Abstract
Objective
To investigate the inflammatory markers in polycystic ovary syndrome (PCOS) and associations of these markers with obesity and in vitro fertilization (IVF) outcomes.
Methods
A total of 292 women underwent IVF procedure either with PCOS (n = 146) or without PCOS (n = 146, age, and body mass index (BMI) matched controls) were included in the study. All patients were classified according to BMI levels (normal weight: NW, BMI <25 kg/m2 and obese: OB, BMI ≥25 kg/m2). The inflammatory markers were leukocyte count, neutrophil/lymphocyte ratio (NLR), platelet/lymphocyte ratio (PLR), mean platelet volume (MPV).
Results
BMI of PCOS was positively correlated with leukocyte, neutrophil, lymphocyte and MPV (p < 0.05), but negatively correlated with NLR and PLR (p < 0.05). Both NLR and PLR increased significantly in PCOS (p < 0.001). PLR increased significantly in NW-PCOS compared the NW-controls and OB-PCOS. MPV values increased only in OB-PCOS subjects. The logistic regression analyzes showed that MPV was the independent variable in PCOS to effect CPR (p = 0.000; OR 0.1; CI 0.06–0.2).
Conclusions
NLR and PLR were significantly increased in all PCOS subjects compared to the BMI-matched controls. Despite PLR being decreased by adiposity, PLR increased in NW-PCOS. These results are supporting the hypothesis that PCOS is a chronic inflammatory process independent of obesity. MPV levels were independently associated with CPR in PCOS. Further prospective studies concerning inflammation and IVF outcomes of PCOS are needed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Polycystic ovary syndrome (PCOS) is the most common endocrinopathy and affects 5–10 % of women in their reproductive years. It is characterized by a higher prevalence of obesity, ovulatory dysfunction, and hyperandrogenism. The majority of the women with PCOS are overweight or obese and over the long term, they are prone to metabolic abnormalities, dyslipidemia, diabetes mellitus, and cardiovascular diseases [1].
Polycystic ovary syndrome is a complex and multifactorial disease with metabolic dysfunction and the etiopathogenesis is not well established [1]. Emerging data suggest that adiposity and chronic low-grade inflammation are involved in the development of the metabolic dysfunction. The inflammatory mediators in PCOS have been linked to hyperandrogenism, insulin resistance, type 2 diabetes, and cardiovascular risk factors. Prior studies have suggested that PCOS is a proinflammatory condition with increased levels of plasminogen activator inhibitor, TNF-alpha, and interleukin (IL) [2–12].
Adipose tissue is an active endocrine organ that secretes hormones, adipokines, and cytokines [8]. One suggested a mechanism of inflammation is PCOS-related adipocyte hypertrophy that leads to a compression of vessels, hypoxia, and elevated inflammatory markers (TNF, IL) [8]. Previous studies have demonstrated increased TNF and IL-6 levels in follicular fluid of PCOS subjects. TNF is a potential candidate for initiating events in PCOS. It has been suggested that mononuclear cell infiltration and local inflammation in the ovary stimulates ovarian steroidogenic activity. TNF stimulates theca cell proliferation and phosphorylation of the receptor and further increases insulin resistance [13–15]. Thus, adipocyte hypertrophy and chronic inflammation are the mainstays of this syndrome. However, the effect of inflammation independent of adiposity remains to be elucidated [5].
Orio et al. studied 150 women with PCOS who were matched for age and BMI (body mass index) with 150 healthy women. They found significantly increased white cell count (WCC) and C-reactive protein (CRP) levels in PCOS. They suggested that increased leukocytes levels were as a marker of chronic inflammation and early cardiovascular risk in PCOS [9]. Morreale et al. performed a meta-analysis of 31 studies including 2359 women and 1289 controls, and found that CRP levels are higher in PCOS [7]. However, after this study Gonzales et al. stated that elevations in CRP levels also higher in obese women and elevations in PCOS below the range needed to predict metabolic risk [4]. Other studies supporting this issue found that CRP levels correlated with BMI and similar in PCOS and BMI matched controls. Recently NLR (neutrophil/lymphocyte ratio) was used as a marker of inflammation in PCOS [16–18]. NLR levels was increased in PCOS despite similar CRP values [17, 18].
