studies. Different commercial kits and manual methods were used to isolate PRP. Therefore, future well-organized, randomized controlled trials are required to validate standard methods for PRP isolation and effective concentration of PRP for intra-ovarian injection in women with POR.
Conclusion
This systematic review and meta-analysis verified that the intra-ovarian injection of PRP improved ovarian reserve markers with increasing AFC, serum level of and day 3 E2 and decreasing serum level of day 3 FSH. In addition, this treatment improved ART outcomes through the increasing of number total oocytes, number of MII oocytes, number of cleavage-stage embryos and number of day 5 embryos in POR women. Even though the treatment of POR women remains a challenge, the usage of intra-ovarian injection of autologous PRP in POR patients before the IVF/ICSI cycles brings a sign of new hope in increasing the success of IVF/ICSI. However, it is important to note that there was no difference in pregnancy outcomes including clinical, ongoing, and spontaneous pregnancy before and after PRP injection, despite some improvement in the ovarian reserve and ART parameters. In addition, we should clearly state that intra-ovarian injection of PRP is an experimental method and further well-organized, randomized controlled trials are required to corroborate its therapeutic potential and long-term safety as part of routine treatment in women with POR.
Data availability
The data of this study can be available on formal and reasonable request to corresponding author by email for academic works by faculty members.
References
Aflatoonian A, Lotfi M, Saeed L, Tabibnejad N (2021) Effects of intraovarian injection of autologous platelet-rich plasma on ovarian rejuvenation in poor responders and women with primary ovarian insufficiency. Reprod Sci 28(7):2050–2059. https://doi.org/10.1007/s43032-021-00483-9
Bastami F, Vares P, Khojasteh A (2017) Healing effects of platelet-rich plasma on peripheral nerve injuries. J Craniofac Surg 28(1):e49–e57. https://doi.org/10.1097/scs.0000000000003198
Berkkanoglu M, Ozgur K (2010) What is the optimum maximal gonadotropin dosage used in microdose flare-up cycles in poor responders? Fertil Steril 94(2):662–665. https://doi.org/10.1016/j.fertnstert.2009.03.027
Cakiroglu Y, Saltik A, Yuceturk A, Karaosmanoglu O, Kopuk SY, Scott RT, Seli E et al (2020) Effects of intraovarian injection of autologous platelet rich plasma on ovarian reserve and IVF outcome parameters in women with primary ovarian insufficiency. Aging (Albany, NY) 12(11):10211–10222. https://doi.org/10.18632/aging.103403
Cakiroglu Y, Yuceturk A, Karaosmanoglu O, Kopuk SY, Korun ZEU, Herlihy N, Seli E et al (2022) Ovarian reserve parameters and IVF outcomes in 510 women with poor ovarian response (POR) treated with intraovarian injection of autologous platelet rich plasma (PRP). Aging (Albany, NY) 14(6):2513–2523. https://doi.org/10.18632/aging.203972
Chang Y, Li J, Chen Y, Wei L, Yang X, Shi Y, Liang X (2015) Autologous platelet-rich plasma promotes endometrial growth and improves pregnancy outcome during in vitro fertilization. Int J Clin Exp Med 8(1):1286–1290
Conforti A, Esteves SC, Cimadomo D, Vaiarelli A, Di Rella F, Ubaldi FM, Alviggi C et al (2019) Management of women with an unexpected low ovarian response to gonadotropin. Front Endocrinol (Lausanne) 10:387. https://doi.org/10.3389/fendo.2019.00387
Dhurat R, Sukesh M (2014) Principles and methods of preparation of platelet-rich plasma: a review and author’s perspective. J Cutan Aesthet Surg 7(4):189–197. https://doi.org/10.4103/0974-2077.150734
Doghaim NN, El-Tatawy RA, Neinaa YME (2019) Assessment of the efficacy and safety of platelet poor plasma gel as autologous dermal filler for facial rejuvenation. J Cosmet Dermatol 18(5):1271–1279. https://doi.org/10.1111/jocd.12876
Elnashar AM (2021) Intraovarian platelet-rich plasma: current status. Middle East Fertil Soc J 26(1):30. https://doi.org/10.1186/s43043-021-00077-0
Esteves SC, Conforti A, Sunkara SK, Carbone L, Picarelli S, Vaiarelli A, Alviggi C et al (2021) Improving reporting of clinical studies using the POSEIDON criteria: POSORT guidelines. Front Endocrinol (Lausanne) 12:587051. https://doi.org/10.3389/fendo.2021.587051
Everts P, Onishi K, Jayaram P, Lana JF, Mautner K (2020) Platelet-rich plasma: new performance understandings and therapeutic considerations in 2020. Int J Mol Sci 21(20):7794. https://doi.org/10.3390/ijms21207794
