Abstract
Background
Chemotherapy negatively affects gonadal function, often resulting in premature ovarian failure (POF) due to ovarian reserve depletion. Mechanisms of gonadotoxicity, such as primordial follicle overactivation and “burnout”, remain to be established. Ovarian tissue cryopreservation (OTC) before treatment plays an important role in safeguarding fertility.
Methods
This is a prospective observational study that aims to evaluate the feasibility of OTC after chemotherapeutic treatment initiation. Patients were divided into 2 groups depending on whether they received chemotherapy before the harvesting procedure (Group 1) or not (Group 2). The main outcomes of this study are serum anti-Mullerian hormone (AMH) levels and histological follicular counts on ovarian tissue biopsies.
Results
Between 2012 and 2020, 79 patients underwent OTC at our Hospital. Follicular counts from the ovarian biopsies of 30 post-pubertal patients and respective serum AMH levels were included in the analysis. AMH levels did not significantly differ between the 2 groups (P = 0.70) as well as the number of primordial follicles (P = 0.73). Ovarian biopsies of patients from Group 1 showed a higher number of primary follicles (P = 0.04) and atretic follicles (P = 0.05) with respect to Group 2.
Conclusions
In conclusion, OTC appears to be feasible even after the start of chemotherapeutic treatment, since in treated patients, the main ovarian reserve indicators (number of primordial follicles and serum AMH levels) were not significantly reduced compared to untreated patients. The “burnout” theory of chemotherapeutic damage to the ovary seems to be supported by the higher number of primary follicles found in the ovaries of patients who received chemotherapy before OTC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Cryopreservation of ovarian tissue after the beginning of chemotherapeutic treatment could be offered to young patients. In chemo-treated patients the number of primordial follicles and serum anti-mullerian hormone (AMH) levels were not significantly reduced compared to untreated patients. |
Introduction
Chemotherapy is one of the main tools modern medicine possesses to tackle cancer and a number of non-oncological pathologies [1]. Thanks to its crucial role in improving disease-free survival rates of cancer patients in recent years [2], more interest has been devoted to quality of life issues of young survivors.
Many female patients experience premature ovarian failure (POF) as a consequence of cytotoxic treatments [3]. Gonadotoxicity varies depending on the type of drug. In particular, alkylating agents, which act independent of the cell cycle, appear to be the most gonadotoxic [3]. The specific mechanisms of chemotherapeutic gonadotoxicity are still not fully understood. The short-term effect of chemotherapy on the ovary is temporary amenorrhea, mainly caused by atresia of growing follicles [4]. The levels of anti-Mullerian hormone (AMH), secreted by growing follicles, have been found to be significantly lower in cancer patients post-treatment compared to age-matched controls [5]. Temporary amenorrhea post-treatment seems to predict earlier onset of menopause, and while it is possible for menses to resume after the end of treatment, there still may be underlying damage to the resting primordial follicle pool [6].
There is a finite number of primordial follicles in humans [7] and an untimely reduction in this non-growing follicular pool is thought to be responsible for the long-term impact of chemotherapy on ovarian function [8]. One of the main mechanisms thought to be involved in long-term chemotherapy-induced ovarian damage is the accelerated activation of primordial follicles, called “follicular burnout” [8].
To safeguard reproductive potential in young female survivors, embryo and mature oocyte cryopreservation are standard practice [9]. Both techniques, which require controlled ovarian stimulation (COS), have successful results in oncological patients [10]. Ovarian tissue cryopreservation (OTC), however, is the only method available to pre-pubertal girls and to those women who cannot delay treatment by the 10–14 days required for COS and oocyte pick-up [9]. There may also be situations in which patients have a real urgency of starting treatment [11], or clinical contraindications to the procedure, such as bulky mediastinal tumors, so that they could resort to OTC only after an initial round of chemotherapy. This also includes patients initially treated with a low-gonadotoxicity regimen developing chemoresistance and needing to switch to a different, more toxic chemotherapy.