The inflammatory markers from hematologic indices such as leucocytes, the NLR, and the platelet/lymphocyte ratio (PLR) which have been documented to predict mortality from cerebrovascular events, cardiovascular diseases and malignancies [19–24]. NLR is a measure of systemic inflammation [22]. Recently, the PLR was introduced as a biological indicator that demonstrates the balance between thrombosis and inflammation. Increased proliferation of the megakaryocytic series and relative thrombocytosis are two results of the ongoing proinflammatory state. High platelet counts and low lymphocyte counts have been suggested as risk indicators and reflect both aggregation and an inflammation pathway [23]. Platelet size is related with platelet function and activation. The MPV is an important indicator of platelet activation and previous studies documented MPV as a risk factor for cardiovascular diseases [24]. In the literature NLR, PLR and MPV (mean platelet volume) are increasingly used as markers of chronic inflammation. [22–24]. There are some reports about NLR [16–18] and MPV in PCOS [25–28]. However, there is no prior report of PLR in PCOS. To the best of our knowledge, this paper is the first search using together NLR, PLR, and MPV in PCOS. Moreover, there is no study searching the association of inflammatory markers in IVF outcome of PCOS.
Growing evidence have been documented that PCOS is a chronic inflammatory status, but it remains to be seen whether increased inflammatory response in PCOS relates to adiposity. It also remains to be seen whether increased inflammatory response in PCOS relates to success rates of IVF treatment. The aim of this paper was to investigate inflammatory markers in PCOS and associations of the inflammatory markers with obesity and IVF outcomes.
Materials and methods
Study design
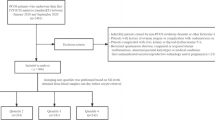
This retrospective case–control study was performed in the Assisted Reproduction Unit of Kocaeli University School of Medicine, Kocaeli, Turkey. Data were collected from the hospital files of women who had undergone a controlled ovarian hyperstimulation (COH) and an IVF (in vitro fertilization)/ICSI (intracytoplasmic sperm injection) procedure between May 2011 and September 2013. The local ethics committee approved the study (KOÜ-KAEK 2015/131).
Patient selection
All patients underwent IVF/ICSI with an ovarian hyperstimulation using a flexible multi-dose gonadotropin-releasing hormone (GnRH) antagonist protocol. All patients had clinical, laboratory, and ultrasonographic evaluations prior to the stimulation program. PCOS patients who met the criterion of Rotterdam (ESHRE/ASRM consensus, 2004) were included [29] (Rotterdam criteria: oligo and or anovulation, clinical and/or biochemical signs of hyperandrogenism, presence of 12 or more follicles in each ovary measuring 2–9 mm in diameter, and/or increased ovarian volume (>10 ml). The presence of at least two of the defining criteria is required for PCOS. In addition, known disorders that mimic the PCOS, such as congenital adrenal hyperplasia, androgen-secreting tumors, Cushing’s syndrome should be excluded) [29]. The control group (nonPCOS) was chosen from the age and BMI-matched women with unexplained infertility and regular menstruations. Exclusion criteria for this study included those with any unavailable data, severe male factor infertility, tubal factor infertility, smoking, endometriosis, poor ovarian reserves (FSH >12 mIU/ml, AMH <1 ng/ml or AFC <5), and medications related to carbohydrate metabolism or hormones were excluded from the study. Additional exclusion criteria included the presence of chronic diseases, including endocrine problems, chronic hypertension, diabetes mellitus, renal disorders, autoimmune diseases, malignancy, chronic liver diseases, pelvic inflammatory diseases, hematologic diseases, splenectomy history, or other chronic inflammatory conditions.
A total of 292 women either with (n = 146) or without PCOS (n = 146 who were age and BMI-matched) was included in the study. All patients were classified into subgroups as normal weight (NW: BMI <25 kg/m2) and obese (OB: BMI ≥25 kg/m2). NW-PCOS (n = 69) and OB-PCOS (n = 77) subjects were compared to BMI-matched controls (NW-Control, n = 69; and OB-Control, n = 77, respectively) for inflammatory markers and IVF outcomes.
