Abstract
Purpose
Polycystic ovary syndrome (PCOS) is a common endocrine disorder often linked to metabolic syndrome (MS), raising the risk of cardiovascular disease and type II diabetes. Certain indicators, such as the lipid accumulation product (LAP) and homeostatic model assessment for insulin resistance (HOMA-IR), can predict MS in PCOS patients. This study aimed to assess the predictive power of the visceral adiposity index (VAI) in comparison to LAP and HOMA-IR as predictors of MS in PCOS patients.
Methods
In this cross-sectional observational study, data from 317 diagnosed PCOS women were analyzed. VAI, LAP, and HOMA-IR were computed as indexes. Participants were categorized into two groups for index accuracy comparison: PCOS patients with and without MS. The data were assessed using a ROC curve.
Results
Among PCOS women with MS, 92.3% had abnormal VAI results, 94.5% had abnormal LAP results, and only 50.5% had abnormal HOMA-IR results. Conversely, the majority of PCOS women without MS had normal HOMA-IR (64.6%). When comparing these indexes using the ROC curve, VAI displayed the highest accuracy, followed by LAP and HOMA-IR.
Conclusion
The VAI index proved to be a superior predictor of metabolic MS in PCOS women when compared to other indexes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The visceral adiposity index (VAI) is a superior predictor of metabolic syndrome (MS) in women with polycystic ovary syndrome (PCOS), when compared to the lipid accumulation index (LAP) and homeostatic model assessment of insulin resistance (HOMA-IR). Therefore, VAI can be a valuable tool in the early identification of cardiovascular risks and type II diabetes in PCOS patients. |
Introduction
Polycystic ovary syndrome (PCOS) is the most common endocrine disorder among women of reproductive age, with a prevalence ranging 5% and 20%, depending on the diagnostic criteria employed [1]. According to the Rotterdam criteria, PCOS is characterized by the presence of at least two out of three criteria: clinical and/or laboratorial hyperandrogenism, oligo/anovulation, and polycystic ovarian morphology on ultrasound [1], after excluding other causes of anovulation and hyperadrogenism such as Cushing’s syndrome, congenital adrenal hyperplasia, hyperprolactinemia, thyroid dysfunction, androgen-producing tumors, and ovarian hyperthecosis have been excluded [2, 3]. PCOS is characterized by dysfunction in the hypothalamic-pituitary-ovary axis and anovulation. However, unlike other causes of ovulatory dysfunction, which involve insufficient ovarian follicle development or suppressed gonadotropin secretion (or both), PCOS typically presents with excess androgens and subtle deviations in serum levels of gonadotropins and estrogens that are not readily detected by routine tests. PCOS carries the potential for significant consequences, including an elevated risk of developing endometrial hyperplasia and neoplasia. Additionally, extra-reproductive manifestations of PCOS encompass insulin resistance (IR), metabolic syndrome (MS), and low-grade chronic inflammation [3,4,5,6,7,8,9]. The diagnosis of PCOS has been a subject of debate, as it involves difficulties in clearly defining the specific aspects within the diagnostic criteria. Additionally, there is substantial clinical diversity within PCOS, which is further compounded by variations across different ethnicities and alterations in clinical characteristics over the course of one's life [3].
Insulin resistance (IR) is detected in up to 70% of women with PCOS and is considered one of the main pathophysiological factors contributing to reproductive and metabolic disturbances in PCOS [10]. IR and disturbances in glucose metabolism are currently believed to contribute to the pathogenesis of the disease. IR results in compensatory hyperinsulinemia, which, in turn, amplifies ovarian androgen production through both direct ovarian effects and by triggering LH secretion. Additionally, women with PCOS often exhibit a higher prevalence of impaired glucose tolerance (IGT), obesity and metabolic syndrome (MS). This cluster of factors has been identified as a predictor of increased risk for future cardiovascular events and type 2 diabetes [11, 12]. The combination of obesity, particularly abdominal obesity, and PCOS has been shown to synergistically promote premature atherosclerosis and elevate cardiovascular mortality [13,14,15,16,17]. These findings underscore the importance of assessing cardiometabolic risk in women with PCOS in clinical practice [3, 11].
Despite its clinical relevance, diagnosing MS in PCOS patients poses challenges due to the heterogeneity of PCOS and variations in diagnostic criteria. Current predictive markers, such as BMI, fasting insulin levels, and lipid profiles, have limitations in accurately identifying MS in PCOS. These markers may not fully capture the unique metabolic complexities and variations present in PCOS patients.