Farimani M, Nazari A, Mohammadi S, Anvari Aliabad R (2021) Evaluation of intra-ovarian platelet-rich plasma administration on oocytes-dependent variables in patients with poor ovarian response: a retrospective study according to the POSEIDON criteria. Reprod Biol Endocrinol 19(1):137. https://doi.org/10.1186/s12958-021-00826-w
Ferraretti AP, Gianaroli L (2014) The Bologna criteria for the definition of poor ovarian responders: is there a need for revision? Hum Reprod 29(9):1842–1845. https://doi.org/10.1093/humrep/deu139
Grisendi V, Mastellari E, La Marca A (2019) Ovarian reserve markers to identify poor responders in the context of Poseidon classification. Front Endocrinol (Lausanne) 10:281. https://doi.org/10.3389/fendo.2019.00281
Herraiz S, Romeu M, Buigues A, Martínez S, Díaz-García C, Gómez-Seguí I, Pellicer A et al (2018) Autologous stem cell ovarian transplantation to increase reproductive potential in patients who are poor responders. Fertil Steril 110(3):496-505.e491. https://doi.org/10.1016/j.fertnstert.2018.04.025
Hersant B, Sid-Ahmed M, Braud L, Jourdan M, Baba-Amer Y, Meningaud J-P, Rodriguez A-M (2019) Platelet-rich plasma improves the wound healing potential of mesenchymal stem cells through paracrine and metabolism alterations. Stem Cells Int 2019:1234263. https://doi.org/10.1155/2019/1234263
Hosseinisadat R, Nejad AF, Mohammadi F (2023) Intra-ovarian infusion of autologous platelet-rich plasma in women with poor ovarian reserve: a before and after study. Eur J Obstet Gynecol Reprod Biol 280:60–3
Keikha F, Shahsavari S, Salari Y, Roozbeh N, Haghollahi F, Tarazjani MD et al (2022) One side ovarian rejuvenation: a quasi-experimental study of the effect of the autologous platelet rich plasma in poor ovarian responders in IVF. Ethiop J Health Sci 32(6):1133–40
Koupenova M, Clancy L, Corkrey HA, Freedman JE (2018) Circulating platelets as mediators of immunity, inflammation, and thrombosis. Circ Res 122(2):337–351. https://doi.org/10.1161/circresaha.117.310795
Li XH, Zhou X, Zeng S, Ye F, Yun JL, Huang TG, Li YM et al (2008) Effects of intramyocardial injection of platelet-rich plasma on the healing process after myocardial infarction. Coron Artery Dis 19(5):363–370. https://doi.org/10.1097/MCA.0b013e3282fc6165
Maleki-Hajiagha A, Razavi M, Rouholamin S, Rezaeinejad M, Maroufizadeh S, Sepidarkish M (2020) Intrauterine infusion of autologous platelet-rich plasma in women undergoing assisted reproduction: a systematic review and meta-analysis. J Reprod Immunol 137:103078. https://doi.org/10.1016/j.jri.2019.103078
Na JI, Choi JW, Choi HR, Jeong JB, Park KC, Youn SW, Huh CH (2011) Rapid healing and reduced erythema after ablative fractional carbon dioxide laser resurfacing combined with the application of autologous platelet-rich plasma. Dermatol Surg 37(4):463–468. https://doi.org/10.1111/j.1524-4725.2011.01916.x
Navali N, Sadeghi L, Farzadi L, Ghasemzadeh A, Hamdi K, Hakimi P, Niknafs B (2022) Intraovarian injection of autologous platelet-rich plasma improves therapeutic approaches in the patients with poor ovarian response: a before-after study. Int J Fertil Steril 16(2):90–94. https://doi.org/10.22074/ijfs.2021.533576.1154
Nazari L, Salehpour S, Hosseini S, Sheibani S, Hosseinirad H (2022) The effects of autologous platelet-rich plasma on pregnancy outcomes in repeated implantation failure patients undergoing frozen embryo transfer: a randomized controlled trial. Reprod Sci 29(3):993–1000. https://doi.org/10.1007/s43032-021-00669-1
Olesen H, Pors SE, Adrados CS, Zeuthen MC, Mamsen LS, Pedersen AT, Kristensen SG (2023) Effects of needle puncturing on re-vascularization and follicle survival in xenotransplanted human ovarian tissue. Reprod Biol Endocrinol 21(1):28. https://doi.org/10.1186/s12958-023-01081-x
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Moher D et al (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372:n71. https://doi.org/10.1136/bmj.n71
Pantos K, Simopoulou M, Pantou A, Rapani A, Tsioulou P, Nitsos N, Sfakianoudis K et al (2019) A case series on natural conceptions resulting in ongoing pregnancies in menopausal and prematurely menopausal women following platelet-rich plasma treatment. Cell Transplant 28(9–10):1333–1340. https://doi.org/10.1177/0963689719859539
Park HB, Yang JH, Chung KH (2011) Characterization of the cytokine profile of platelet rich plasma (PRP) and PRP-induced cell proliferation and migration: Upregulation of matrix metalloproteinase-1 and -9 in HaCaT cells. Korean J Hematol 46(4):265–273. https://doi.org/10.5045/kjh.2011.46.4.265
Parvanov D, Ganeva R, Vidolova N, Nikolova K, Vasileva M, Stoykov I et al (2020) Ovarian autologous platelet-rich plasma (PRP) treatment improves oocyte and embryo quality in women with poor ovarian response. Fertil Steril 114(3):E452–53
