Abstract
Purpose
Determine if intravenous iron for antenatal anemia is associated with reduced incidence of postnatal depression (PND) within 12 months.
Methods
This retrospective cohort study included adult women with antenatal anemia (hemoglobin value of < 11.0 g/dL within 3 months before delivery). PND was defined as Edinburgh Postnatal Depression Scale (EPDS) or Patient Health Questionnaire-9 (PHQ-9) ≥ 10. Data on intravenous iron, lowest hemoglobin concentration, EPDS and PHQ-9 scores, insurance status, history of anxiety, depression, chronic pain, and substance use, obstetric complications, labor analgesia, and mode of delivery were obtained. Standardized mean difference (SMD) was estimated and multivariable logistic regression models were constructed with adjustment for potential confounders with absolute SMD of ≥ 0.1.
Results
Data from 3988 women were analyzed. The 368 (9.2%) women who received intravenous iron therapy had lower antenatal hemoglobin levels, were more likely to be African American or single/widowed women, and more commonly had Medicaid coverage, repeat cesarean delivery, and history of depression compared to those who did not receive intravenous iron therapy. Unadjusted analysis showed women who received intravenous iron had higher incidence of PND (18.5%) than those who did not (13.4%) (p = 0.008). Multivariable analysis showed no significant association between intravenous iron and PND incidence (aOR 1.21, 95%CI 0.89–1.63, p = 0.232), although history of depression (aOR 2.42, 95%CI 1.91–3.08, p < 0.001), higher gravidity (aOR 1.09, 95%CI 1.02–1.17, p = 0.016), and Medicaid insurance (aOR 1.44, 95%CI 1.16–1.80, p = 0.001) were independently associated with PND.
Conclusion
Intravenous iron for antenatal anemia was not associated with significant change in the incidence of PND.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Postnatal depression (PND, onset of depression within 12 months after childbirth) is a significant clinical and societal issue. With a prevalence of 12–19% [1] and over 140 million births annually, a large population of postnatal women is at risk of debilitating morbidity including breastfeeding difficulty, impaired social function, and increased risk of substance abuse [2], while their neonates suffer from poor growth or cognitive development, and child abuse [3].
Several factors are associated with PND, including pregnancy-related hormonal changes (fluctuations in estradiol and glucocorticoid levels), psychosocial factors (poor socioeconomic status, lack of social support), poor nutrition (inadequate vitamin D, magnesium, or zinc levels), patient characteristics (higher body mass index, race/ethnicity, lower physical activity level), obstetric factors (repeat cesarean delivery, severe antenatal/postnatal pain), and comorbidity (post-dural puncture headache, preeclampsia, preterm delivery, chorioamnionitis, need for intensive care, history of depression, anxiety, chronic pain, and illicit drug, smoking, or alcohol use) [1, 4,5,6,7,8]. However, many of these factors are not amenable to routine clinical assessment or modification, which limit their utility in predicting or preventing PND.
Antenatal anemia has been associated with PND (OR 1.53, 95%CI 1.32–1.78) [1]. Defined as hemoglobin value of < 11.0 g/dL, antenatal anemia affects approximately 30% of pregnant women [9]. Iron deficiency is the underlying etiology for approximately 75% of antenatal anemia [10], and is postulated to be a PND risk factor as it also alters neurotransmitter homeostasis and monoamine metabolism, and is associated with downregulation of dopamine receptor-1 expression [11, 12], reduction in dopamine and gamma-aminobutyric acid (GABA) levels [11, 12], and suppression of norepinephrine and serotonin signaling [13], all of which have been associated with mood alterations and increased risk of depression [1].
At present, few studies investigated the potential associations between antenatal iron deficiency or iron therapy with PND, particularly within the USA [14]. A case–control study conducted in Tehran [15] reported no association between antenatal oral iron supplementation with PND, and another study conducted in Saudi Arabia [16] showed no association between antenatal anemia and PND. The lack of studies conducted in the USA is concerning, as socioeconomic and environmental factors have great influence on PND incidence [1, 17] and limits the generalizability of existing data to this population. Furthermore, prior studies examined oral rather than intravenous iron formulations. Oral iron is associated with delayed onset of clinical effects of up to two months, poor duodenal absorption, and gastrointestinal adverse effects, which may limit compliance and efficacy in treating iron deficiency [18]. Hence, this study focused on the use of intravenous iron therapy, which has a shorter onset time, better efficacy, and fewer adverse effects compared to oral formulations [18].
Given the biological link between iron deficiency and PND, and the possibility that pre-emptive antenatal iron therapy may forestall PND onset, this study aimed to investigate the potential association between intravenous iron therapy for antenatal anemia and the incidence of PND within 12 months following childbirth. We hypothesized that intravenous iron will be associated with clinically significant reduction in PND incidence of at least 10% compared to anemic women who did not receive intravenous iron therapy.