Laboratory evaluation
-
1.
Inflammatory markers: The inflammatory markers were WCC (white cell count), NLR (neutrophil to lymhocyte ratio), PLR (platelet to lymhocyte ratio), and MPV (mean platelet volume). Blood samples were collected before controlled ovarian stimulation protocol during the follicular phase. A blood analyzer (Cell-Dyn 3700.Abbott®, USA) was used for the determination of the complete blood cell counts.
-
2.
Glucose and insulin levels: Plasma glucose levels was estimated by spectrophotometric method (Abbott Architect C 1600®, USA). Serum insulin was detected by chemiluminescence (Unicel DxI 800, Beckman Coulter®, USA).
-
3.
Insulin resistance: Insulin resistance was determined by using the homeostasis model assessment HOMA-IR formula for insulin resistance. The formula used is as follows: (HOMA-IR) formula [HOMA-IR = fasting insulin (mIU/mL) × fasting glucose (mg/dL)/18)/22.5].
-
4.
Hormonal analysis: All patients had measures of day three follicle stimulating hormone (FSH), luteinizing hormone (LH), estradiol (E2), and the FSH/LH ratio. The anti-Mullerian hormone (AMH) levels analyzed on a random day. All patients were measured for their levels of thyroid stimulating hormone (TSH), free thyroxine (f T4), prolactin, testosterone, and dehydroepiandrosterone sulfate (DHEAS) via chemiluminescence (Unicel DxI 800, Beckman Coulter®, USA). AMH measurements were performed by the Gen II microELISA method (Beckman Coulter®, Kraemer Blvd. Brea, CA 92821 USA). The immunoassay method used to measure FSH, LH, and E2 levels (Siemens Advia Centaur XP®, Ireland).
Transvaginal ultrasonography
All patients had basal ultrasonographic evaluation to exclude any pelvic pathology, and to measure antral follicle counts (AFC: total count of antral follicles with 2–5 mm diameter). Each patient underwent serial ultrasonographic examination during COH protocol. The ultrasonographic scans were performed by using a 6.5 MHz transvaginal transducer. (Sonoace X8, Samsung Medison Co.Ltd, South Korea).
COH protocol and IVF/ICSI procedure
All subjects underwent flexible GnRH antagonist COH protocol by using rec FSH (follitropin alfa (r-h FSH); Gonal-F®, Merck KGaA, Darmstadt, Germany) and Cetrorelix (Cetrorelix acetate; Cetrotide® 0.25 mg; Merck KGaA, Darmstadt, Germany). Patients monitored during COH protocol via serial measurements of serum E2, LH, progesterone level and ultrasonographic examinations. The rhCG (recombinant-choriogonadotropin alpha; Ovitrelle® 250 µg; Merck KGaA, Darmstadt, Germany) was administered if two follicles (18 mm) or 3 or more follicles (17 mm) develop during COH protocol. The oocytes retrieval (OR) was carried out 36 h after hCG injection. Than, embryo transfer was performed on 3–5 days. The visualization of a intrauterine gestational sac and fetal cardiac activity via ultrasonography was defined as clinical pregnancy. Fertilization rate was defined as number of oocytes that be fertilized by sperm. Implantation rate was defined as the number of gestational sac per number of transferred embryo. Miscarriage defines the pregnancy loss before 20 weeks of gestation. The total number of oocyte retrieved, total number of MII (mature) oocytes, fertilization rate, implantation rate, miscarriages and clinical pregnancy rates (CPR) were the IVF outcomes that were searched.
Analysis
The Statistical Package for Social Sciences for Windows (SPSS 18, Chicago, IL, USA) was used. All measurements were performed within a 95 % confidence interval. A p value less than 0.05 was considered for statistically significant. Descriptive data were expressed as means, standard deviations, and percentages. The Levene and Welch tests were used to test for homogeneity of variances. According to distribution of data, the comparisons of variables were performed by the ANOVA test, Student’s t test, Mann–Whitney U test, Fisher’s exact test, and the χ 2-test, when appropriate. The relationships between the data were evaluated using Pearson’s correlations. Logistic regression analysis was used to measure the association between dependent and independent variables.