OTC, unlike oocyte cryopreservation, could be performed after the beginning of chemotherapy due to the relative resistance of primordial follicles to cytotoxic damage [12]. However, current indications for OTC do not include patients receiving chemotherapy prior to tissue harvest [13]. Births after transplantation of tissue exposed to chemotherapy have actually been reported [12, 12] and a recent study found that chemotherapy before OTC did not influence ovarian function recovery and reproductive outcomes following transplantation [15].
The aim of the present study is to define whether post-chemotherapy OTC is feasible and safe by analyzing the morphologic effects of chemotherapy on biopsies of human ovarian cortical tissue obtained from patients undergoing the procedure, and by comparing histological follicular counts and AMH serum levels with those of patients not exposed to chemotherapy. A comprehensive view of follicular condition post-chemotherapy could also provide deeper knowledge regarding the mechanisms of chemotherapeutic-induced damage to the ovary.
Materials and methods
Study design and ethical approval
This is a prospective observational study evaluating the outcomes of fertility preservation procedures carried out in patients with cancer at the Onco-fertility Unit of San Raffaele Hospital, Milan, Italy from 2012 to 2020. These procedures include fertility-sparing surgery, cryopreservation of oocytes and cryopreservation of ovarian tissue.
The study was approved by the Institutional Review Board of San Raffaele Hospital (protocol Onco-fertility v2.0). All patients included in the analysis signed a written informed consent for the fertility preservation procedure and a consent for the use of personal data and publication.
Participants and study groups
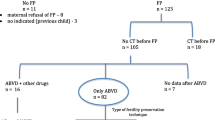
From January 2012 to March 2020, 79 patients underwent OTC at our Unit.
Only patients aged 15 years or older at the time of the procedure were included in this study. Patients with underlying ovarian pathologies such as endometriosis, FOXL2 mutation or ovarian fibrothecoma were excluded. A total of 30 women met the above-mentioned inclusion and exclusion criteria. Out of 30 recruited patients undergoing OTC, the majority [29] had a malignant tumor diagnosis, whereas one had multiple sclerosis, for which chemotherapy had been indicated.
Patients were divided into 2 groups depending on whether they underwent chemotherapy before surgical retrieval of ovarian tissue. Group 1 (n = 15) included patients who received chemotherapy, while Group 2 (n = 15) included those who did not receive chemotherapy.
The majority of Group 1 patients (11 out of 15) received a highly gonadotoxic regimen (i.e., containing alkylating agents) or an intermediate-risk regimen (i.e., containing platinum or doxorubicin) [16]. Pre-procedure AMH serum levels were available for 20 patients. Data on type of tumor and chemotherapeutic regimen were collected.
Histological analysis
Ovarian tissue was retrieved by laparoscopic excision. Ovarian cortex specimens were cut into strips and transported to the storage site at San Raffaele hospital for slow freezing and histological analysis.
All tissue samples were analyzed by the same histopathologist (G. L. T.), who was blind to the chemotherapeutic status of the patients’ tissue. A histological follicular count was performed on 10 different and non-consecutive areas of 1 mm2, using hematoxylin and eosin staining. The thickness of the sections was 5 microns and the distance from one another was 50 microns. Ovarian follicles were classified as primordial follicles (Fig. 1), primary follicles (Fig. 2), early secondary follicles (Fig. 3), late secondary follicles (comprising antral follicles and pre-ovulatory follicles), atretic follicles and dysfunctional follicles. A total of functional follicles in 10 areas of 1 mm2 and 1 mm3 were then calculated. If reports of biopsies of two different strips of tissue from the same patient were found in clinical records, a mean of the number of follicles was considered.
Fibrosis and sclerosis were analyzed in all the 30 available biopsies, with fibrosis indicating the mechanism in which, after any injury, parenchymal ovarian cortical tissue is replaced by connective tissue and sclerosis meaning the hardening of the tissue, not necessarily linked to fibrous tissue deposition [17].