Some markers, including the lipid accumulation product (LAP), homeostatic model assessment for insulin resistance (HOMA-IR), and visceral adiposity index (VAI) have been proposed as predictors of cardiovascular complications and insulin resistance even prior to the diagnosis of metabolic syndrome (MS). Early identification of MS in PCOS patients has the potential to prevent further complications associated with metabolic disturbances, such as cardiovascular disease (CVD) [1, 18].
The homeostasis model assessment of insulin resistance (HOMA-IR), which utilizes fasting glucose and fasting insulin levels, serves as an alternative to the glucose clamp method for assessing insulin resistance. Despite the widespread use of HOMA-IR, there is a lack of consensus regarding the cutoff points for classifying insulin resistance. Some researchers have attempted to determine HOMA-IR cutoffs in subjects exhibiting tendencies towards insulin resistance or MS, but their findings have been inconsistent [18]. While insulin resistance may play a central role in the cluster of metabolic abnormalities characterizing MS, previous studies indicate that MS is not always synonymous with insulin resistance [18], which may limit the utility of this index as a predictor of MS.
The lipid accumulation product (LAP) was introduced by Kahn in 2005 as a superior indicator of cardiovascular diseases (CVD) risk compared to BMI [19]. Numerous studies have demonstrated a strong association between LAP and metabolic syndrome (MS) according to various diagnostic criteria [20]. LAP is a cardiovascular risk index based on the combination of waist circumference (WC) and fasting triglyceride (TG) levels. However, despite its potential, there is a scarcity of clinically relevant studies assessing the utility of LAP.
The visceral adiposity index (VAI) is an empirical-mathematical model that is gender-specific and relies on anthropometric measurements such as BMI and waist circumference, as well as biochemical parameters including triglyceride (TG) and high-density lipoprotein cholesterol (HDL-C) concentrations [21]. This index enables accurate identification of visceral adipose tissue and predicts the likelihood of obesity-related complications even before the diagnosis of metabolic syndrome [1, 20, 22, 23]. A VAI value greater than 1.675 enables differentiation between women with metabolically unhealthy polycystic ovary syndrome (MU-PCOS) and those with metabolically healthy polycystic ovary syndrome (MH-PCOS) [22].
This study aimed to assess the predictive role of VAI compared to LAP and HOMA-IR indexes in identifying metabolic syndrome (MS) among patients with polycystic ovary syndrome (PCOS). This study aims to address the critical need for more accurate predictive markers for MS in PCOS, intending to overcome the limitations of current markers and provide a more precise assessment of MS risk in PCOS patients.
Material and methods
This cross-sectional observational study was conducted at the Hyperandrogenism Outpatient Clinic of the Clinical Hospital of the Federal University of Minas Gerais (UFMG), Belo Horizonte, MG, Brazil. The study population consisted of 317 women aged 18–40 years, diagnosed with polycystic ovary syndrome (PCOS) according to the Rotterdam criteria, between November 2009 and June 2019. A secondary database was utilized, and data were included for secondary analysis if all the prerequisite variables required for calculating the indexes were available.
The collected data from the database included BMI, weight, height, waist circumference (WC), total cholesterol, HDL, LDL, total testosterone, triglyceride (TG), homeostatic model assessment for insulin resistance (HOMA-IR), lipid accumulation product (LAP), and visceral adiposity index (VAI). The indexes were calculated using the following formulas: HOMA-IR = fasting blood glucose × fasting insulin/405, LAP = (WC [cm] − 58) × TG (mmol/L) and VAI = (WC/36.58 + [1.89 × BMI]) × (TG/0.81) × (1.52/HDL). Reference values for the indexes were defined as VAI ≤ 1.67, LAP < 34.5, and HOMA-IR < 2.7.
Metabolic syndrome (MS) was diagnosed based on the National Cholesterol Education Program Adult Treatment Panel (NCEP-ATP) III criteria, which required the presence of at least three of the following criteria: waist circumference (WC) > 88 cm, triglyceride level ≥ 150 mg/dl, high-density lipoprotein (HDL) < 50 mg/dl, blood pressure ≥ 130/85 mmHg, and fasting glucose level ≥ 110 mg/dl [24].
The participants were categorized into two groups: PCOS patients with metabolic syndrome (MS) and PCOS patients without metabolic syndrome.
Ethical aspects
The present research was approved by research ethics committees from UFMG (Federal University of Minas Gerais). All the participants signed a term of informed consent.
Statistical analyses
Sample calculation considered a prevalence of 10% for PCOS and, among these patients, 60% for MS [2]. A 95% confidence interval and a maximum error of 0.03 were defined to estimate the true value. The data from 238 patients were required in this study.