Results
Table 1 presents the baseline characteristics of all patients. Age, BMI, HOMA-IR, and obstetric characteristics were similar between groups. AFC, LH, and AMH levels were significantly higher in the NW-PCOS group and AFC and AMH were higher in the OB-PCOS group compared to the control groups. Total oocytes retrieved, and MII oocyte counts were significantly increased in the OB-PCOS compared to the OB-controls. Comparisons of fertilization rates, pregnancy rates, miscarriage rates were similar (p > 0.05).
Table 2 presents the comparison of inflammatory markers between PCOS and control groups. NLR and PLR were significantly increased in NW-PCOS even though the WCC (white cell count) did not rise. The MPV and platelet counts were similar. The WCC, NLR, PLR, and MPV were significantly increased in the OB-PCOS compared to OB-Control (p < 0.05).
Table 3 shows the comparison between NW-PCOS and OB-PCOS with regard to inflammatory markers. OB-PCOS had significantly increased WCC, neutrophil, and lymphocyte levels, and a significantly decreased PLR compared to NW-PCOS. NLR ratios and platelet counts were similar in OB and NW-PCOS (p > 0.05). Although OB-PCOS had an increased MPV compared to NW-PCOS, this increase did not reach statistical significance (p > 0.05).
Correlation analysis
-
1.
BMI and day 3 hormones: The correlations of inflammatory markers with basal hormonal levels of PCOS women showed no relationship with FSH, estradiol, testosterone, DHEAS, and AMH levels. However, LH levels were negatively correlated with neutrophil (r = −0.198, p < 0.05) and lymphocyte count (r = −0.189, p < 0.05) and positively correlated with PLR (r = 0.192, p < 0.05). Prolactin levels were negatively correlated with NLR (r = −0.208, p < 0.05). BMI levels of PCOS were positively correlated with WCC (r = 0.789, p = 0.00), neutrophil (r = 0.741, p = 0.00), lymphocyte (r = 0.486, p = 0.00) and MPV (r = 0.236, p = 0.005). BMI levels (PCOS) were negatively correlated with NLR (r = −0.203, p = 0.01), and PLR (r = −0.321, p = 0.00). Figure 1 presents the correlations between BMI and inflammatory markers in PCOS. Figure 2 presents the correlations between BMI and inflammatory markers in control group.
Fig. 1 -
2.
IVF outcomes of PCOS: Total oocyte numbers were negatively related WCC (r = −0.210, p = 0.01), neutrophil (r = −0.180, p = 0.03). M II oocyte counts were negatively related to WCC (r = −0.236, p = 0.005), neutrophil (r = −0.214, p = 0.01), lymphocytes (r = −0.179, p = 0.03). The correlations of inflammatory markers (WCC, neutrophils, lymphocytes, NLR, platelet, PLR) with CPR were insignificant (p < 0.05). The correlations of neutrophil, leukocyte, lymphocyte and NLR with miscarriage were insignificant (p < 0.05). However, PLR was significantly positively correlated with miscarriage rates (r = 0.185, p < 0.05). MPV was significantly negatively correlated with CPR (r = −0.513, p < 0.001) and implantation rates (r = −0.470, p < 0.001).
Logistic regression analyzes were performed for various associations of pregnancy rates in PCOS and control subjects. Age, BMI, HOMAIR, WCC, NLR, PLR, and MPV were the independent variables to analyze dependent variable clinical pregnancy rates. MPV was the only independent variable in PCOS to effect CPR (p = 0.000; OR 0.1; CI 0.06–0.2). Age was the only independent variable affecting pregnancy rates in the control group (p = 0.03; OR 0.9; CI 0.8–0.9). Table 4 shows the details.