Outcomes
The primary outcomes of the study were AMH serum levels and histological follicular counts on ovarian tissue biopsies (number of primordial, primary, early and late secondary, atretic and dysfunctional follicles).
Statistical analysis
Statistical analyses were carried out using IBM SPSS Statistics software, version 26 for Mac. Descriptive statistics were used to evaluate data distribution. Shapiro–Wilk test was performed to verify normal distribution of data. Spearman correlation analysis was performed to test for the influence of age on histological follicular count. Mann–Whitney U test was used to compare age and AMH levels between the 2 groups. Histological follicular counts were compared between patients who received chemotherapy and patients without prior treatment using logistic regression analysis to adjust for the effect of age. All calculated p-values were two sided and p-values ≤ 0.05 were considered statistically significant.
Results
Patients’ age and pre-procedural AMH levels are reported in Table 1. Age was not significantly different between the two groups.
Correlation analysis was performed to evaluate any correlations between age and histological follicular count. Age was found to be negatively correlated with the number of primordial follicles (r = − 0.56, P = 0.01) and with the total number of functional follicles in 10 areas of 1 mm2 and in 1 mm3 (r = − 0.9, P = 0.006).
In the whole population, 14 patients (46.7%) had a sarcoma diagnosis, 4 patients (13.3%) had a gynecological cancer (1 breast cancer, 2 cervical cancer, 1 endometrial cancer), 5 patients (16.6%) had a lymphoma, 2 patients (6.7%) had a germ cell tumor, 2 patients (6.7%) had a neurological tumor, 2 patients (6.7%) had an unspecified tumor, and 1 patient (3.3%) had multiple sclerosis.
Pathology distribution among the 15 patients receiving chemotherapy, along with pre-OTC chemotherapeutic protocols or drugs can be seen in Table 2.
Seven patients (46%) were treated with a highly gonadotoxic regimen (i.e., containing alkylating agents), 4 patients (27%) with an intermediate-risk regimen (containing platinum compounds or doxorubicin) and 4 patients (27%) received a low-risk regimen (adriamycin/doxorubicin, bleomycin, vinblastine, dacarbazine [ABVD]) (16).
Timing of OTC among chemo-treated patients ranged from 17 days after last chemotherapy administration to 15 months. However, in 10 out of 15 patients (66%), the procedure was carried out less than a month after the last dose.
Values of pre-procedural serum AMH levels and histological follicular counts, corrected by age, were compared between the 2 groups and are reported in Table 3.
AMH values (P = 0.70) and number of primordial follicles (P = 0.73) were slightly lower in Group 1 with respect to Group 2 but still devoid of significance. The number of total functional follicles was similar between the 2 groups.
Ovarian tissue biopsies of patients from Group 1 showed a significantly higher number of primary follicles (P = 0.04) compared to Group 2. The number of atretic follicles was also significantly higher among Group 1 patients (P = 0.05).
Within histological reports from Group 1, 9 (60%) showed a combination of fibrosis and sclerosis and in 6 samples (40%) sclerosis was reported. In Group 2, 9 reports (60%) noted the concurrent presence of fibrosis and sclerosis, 3 (20%) showed fibrosis, 2 (13%) showed sclerosis and in 1 report (7%) neither fibrosis nor sclerosis were present. In 6 biopsies from Group 1 (40%) and 2 biopsies from Group 2 (13%), vascular proliferation was observed.
Discussion
Chemotherapeutic agents, while undoubtedly an essential weapon against cancer and other non-oncological pathologies, give rise to harmful side effects to patients of reproductive age, due to their varying levels of gonadotoxicity. Knowledge about how chemotherapy exactly affects ovarian activity and fertility is fundamental to optimize research about new pharmacological methods to shield the ovary from gonadotoxic agents and to refine the indications for different existing fertility preservation techniques. Among the available options, ovarian cortex cryopreservation, with its subsequent transplant, has the added benefit of restoration of endocrine function [9]. However, having already received chemotherapy is currently a limitation or even a contraindication to the technique.