Descriptive (frequencies, percentages, means, standard deviation) and comparative analyses were used for the statistical analyses. Comparative analyses included the Student’s t-tests to compare the means of two independent groups and the chi-squared test to compare categorical variables. Statistically significant associations were considered when the P-value was less than or equal to 0.05.
Results
Figure 1 presents the characteristics of the 317 included PCOS patients, with 28.7% having metabolic syndrome (MS) and 71.3% without MS.
Table 1 describes the clinical and laboratory data of the study population, presenting basic statistical information.
The comparison of variables between patients with and without MS (Table 2) revealed no significant differences in height, insulin level, Ferriman score, and total testosterone level. However, patients with MS had higher mean values of weight, waist circumference (WC), body mass index (BMI), fasting blood glucose, and triglycerides. Conversely, patients without MS had a higher mean value of high-density lipoprotein (HDL) compared to patients with MS.
The association between VAI and MS was assessed using the chi-squared test (Table 3), which demonstrated a statistically significant difference between the groups with and without MS (P = 0.000). The majority of patients exhibited elevated VAI values, albeit in varying proportions. Specifically, VAI changed in 92.3% of patients with MS and 53.1% of patients without MS. The odds ratio indicated that an MS patient was less likely to have a normal VAI compared to a changed VAI (odds ratio = 0.094).
The chi-squared test also revealed a significant difference between patients with and without MS regarding LAP (P = 0.000) (Table 4). The majority of patients had elevated LAP values, with 94.5% of women with MS and 56.2% of women without MS showing a change. The odds ratio indicated that an MS patient had a lower likelihood of having a normal LAP compared to a changed LAP (odds ratio = 0.075).
Regarding HOMA-IR, the chi-squared test showed a significant difference between patients with and without MS (P = 0.013) (Table 5). Among patients with MS, 50.5% had a changed HOMA-IR, while 49.5% had a normal HOMA-IR. The majority of patients without MS exhibited a normal HOMA-IR. The odds ratio indicated that an MS patient had a lower likelihood of having a normal HOMA-IR compared to a changed HOMA-IR (odds ratio = 0.536).
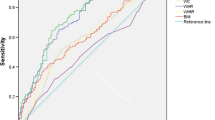
The receiver operating characteristic (ROC) curve analysis comparing the accuracy of VAI, LAP, and HOMA-IR indexes demonstrated that VAI had the highest accuracy, followed by LAP, and subsequently HOMA-IR (Fig. 2).
Discussion
The present study provides evidence that VAI is a robust predictor of metabolic syndrome (MS) in polycystic ovary syndrome (PCOS) patients, surpassing the accuracy of LAP and HOMA-IR. Moreover, a positive association was observed among the three indexes.
Previous research by Schuster et al. demonstrated a direct correlation between the diagnostic criteria for MS and VAI in young adults, suggesting VAI as a reliable predictor of the condition [25]. Joshi et al. reported significantly higher VAI values in the PCOS patient group compared to the control group, highlighting its effectiveness as a predictive biomarker in the studied population [26]. Similarly, Techatraisak et al., in a study comprising 399 PCOS patients and 42 controls, found an association between the recommended reference value for VAI in the literature and MS. However, their ROC curve analysis suggested the need for a new cutoff value. Furthermore, the prevalence of MS in PCOS (24.6%) reported by Techatraisak et al. aligns with the findings of the present study, where 28.7% of PCOS patients presented MS, while 71.3% did not.
Although this study did not observe a statistical difference in HOMA-IR values between patients with and without MS, significant differences were detected in patients without MS. Pontes et al. conducted a study involving 189 PCOS patients, demonstrating a strong association between HOMA-IR and LAP as biomarkers for insulin resistance [28]. The prevalence of insulin resistance varied according to the assessment method and was directly related to BMI. Existing literature indicates that increased HOMA-IR and LAP values are commonly associated with insulin resistance.
Soares's study indicated that LAP is a reliable predictor of MS in adults, with an area under the ROC curve greater than 0.85 [29]. Similarly, Ribeiro et al. conducted a cross-sectional study revealing that both LAP and VAI can serve as sensitive predictors of MS and insulin resistance in PCOS patients [30]. However, the present study found LAP to be a more sensitive index than VAI, emphasizing its simplicity and practicality, which makes it an encouraging choice for clinical application.
Strengths of this study include a sample size surpassing the minimum requirement and comparable populations in various measures, such as height, insulin, and indicators of clinical and laboratory hyperandrogenism (Ferriman score and total testosterone, respectively). Additionally, the study introduced a novel index that is cost-effective, user-friendly, and highly accurate in predicting MS in PCOS patients, adding value to the assessment of PCOS.