Discussion
Polycystic ovary syndrome is a complex phenomenon accompanied by metabolic dysfunction, adiposity and low-grade chronic inflammation [5]. To date studies have been limited concluding whether chronic inflammation is secondary to obesity or not [5–11]. In the present paper, we investigated inflammatory markers in PCOS women relative to BMI and determined associations of these markers with IVF outcomes. This study found that NLR and PLR were the inflammatory markers that were increased both in NW and OB-PCOS compared to BMI-matched controls. PLR was the marker significantly that was increased in NW-PCOS compared to OB-PCOS and NW-control. MPV values only increased in OB-PCOS. Despite the increased number of retrieved oocytes and MII oocytes, CPR were similar between PCOS and control groups. MPV levels were negatively associated with pregnancy rates. PLR levels were positively associated with miscarriages. Logistic regression analysis showed that MPV was the independent variable in PCOS to effect CPR.
Growing evidence supports that PCOS is associated with low-grade chronic inflammation [2–18] and inflammation is also related with prominent features of PCOS such as insulin resistance and cardiovascular diseases [1, 5]. Inflammation is considered to be the key feature of endothelial dysfunction and atherosclerosis [20]. Several studies have shown that increased WCC and subtypes are related to the severity of cardiovascular diseases and malignancies [19–24]. Orio et al. demonstrated the elevations of WCC in PCOS women [9]. After this study of Orio et al., several studies confirmed the elevations of WCC in PCOS [5, 7, 16]. In the present study, OB-PCOS group had significantly elevated WCC (8440.4 ± 1004.3 vs 7938.9 ± 1084.6, p = 0.004) compared to OB-control. However, the WCC did not rise in NW-PCOS. Apart from the study of Orio et al., we found that WCC increased in OB-PCOS women, not NW-PCOS.
Morreale et al. found that CRP levels are higher in PCOS, but many other studies found that CRP levels are also high in obese patients [7, 17, 18]. Recent studies found similar CRP levels in PCOS and controls [17, 18]. NLR was introduced as a new marker of inflammation in PCOS [16–18]. Kurt et al. compared NLR ratio between PCOS (n = 62) and control group (n = 60) [16]. They reported CRP, NLR, neutrophil and leucocytes were higher in PCOS group. Their study suggested the inflammation in PCOS is independent of obesity [16]. Recently, Yılmaz et al. [17] and Ağaçayak et al. [18] reported that NLR levels were increased in PCOS despite similar CRP levels. In this study, both NLR and PLR were significantly increased in all PCOS subjects. Our study confirmed the prior studies [16–18] that NLR levels increased in PCOS women. There is no prior report of PLR in PCOS. In our study, despite a subtle rise of NLR, the rise in PLR was prominent in PCOS. PLR was significantly higher in NW-PCOS compared to NW-control and OB-PCOS. Briefly, this study found that OB-PCOS had significantly increased WCC, NLR and PLR levels. However, NLR and PLR were significantly rised in NW-PCOS, eventhough WCC did not rise. These results suggest that the inflammatory status of PCOS is independent of obesity.
The activation of platelets at the site of vascular injury is central to the pathogenesis of occlusive arterial diseases. High MPV levels might contribute to the endothelial injury by platelet activation and higher plasma concentrations of thromboxane A2. Large sized platelets have increased hemostatic functions and previous studies have documented the clinical utility of MPV in thrombosis [24, 26]. There were studies searching MPV levels in PCOS [25–28]. Yılmaz et al. reported recently that lean PCOS group had higher MPV than controls [17]. However, prior two searches found that MPV values did not differ in NW- PCOS and controls [25, 26]. Doğan et al. suggested that in the studies that included PCOS and non-PCOS women with and without obesity, the effect of obesity on MPV could be better understood [25]. Similar to prior reports by Doğan et al. and Silfeler et al., our study confirmed that MPV values did not differ in NW-PCOS and control subjects [25, 26]. In our study, OB-PCOS had significantly increased MPV levels (p < 0.05).