The purpose of this study was to assess the feasibility of cryopreservation of chemotherapy-exposed ovarian tissue by analyzing ovarian cortex biopsies from patients undergoing cryopreservation and comparing tissue samples which were subject to chemotherapy with those who were not.
It is particularly difficult to obtain human ovarian tissue for research and the amount of donated tissue is small; therefore, the majority of experiments in this field are conducted either on animals or on cultured tissue. Only one study investigating cryopreservation of chemotherapy-exposed human ovarian tissue has been performed so far. This study concluded that pre-chemotherapy OTC is to be preferred, but post-chemotherapy cryopreservation can be considered in younger patients [18]. However, one important limitation of this study was the inclusion of pre- and post-pubertal patients in the same analysis: due to the substantial differences between pre- and post-pubertal ovarian tissue, we decided to focus our analysis only on post-pubertal patients.
The main strength of our study is the evaluation of the effects of chemotherapy on human ovarian cortical biopsies stored at a single center and examined by the same pathologist, who was unaware of the chemotherapeutic status of the patients.
Our results showed that, when comparing histological follicular counts between individuals who had undergone chemotherapy before tissue retrieval and those who had not, most of the differences were non-significant. As expected, the number of atretic follicles was significantly higher in the former cohort and secondary follicles were slightly lower, although not significantly, in the group subject to chemotherapy. These results could support the established concept that chemotherapy reduces the developing follicle pool.
A number of studies have shown a depletion of primordial follicles post-chemotherapy, in human biopsies [19], in xenografts [20] and in cyclophosphamide-treated mice [21]. Although slightly lower in the group whose tissue was exposed to chemotherapy, the mean number of primordial follicles in our study was similar in the 2 groups and serum AMH values were also comparable, consistent with the strong correlation between AMH and the primordial follicle pool [22]. Therefore, our study did not confirm a significant chemotherapy-induced depletion of the ovarian reserve, which is made up of resting follicles. This supports the case for post-chemotherapy OTC to be acceptable for those patients who need to receive chemotherapy immediately after diagnosis and cannot undergo fertility preservation before the initiation of such treatment.
Last but not least, we observed that primary follicles were significantly higher in the group of patients who underwent ovarian tissue retrieval post-chemotherapy. This is curious as primary follicles, intuitively, should follow the same trend as the primordial follicles.
In the ovary, there is a fine balance between suppressing the activation of the majority of dormant primordial follicles via AMH and promoting growth of few at a time, primarily through the PI3K/Akt/mTOR signaling pathway [23]. Chemotherapy is able to disrupt this balance and upregulate the PI3K pathway, initiating a massive over-recruitment of dormant follicles which grow and then die, causing a premature burnout of the reservoir [24]. This phenomenon has been observed in mice, following the administration of different agents [25,26,27,28] and in human follicles in culture [29].
In our data, due to the slightly lower number of primordial follicles in the group who had received chemotherapy, along with the higher number of primary follicles, the implication could be perhaps that resting follicles were recruited and started to develop prematurely. If apoptotic staining in primordial follicles had been performed and had turned out negative, our study would have more confidently backed the “burnout” theory of ovarian reserve depletion.
The main limitations of this study are the relatively small sample of patients included, and the lack of data regarding stromal damage and vascularization. It has been hypothesized that an impairment of ovarian stroma and vessels can be detrimental on the health of follicular reserve itself and interfere with follicular development [30]. In our study, a significant percentage of histological reports from both cohorts showed sclerosis and fibrosis of ovarian tissue.
Within the patients who received chemotherapy, different protocols were employed as shown in Table 2, and for this reason, it was not possible to evaluate any differences between protocols containing specific drugs or identifying dose thresholds to access cryopreservation according to chemotherapeutic schedule. Also, elapsed time between chemotherapeutic treatment and OTC varied consistently among patients.
Finally, another important limitation in this study is the lack of outcomes regarding reprisal of ovarian function and pregnancy rates, as all the patients included in the study have yet to resort to transplantation. Live-births from cryopreserved ovarian tissue have been reported in recent series [31, 32].