Limitations of the study include its cross-sectional design, reliance on a secondary database with data collected by different professionals, and the possibility of information bias. Furthermore, the study highlighted the non-comparability of variables such as BMI, HDL, TG, WC, weight, and fasting blood glucose between groups, which was expected as these variables were used in the calculation of the studied indexes.
In conclusion, this study establishes VAI as a superior biomarker compared to other indexes, proving its efficacy in predicting MS in PCOS patients. Its simplicity and accuracy make it a valuable tool in the evaluation of PCOS. However, further research is necessary to explore the applicability of VAI due to its relatively recent introduction in the field.
Data availability
Data supporting this paper are available on request.
References
Amato MC, Magistro A, Gambino G, Vesco R, Giordano C (2015) Visceral adiposity index and DHEAS are useful markers of diabetes risk in women with polycystic ovary syndrome. Eur J Endocrinol 172(1):79–88. https://doi.org/10.1530/EJE-14-0600
ESHRE Rotterdam ASRM-Sponsored (2004) Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod 19:41–47
Mousa A, Tay CT, Teede H (2023) Technical report for the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Monash University, Melbourne
Çakıroğlu Y, Vural F, Vural B (2016) The inflammatory markers in polycystic ovary syndrome: association with obesity and IVF outcomes. J Endocrinol Invest 39(8):899–907
de Leo V, Musacchio MC, Cappelli V et al (2016) Genetic, hormonal and metabolic aspects of PCOS: an update. Reprod Biol Endocrinol 14(1):38
Durmus U, Duran C, Ecirli S (2017) Visceral adiposity index levels in overweight and/or obese, and non-obese patients with polycystic ovary syndrome and its relationship with metabolic and inflammatory parameters. J Endocrinol Invest 40(5):487–497
McCartney CR, Marshall JC (2016) Clinical practice polycystic ovary syndrome. N Engl J Med 375(1):54–64
Spritzer PM, Lecke SB, Satler F et al (2015) Adipose tissue dysfunction, adipokines, and low-grade chronic inflammation in polycystic ovary syndrome. Reproduction 149(5):R219–R227
Rocha AL et al (2019) Recent advances in the understanding and management of polycystic ovary syndrome. F1000Res. https://doi.org/10.12688/f1000research.15318.1
Kakoly NS, Khomami MB, Joham AE, Cooray SD, Misso ML, Norman RJ, Harrison CL, Ranasinha S, Teede HJ, Moran LJ (2018) Ethnicity, obesity and the prevalence of impaired glucose tolerance and type 2 diabetes in PCOS: a systematic review and meta-regression. Hum Reprod Update 24(4):455–467. https://doi.org/10.1093/humupd/dmy007. (PMID: 29590375)
Amato MC, Verghi M, Galluzzo A, Giordano C (2011) The oligomenorrhoic phenotypes of polycystic ovary syndrome are characterized by a high visceral adiposity index: a likely condition of cardiometabolic risk. Hum Reprod 26(6):1486–1494. https://doi.org/10.1093/humrep/der088. (Epub 2011 Mar 29 PMID: 21447694)
Reaven G (2002) Metabolic syndrome: pathophysiology and implications for management of cardiovascular disease. Circulation 106:286–288
Guzick DS, Talbott EO, Sutton-Tyrrell K, Herzog HC, Kuller LH, Wolfson SKJr (1996) Carotid atherosclerosis in women with polycystic ovary syndrome: initial results from a case–control study. Am J Obstet Gynecol 174:1224–1232
Birdsall MA, Farquhar CM, White HD (1997) Association between polycystic ovaries and extent of coronary artery disease in women having cardiac catheterization. Ann Intern Med 126:32–35
Pierpoint T, McKeigue PM, Isaacs AJ, Wild SH, Jacobs HS (1998) Mortality of women with polycystic ovary syndrome at long-term follow-up. J Clin Epidemiol 51:581–586
Wild S, Pierpoint T, McKeigue P, Jacobs H (2000) Cardiovascular disease in women with polycystic ovary syndrome at long-term follow-up: a retrospective cohort study. Clin Endocrinol 52:595–600
Christian RC, Dumesic DA, Behrenbeck T, Oberg AL, Sheedy PF, Fitzpatrick LA (2003) Prevalence and predictors of coronary artery calcification in women with polycystic ovary syndrome. J Clin Endocrinol Metab 88:2562–2568
Esteghamati A, Ashraf H, Khalilzadeh O, Zandieh A, Nakhjavani M, Rashidi A, Haghazali M, Asgari F (2010) Optimal cut-off of homeostasis model assessment of insulin resistance (HOMA-IR) for the diagnosis of metabolic syndrome: third national surveillance of risk factors of non-communicable diseases in Iran (SuRFNCD-2007). Nutr Metab (Lond) 7(7):26. https://doi.org/10.1186/1743-7075-7-26.PMID:20374655;PMCID:PMC2857836