Polycystic ovary syndrome is a heterogenoues syndrome with endocrine abnormalities and metabolic dysfuntion that affect reproduction from folliculogenesis to implantation [30, 31]. Prior data showed that ovarian response and IVF outcomes adversely affected and miscarriages are higher in PCOS [31, 32]. These adverse outcomes may partially attribute to hyperandrogenemia and increased intrafollicular androgen levels, thereby increased follicular degeneration [31]. The link between insulin resistance, hyperandrogenism, and ovulatory disorder is very complex in PCOS [30–33]. Furthermore, the impact of adiposity and insulin resistance on IVF outcomes of PCOS is dabatable issue [31]. Our results confirmed the previous data [30] that despite increased total oocyte retrieved in PCOS group, fertilization rate, and clinical pregnancy rates were similar. However, it should be emphasized that these results are COH protocol findings, and could not be generalized to all PCOS population including pregnancy rates of spontaneous cycle or ovulation induction drugs without IVF.
Apart from the other studies, we investigated associations between inflammatory markers and IVF success. The fecundability of women decrease after 35 years. Studies have reported poor IVF outcomes in general population over the age of 40 years [34]. Our data confirmed the prior studies that age was the independent variable affecting pregnancy rates in control group. In PCOS subjects, MPV values were significantly correlated with CPR and implantation rate. PLR levels were positively associated with miscarriages. The inflammatory markers (WCC, neutrophils) were negatively correlated to retrieved oocyte count and oocyte quality (p < 0.05). Logistic regression analysis showed that MPV associated with CPR independent of age, BMI, HOMA-IR levels in PCOS women. These results suggest that inflammation of PCOS is related to adverse IVF outcomes. These preliminary findings need to be supported by further studies.
The limitations of our paper included the use of single center data, infertile population, and retrospective design. To minimize a bias risk the control patient selection, we strictly obeyed the exclusion criteria, and both age and BMI matched women were selected. Another limitation of this study was the determination of adiposity levels that relied on BMI values. Further studies with more specific body fat analysis and comparing NLR and PLR values with CRP, TNF and IL will give us more valuable results. Despite these limitations, this was the first study using NLR, PLR and MPV as markers to determine their associations with IVF outcomes.
In conclusion, NLR and PLR were significantly increased in all PCOS subjects compared to controls. PLR was significantly increased in NW-PCOS compared to control and OB-PCOS. MPV negatively associated with CPR independent of age, BMI and HOMA-IR. These results are supporting the hypothesis that PCOS is a chronic inflammatory process independent of obesity. These results also suggest that inflammation may have a negative impact on IVF outcomes. Future prospective studies with the larger population are needed to elucidate the impact of inflammation on IVF succes.
References
Legro RS, Arslanian SA, Ehrmann DA, Hoeger KM, Murad MH, Pasquali R, Welt CK, Endocrine Society (2013) Diagnosis and treatment of polycystic ovary syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 98(12):4565–4592. doi:10.1210/jc.2013-2350
Orio F Jr, Palomba S, Cascella T, De Simone B, Di Biase S, Russo T, Labella D, Zullo F, Lombardi G, Colao A (2004) Early impairment of endothelial structure and function in young normal-weight women with polycystic ovary syndrome. J Clin Endocrinol Metab 89:4588–45931