Conclusions
Prior chemotherapy, in fact, could no longer be considered a limitation for OTC. The relative increase of primary follicles we observed in chemotherapy-treated tissue could indicate that primordial follicles, following the chemotherapeutic insult, were recruited into the growing pool. However, to confirm the “burnout” hypothesis of primordial follicle depletion in human tissue, which, to our knowledge, has only been observed in mice [25,26,27,28] and in human ovarian tissue cultured with cyclophosphamide in vitro [29], specifically designed molecular assays are required.
References
DeVita VT, Chu E (2008) A history of cancer chemotherapy. Cancer Res 68(21):8643–8653
American cancer society cancer treatment & survivorship facts & figures 2019–2021 Atlanta: american cancer society 2019 Available from: https://www.cancer.org/research/cancer-facts-statistics/survivor-facts-figures.html Accessed on 26/02/2020
Meirow D, Nugent D (2001) The effects of radiotherapy and chemotherapy on female reproduction. Hum Reprod Update 7(6):535–543
Liedtke C, Kiesel L (2012) Chemotherapy-induced amenorrhea an update Geburtshilfe und Frauenheilkunde. Geburtshilfe Frauenheilkd 72(9):809–818
Peigné M, Decanter C (2014) Serum AMH level as a marker of acute and long-term effects of chemotherapy on the ovarian follicular content: A systematic review. Reprod Biol Endocrinol 26(12):26
Partridge A, Gelber S, Gelber RD, Castiglione-Gertsch M, Goldhirsch A, Winer E (2007) Age of menopause among women who remain premenopausal following treatment for early breast cancer: long-term results from International Breast Cancer Study Group Trials V and VI. Eur J Cancer 43(11):1646–1653
Wallace WHB, Kelsey TW (2010) Human ovarian reserve from conception to the menopause. PLoS ONE 5(1):1–9
Meirow D, Biederman H, Anderson RA, Wallace WHB (2010) Toxicity of chemotherapy and radiation on female reproduction. Clin Obstet Gynecol 53(4):727–739
Donnez J, Dolmans MM (2017) Fertility preservation in women. N Engl J Med 377(17):1657–1665
Cardozo ER, Thomson AP, Karmon AE, Dickinson KA, Wright DL, Sabatini ME (2015) Ovarian stimulation and in-vitro fertilization outcomes of cancer patients undergoing fertility preservation compared to age matched controls: a 17-year experience. J Assist Reprod Genet 32(4):587–596
Meirow D, Ra’anani H, Shapira MB et al (2016) Transplantations of frozen-thawed ovarian tissue demonstrate high reproductive performance and the need to revise restrictive criteria. Fertil Steril 106(2):467–474
Practice Committee of the American Society for Reproductive Medicine (2019) Fertility preservation in patients undergoing gonadotoxic therapy or gonadectomy: a committee opinion. Fertil Steril 112(6):1022–1033
Anderson RA, Mitchell RT, Kelsey TW, Spears N, Telfer EE, Wallace WH (2015) Cancer treatment and gonadal function: experimental and established strategies for fertility preservation in children and young adults. Lancet Diabetes Endocrinol 3(7):556–567
Meirow D, Levron J, Eldar-Geva T et al (2005) Pregnancy after transplantation of cryopreserved ovarian tissue in a patient with ovarian failure after chemotherapy. N Engl J Med 353(3):318–321
Poirot C, Fortin A, Lacorte JM et al (2019) Impact of cancer chemotherapy before ovarian cortex cryopreservation on ovarian tissue transplantation. Hum Reprod 34(6):1083–1094
Bedoschi G, Navarro PA, Oktay K (2016) Chemotherapy-induced damage to ovary: mechanisms and clinical impact. Futur Oncol 12(19):2333–2344
Wynn TA, Ramalingam TR (2012) Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat Med 18(7):1028–1040
Abir R, Ben-Haroush A, Felz C et al (2008) Selection of patients before and after anticancer treatment for ovarian cryopreservation. Hum Reprod 23(4):869–877