Kahn HS (2005) The “lipid accumulation product” performs better than the body mass index for recognizing cardiovascular risk: a population-based comparison. BMC Cardiovasc Disord 5:26
Naghshband Z, Kumar L, Mandappa S, Niranjana Murthy AS, Malini SS (2021) Visceral adiposity index and lipid accumulation product as diagnostic markers of metabolic syndrome in south indians with polycystic ovary syndrome. J Hum Reprod Sci. 14(3):234–243
Amato MC, Giordano C, Galia M et al (2010) AlkaMeSy study group: visceral adiposity index: a reliable indicator of visceral fat function associated with cardiometabolic risk. Diabetes Care 33:920–922
Brończyk-Puzoń A, Jagielski P, Kulik-Kupka K, Koszowska A, Nowak J, Zubelewicz-Szkodzińska B (2017) Usefulness of a new anthropometric indicator – VAI (visceral adiposity index) in the evaluation of metabolic and hormonal disorders in women with polycystic ovary syndrome. Adv Clin Exp Med 26(5):825–828
Abruzzese GA, Cerrone GE, Gamez JM, Graffigna MN, Belli S, Lioy G et al (2017) Lipid accumulation product (LAP) and visceral adiposity index (VAI) as markers of insulin resistance and metabolic associated disturbances in young argentine women with polycystic ovary syndrome. Horm Metab Res 49:23–29
Expert panel on detection, evaluation, and treatment of high blood cholesterol in adults. executive summary of the third report of the national cholesterol education program (NCEP) (2001) Expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III). J Am Med Assoc 2001(285):2486–2497. https://doi.org/10.1001/jama.285.19.2486
Schuster J, Vogel P, Eckhardt C, Morelo SDB (2014) Simone Applicability of the visceral adiposity index (VAI) in predicting components of metabolic syndrome in young adults. Nutr Hosp 30(4):806–812
Joshi B, Lakhan T, Mukherjee S, Patil A, Unisa S (2018) Visceral adiposity index among young girls with PCOS and its association with phenotypes and metabolic risk. Int J Reprod Contracept Obstet Gynecol 7(2):513–518
Techatraisak K, Wongmeerit K, Dangrat C, Wongwananuruk T, Indhavivadhana S (2016) Measures of body adiposity and visceral adiposity index as predictors of metabolic syndrome among Thai women with PCOS. Gynecol Endocrinol 32(4):276–280
Pontes AG, Rehme MFB, Martins AMVC, Micussi MTABC, Maranhão TMO, Pimenta WP et al (2012) Resistência à insulina em mulheres com síndrome dos ovários policísticos: relação com as variáveis antropométricas e bioquímicas. Rev Bras Ginecol Obstet 34:74–79
Soares LM (2016) Produto de acumulação lipídica: acurácia para identificação de portadores da síndrome metabólica em adultos. Universidade Federal de Minas Gerais, Belo Horizonte
Ribeiro VB, Kogure GS, Lopes IP, Silva RC, Pedroso DCC, Ferriani RA et al (2019) Association of measures of central fat accumulation indices with body fat distribution and metabolic, hormonal, and inflammatory parameters in women with polycystic ovary syndrome. Arch Endocrinol Metab 63(4):417–426
Funding
This study and all authors have received no funding.
Author information
Authors and Affiliations
Contributions
BT: conceptualization, data curation, writing—original draft preparation; NIR; CJM; CJD; OMA; CMG: data curation, writing—original draft preparation; ARC: conceptualization, data curation, writing—review & editing; OFR: data curation—original draft preparation; CFV: data curation, writing—original draft preparation; CAL: project administration, supervision, writing—review & editing; RFM and R ALL: conceptualization, data curation, methodology, supervision, writing—review & editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors certify that they have NO affiliations with or involvement in any organization or entity with any financial, or non-financial interest in the subject matter or materials discussed in this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rocha, A.L., Baêta, T., Nazareth, I.R. et al. The role of the visceral adiposity index in the assessment of metabolic syndrome of polycystic ovary syndrome patients: a new anthropometric index. Arch Gynecol Obstet 309, 1643–1649 (2024). https://doi.org/10.1007/s00404-023-07328-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-023-07328-7