Giallauria F, Orio F, Lombardi G, Colao A, Vigorito C, Tafuri MG, Palomba S (2009) Relationship between heart rate recovery and inflammatory markers in patients with polycystic ovary syndrome: a cross-sectional study. J Ovarian Res 2:3. doi:10.1186/1757-2215-2-3
González F, Sia CL, Shepard MK, Rote NS, Minium J (2012) Inflammation in response to glucose ingestion is independent of excess abdominal adiposity in normal-weight women with polycystic ovary syndrome. J Clin Endocrinol Metab 97(11):4071–4079. doi:10.1210/jc.2012-2131
Duleba AJ, Dokras A (2012) Is PCOS an inflammatory process? Fertil Steril 97(1):7–12. doi:10.1016/j.fertnstert.2011.11.023
González F (2012) Inflammation in polycystic ovary syndrome: underpinning of insulin resistance and ovarian dysfunction. Steroids 77(4):300–305. doi:10.1016/j.steroids.2011
Escobar-Morreale HF, Luque-Ramírez M, González F (2011) Circulating inflammatory markers in polycystic ovary syndrome: a systematic review and metaanalysis. Fertil Steril 95(3):1048e1-2–1058e1-2. doi:10.1016/j.fertnstert.2010.11.036
Spritzer PM, Lecke SB, Satler F, Morsch DM (2015) Adipose tissue dysfunction, adipokines, and lowgrade chronic inflammation in polycystic ovary syndrome. Reproduction 149(5):R219–R227. doi:10.1530/REP-14-0435
Orio F Jr, Palomba S, Cascella T, Di Biase S, Manguso F, Tauchmanovà L, Nardo LG, Labella D, Savastano S, Russo T, Zullo F, Colao A, Lombardi G (2005) The increase of leukocytes as a new putative marker of low-grade chronic inflammation and early cardiovascular risk in polycystic ovary syndrome. J Clin Endocrinol Metab 90(1):2–5
Shorakae S, Teede H, de Courten B, Lambert G, Boyle J, Moran LJ (2015) The emerging role of chronic low-grade inflammation in the pathophysiology of polycystic ovary syndrome. Semin Reprod Med 33(4):257–269. doi:10.1055/s-0035-1556568
Barcellos CR, Rocha MP, Hayashida SA, Dantas WS, Dos Reis Vieira Yance V, Marcondes JA (2015) Obesity, but not polycystic ovary syndrome, affects circulating markers of low-grade inflammation in young women without major cardiovascular risk factors. Hormones (Athens) 14(2):251–257. doi:10.14310/horm.2002.1584
Kelly CC, Lyall H, Petrie JR, Gould GW, Connell JM, Sattar N (2001) Low grade chronic inflammation in women with polycystic ovarian syndrome. J Clin Endocrinol Metab 86:2453–2455. doi:10.1210/jc.86.6.2453
Vázquez-Vela ME, Torres N, Tovar AR (2008) White adipose tissue as endocrine organ and its role in obesity. Arch Med Res 39(8):715–728. doi:10.1016/j.arcmed.2008.09.005
Lecke SB, Morsch D, Spritzer PM (2013) Circulating levels and subcutaneous adipose tissue gene expression of pigment epithelium-derived factor in polycystic ovary syndrome and normal women: a case control study. Reprod Biol Endocrinol 11:77. doi:10.1186/1477-7827-11-77
Barber TM, Franks S (2013) Adipocyte biology in polycystic ovary syndrome. Mol Cell Endocrinol 373(1–2):68–76. doi:10.1016/j.mce.2012.10.010
Keskin Kurt R, Okyay AG, Hakverdi AU, Gungoren A, Dolapcioglu KS, Karateke A, Dogan MO (2014) The effect of obesity on inflammatory markers in patients with PCOS: a BMI-matched case-control study. Arch Gynecol Obstet 290(2):315–319. doi:10.1007/s00404-014-3199-3
Yilmaz MA, Duran C, Basaran M (2016) The mean platelet volume and neutrophil to lymphocyte ratio in obese and lean patients with polycystic ovary syndrome. J Endocrinol Invest 39(1):45–53. doi:10.1007/s40618-015-0335-2
Agacayak E, Tunc SY, Sak S, Basaranoglu S, Yüksel H, Turgut A, Gul T (2015) Levels of neopterin and other inflammatory markers in obese and non-obese patients with polycystic ovary syndrome. Med Sci Monit 20(21):2446–2455. doi:10.12659/MSM.894368
Lee CD, Folsom AR, Nieto FJ, Chambless LE, Shahar E, Wolfe DA (2001) White blood cell count and incidence of coronary heart disease and ischemic stroke and mortality from cardiovascular disease in African-American and white men and women. Am J Epidemiol 154:758–764. doi:10.1093/aje/154.8.758