Oktem O, Oktay K (2007) Quantitative assessment of the impact of chemotherapy on ovarian follicle reserve and stromal function. Cancer 110(10):2222–2229
Oktem O, Oktay K (2007) A novel ovarian xenografting model to characterize the impact of chemotherapy agents on human primordial follicle reserve. Cancer Res 67(21):10159–10162
Meirow D, Lewis H, Nugent D, Epstein M (1999) Subclinical depletion of primordial follicular reserve in mice treated with cyclophosphamide: clinical importance and proposed accurate investigative tool. Hum Reprod 14(7):1903–1907
Hansen KR, Hodnett GM, Knowlton N, Craig LB (2011) Correlation of ovarian reserve tests with histologically determined primordial follicle number. Fertil Steril 95(1):170–175
Sonigo C, Beau I, Grynberg M, Binart N (2019) AMH prevents primordial ovarian follicle loss and fertility alteration in cyclophosphamide-treated mice. FASEB J 33(1):1278–1287
Roness H, Kashi O, Meirow D (2016) Prevention of chemotherapy-induced ovarian damage. Fertil Steril 105(1):20–29
Kalich-Philosoph L, Roness H, Carmely A et al (2013) Cyclophosphamide triggers follicle activation and “burnout”; AS101 prevents follicle loss and preserves fertility. Sci Transl Med 5(185):185ra62
Chang EM, Lim E, Yoon S et al (2015) Cisplatin induces overactivation of the dormant primordial follicle through PTEN/AKT/FOXO3α pathway which leads to loss of ovarian reserve in mice. PLoS ONE 10(12):1–16
Wang Y, Liu M, Johnson SB et al (2019) Doxorubicin obliterates mouse ovarian reserve through both primordial follicle atresia and overactivation. Toxicol Appl Pharmacol 381(April):114714
Zhou L, Xie Y, Li S et al (2017) Rapamycin Prevents cyclophosphamide-induced over-activation of primordial follicle pool through PI3K/Akt/mTOR signaling pathway in vivo. J Ovarian Res 10(1):1–11
Lande Y, Fisch B, Tsur A et al (2017) Short-term exposure of human ovarian follicles to cyclophosphamide metabolites seems to promote follicular activation in vitro. Reprod Biomed Online 34(1):104–114
Spears N, Lopes F, Stefansdottir A et al (2019) Ovarian damage from chemotherapy and current approaches to its protection. Hum Reprod Update 25(6):673–693
Donnez J, Dolmans MM (2015) Ovarian cortex transplantation: 60 reported live births brings the success and worldwide expansion of the technique towards routine clinical practice. J Assist Reprod Genet 32(8):1167–1170
Jensen AK, Macklon KT, Fedder J, Ernst E, Humaidan P, Andersen CY (2016) 86 successful births and 9 ongoing pregnancies worldwide in women transplanted with frozen-thawed ovarian tissue: focus on birth and perinatal outcome in 40 of these children. J Assist Reprod Genet 34(3):325–336
Funding
The authors have not received any funding to support this study.
Author information
Authors and Affiliations
Contributions
RC’s roles included conceptualization, formal analysis and manuscript drafting; GM. contributed to conceptualization, critical discussion of data and manuscript review; GT. and LC performed data curation and formal analysis, and contributed to manuscript drafting; LP, EP, AB, SF and MC contributed to critical discussion of data and manuscript review. All authors read and approved the final version of the manuscript. The present work was performed by RC in partial fulfillment of the requirements for obtaining the PhD degree at Vita-Salute San Raffaele University, Milano, Italy.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest relevant to the present study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cioffi, R., Cervini, L., Taccagni, G. et al. A prospective, observational study of chemotherapy-induced ovarian damage on follicular reserve and maturation. Arch Gynecol Obstet 306, 1723–1729 (2022). https://doi.org/10.1007/s00404-022-06692-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-022-06692-0