Parvanov D, Ganeva R, Vidolova N, Nikolova K, Vasileva M, Totev T, Stamenov G (2022) Autologous ovarian platelet rich plasma treatment improves oocyte and embryo quality: a before-after prospective study. Biotechnol Biotechnol Equip 36(1):425–432
Pastore LM, Christianson MS, Stelling J, Kearns WG, Segars JH (2018) Reproductive ovarian testing and the alphabet soup of diagnoses: DOR, POI, POF, POR, and FOR. J Assist Reprod Genet 35(1):17–23. https://doi.org/10.1007/s10815-017-1058-4
Raeissadat SA, Ghazi Hosseini P, Bahrami MH, Salman Roghani R, Fathi M, Gharooee Ahangar A, Darvish M (2021) The comparison effects of intra-articular injection of platelet rich plasma (PRP), plasma rich in growth factor (PRGF), hyaluronic acid (HA), and ozone in knee osteoarthritis; a one year randomized clinical trial. BMC Musculoskelet Disord 22(1):134. https://doi.org/10.1186/s12891-021-04017-x
Rasool S, Shah D (2017) Fertility with early reduction of ovarian reserve: the last straw that breaks the Camel’s back. Fertil Res Pract 3:15. https://doi.org/10.1186/s40738-017-0041-1
Romito A, Bardhi E, Errazuriz J, Blockeel C, Santos-Ribeiro S, Vos M, Drakopoulos P et al (2020) Heterogeneity among poor ovarian responders according to Bologna criteria results in diverging cumulative live birth rates. Front Endocrinol (Lausanne) 11:208. https://doi.org/10.3389/fendo.2020.00208
Samy A, Abbas AM, Elmoursi A, Elsayed M, Hussein RS (2020) Effect of autologous platelet-rich plasma transfusion in the treatment of infertile women with thin endometrium and its implications in IVF cycles: a literature review. Middle East Fertil Soc J 25(1):5. https://doi.org/10.1186/s43043-020-0019-5
Sfakianoudis K, Simopoulou M, Grigoriadis S, Pantou A, Tsioulou P, Maziotis E, Pantos K et al (2020) Reactivating ovarian function through autologous platelet-rich plasma intraovarian infusion: pilot data on premature ovarian insufficiency, perimenopausal, menopausal, and poor responder women. J Clin Med 9(6):1809. https://doi.org/10.3390/jcm9061809
Skinner MK (2005) Regulation of primordial follicle assembly and development. Hum Reprod Update 11(5):461–471. https://doi.org/10.1093/humupd/dmi020
Soave I, Lo Monte G, Marci R (2012) Spontaneous pregnancy and unexplained infertility: a gift with many whys. N Am J Med Sci 4(10):512–513. https://doi.org/10.4103/1947-2714.102010
Stojkovska S, Dimitrov G, Stamenkovska N, Hadzi-Lega M, Petanovski Z (2019) Live birth rates in poor responders’ group after previous treatment with autologous platelet-rich plasma and low dose ovarian stimulation compared with poor responders used only low dose ovarian stimulation before in vitro fertilization. Open Access Maced J Med Sci. 7(19):3184–8
Tülek F, Kahraman A (2022) The effects of intra-ovarian autologous platelet rich plasma injection on IVF outcomes of poor responder women and women with premature ovarian insufficiency. J Turk Ger Gynecol Assoc 23(1):14–21. https://doi.org/10.4274/jtgga.galenos.2021.2021.0134
Wang X, Jin L, Mao YD, Shi JZ, Huang R, Jiang YN, Liang XY et al (2021) Evaluation of ovarian reserve tests and age in the prediction of poor ovarian response to controlled ovarian stimulation-a real-world data analysis of 89,002 patients. Front Endocrinol (Lausanne) 12:702061. https://doi.org/10.3389/fendo.2021.702061
Zhang X, Feng T, Yang J, Hao Y, Li S, Zhang Y, Qian Y (2021) A flexible short protocol in women with poor ovarian response over 40 years old. J Ovarian Res 14(1):3. https://doi.org/10.1186/s13048-020-00761-1
Zhu M, Xu M, Zhang J, Zheng C (2023) The role of Hippo pathway in ovarian development. Front Physiol 14:1198873. https://doi.org/10.3389/fphys.2023.1198873
Funding
This work received no grant from any funding agency in the public, commercial, governmental, or academic sectors.
Author information
Authors and Affiliations
Contributions
MV, HH, RV, TD: concept and design; SS, MT, FKH: data screening; RV, JJ, MJ, HH: data analysis; all the authors contributed in manuscript draft; MV, SS, HH, RV and TD revised the final version.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that there are no competing interests related to the subject matter or materials discussed in this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Vahabi Dastjerdi, M., Sheibani, S., Taheri, M. et al. Efficacy of intra-ovarian injection of autologous platelet-rich plasma in women with poor responders: a systematic review and meta-analysis. Arch Gynecol Obstet 309, 2323–2338 (2024). https://doi.org/10.1007/s00404-024-07442-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-024-07442-0