Hoffman M, Blum A, Baruch R, Kaplan E, Benjamin M (2004) Leukocytes and coronary heart disease. Atherosclerosis 172:1–6. doi:10.1016/S0021-9150(03)00164-3. doi:10.1016/S0021-9150(03)00134-5
Templeton AJ, Ace O, McNamara MG, Al-Mubarak M, Vera-Badillo FE, Hermanns T, Seruga B, Ocaña A, Tannock IF, Amir E (2014) Prognostic role of platelet to lymphocyte ratio in solid tumors: a systematic review and meta-analysis. Cancer Epidemiol Biomark Prev 23(7):1204–1212. doi:10.1158/1055-9965
Balta S, Celik T, Mikhailidis DP, Ozturk C, Demirkol S, Aparci M, Iyisoy A (2015) The relation between atherosclerosis and the neutrophil-lymphocyte ratio. Clin Appl Thromb Hemost 1–7. doi:10.1177/1076029615569568
Balta S, Ozturk C (2015) The platelet-lymphocyte ratio: a simple, inexpensive and rapid prognostic marker for cardiovascular events. Platelets 26(7):680–681. doi:10.3109/09537104.2014.979340
Gasparyan AY, Ayvazyan L, Mikhailidis DP, Kitas GD (2011) Mean platelet volume: a link between thrombosis and inflammation? Curr Pharm Des 17:47–58. doi:10.2174/138161211795049804
Dogan BA, Arduc A, Tuna MM, Karakılıc E, Dagdelen I, Tutuncu Y, Berker D, Guler S (2014) Association of mean platelet volume with androgens and insulin resistance in nonobese patients with polycystic ovary syndrome. Int J Endocrinol Metab 12(4):e18642. doi:10.5812/ijem.18642
Silfeler DB, Kurt RK, Yengil E, Un B, Arica S, Baloglu A (2014) Evaluation of Mean Platelet. Volume values in lean women with polycystic ovary syndrome. Pak J Med Sci 30(3):589–592. doi:10.12669/pjms.303.4475
Köşüş N, Köşüş A, Turhan NO (2011) Relationship of ovarian volume with mean platelet volume and lipid profile in patients with polycystic ovary syndrome. Exp Ther Med 2(6):1141–1144
Dasanu CA, Clark BA 3rd, Ichim TE, Alexandrescu DT (2011) Polycystic ovary syndrome: focus on platelets and prothrombotic risk. South Med J 104(3):174–178. doi:10.1097/SMJ.0b013e31820c0172
Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Groupn (2004) Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril 81(1):19–25
Inal HA, Yilmaz N, Gorkem U, Oruc AS, Timur H (2015) The impact of follicular fluid adiponectin and ghrelin levels based on BMI on IVF outcomes in PCOS. J Endocrinol Invest. doi:10.1007/s40618-015-0392-6
Rittenberg V, Seshadri S, Sunkara SK, Sobaleva S, Oteng-Ntim E, El-Toukhy T (2011) Effect of body mass index on IVF treatment outcome: an updated systematic review and meta-analysis. Reprod Biomed Online 23(4):421–439. doi:10.1016/j.rbmo.2011.06.018
Amato MC, Vesco R, Vigneri E, Ciresi A, Giordano C (2015) Hyperinsulinism and polycystic ovary syndrome (PCOS): role of insulin clearance. J Endocrinol Invest 38(12):1319–1326. doi:10.1007/s40618-015-0372-x
Kim CH, Moon JW, Kang HJ, Ahn JW, Kim SH, Chae HD, Kang BM (2012) Effectiveness of GnRH antagonist multiple dose protocol applied during early and late follicular phase compared with GnRH agonist long protocol in non-obese and obese patients with polycystic ovary syndrome undergoing IVF/ICSI. Clin Exp Reprod Med 39(1):22–27. doi:10.5653/cerm.2012.39.1.22
Hull MGR, Fleming CF, Hughes AO, McDermott A (1996) The age-related decline in female fecundity: a quantitative controlled study of implanting capacity and survival of individual embryos after in vitro fertilization. Fertil Steril 65:783–790
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No conflict of interest.
Ethical approval
The local ethics committee approved the study (KOÜ-KAEK 2015/13.
Informed consent
Written informed consent was obtained from the patients.
Rights and permissions
About this article
Cite this article
Çakıroğlu, Y., Vural, F. & Vural, B. The inflammatory markers in polycystic ovary syndrome: association with obesity and IVF outcomes. J Endocrinol Invest 39, 899–907 (2016). https://doi.org/10.1007/s40618-016-0446-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-016-0446-4