Materials and methods
This retrospective cohort study was approved by Duke University Healthcare System (DUHS) Institutional Review Board (Pro00103540) and was exempt from informed patient consent requirements as all patient data were fully de-identified in accordance with the Health Insurance Portability and Accountability Act (HIPAA). This article conforms to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines.
Adult women (≥ 18 years old) who delivered via cesarean or vaginal delivery at DUHS between June 2013 and August 2019, with antenatal anemia (hemoglobin value of < 11.0 g/dL within 3 months prior to delivery), and who had Edinburgh Postnatal Depression Scale (EPDS) or Patient Health Questionnaire-9 (PHQ-9) scores documented within 12 months after childbirth were included. We excluded women with missing delivery date, missing antenatal hemoglobin concentrations, missing EPDS or PHQ-9 scores, those who received transfusion of packed red cells during gestation or within 12 months of childbirth, or with history of chronic anemia requiring iron supplementation, erythropoietin, or blood transfusions.
The principal exposure under investigation is the presence versus absence of antenatal intravenous iron therapy, determined by chart review of the electronic medical record (EMR, EPIC, MaestroCare, Wisconsin). We investigated the association between intravenous iron therapy and PND (primary outcome), defined as EPDS or PHQ-9 ≥ 10 within 12 months after delivery. The routine practice at DUHS is to screen for PND at the first postnatal clinic visit typically within three months after childbirth, during which EPDS or PHQ-9 scores are recorded in the EMR. A minority of women are screened multiple times, and in these women the highest EPDS or PHQ-9 scores were analyzed.
The EPDS is a 10-item questionnaire which is the most widely used assessment tool for PND. EPDS has been extensively studied and validated in both antenatal and postnatal patients [19] and against other diagnostic criteria for depression such as the Diagnostic and Statistical Manual for Mental disorders (DSM) [20]. The EPDS threshold of 10 was chosen to retain high positive and low negative likelihood ratios; a positive likelihood ratio > 5 and negative likelihood ratio < 0.2 would provide evidence to include or exclude PND, respectively [21]. It was estimated that EPDS ≥ 10 yields a positive likelihood ratio of 5.9 and negative likelihood ratio of 0.15 [22]. Similarly, the PHQ-9 is a commonly used screening tool assessing for major depressive symptoms, with a recommended threshold of PHQ-9 ≥ 10 [23].
Known PND risk factors that may confound the association between iron therapy and PND were identified from literature review [4,5,6], and corresponding data were extracted from the EMR using ICD-9 codes. These included patient characteristics (age, body mass index, race, marital status), payor category, clinical factors including lowest recorded hemoglobin level during gestation, gravidity/parity, gestational age, mode of delivery, repeat cesarean delivery, provision of labor analgesia, post-dural puncture headache, preeclampsia, preterm delivery, gestational diabetes, chorioamnionitis, need for intensive care, and history of depression, anxiety, autoimmune disease, chronic opioid use, and chronic pain. We also collected data about the use of oral iron therapy, tobacco, alcohol, and illicit drugs. Accuracy of data collection was assessed via randomized checks by an independent investigator, and discrepancies were resolved by review of the electronic records.
The study cohort was classified according to the presence or absence of antenatal intravenous iron therapy. At DUHS, pregnant women are screened for anemia at approximately 28 weeks’ gestation, and referred to the preoperative anemia clinic (PAC) for diagnosis and treatment of iron-deficiency anemia with intravenous iron as described previously [24]. The PAC began in 2014 as a pilot program for anemic pregnant women deemed at high risk of transfusion (previous cesarean delivery, multiple gestation, severe anemia), and during the study period expanded to accept referrals of all obstetric patients with anemia. Briefly, following laboratory-confirmation of iron-deficiency anemia, women are treated with a single administration of low molecular weight (LMW) iron dextran (1–1.5 g based on the calculated iron deficit). Alternate formulations (ferric gluconate, iron sucrose, and ferumoxytol) are used in women with history of LMW iron dextran allergy or based on their insurance coverage and preference. Those patients with iron deficiency without anemia or with mild iron-deficiency anemia are given oral therapy.
Descriptive statistics were used to examine patient characteristics, obstetric factors or complications, labor analgesia, history of depression, anxiety, or chronic pain, and drug or substance use according to the presence or absence of intravenous iron therapy. Categorical variables are reported as number (percentage) and continuous variables are reported as median [interquartile range]. Variable balance between the groups was assessed using standardized mean differences (SMD), with an absolute value ≥ 0.1 considered to indicate significant imbalance. The SMD is calculated as a ratio between the difference in means or proportions and the pooled standard deviation. Multivariable logistic regression models were constructed with PND as the outcome and including variables with absolute SMD ≥ 0.1 between the groups. Intravenous iron therapy was forced into the model. Interaction terms between the variables and presence or absence of intravenous iron therapy were assessed and included if statistically significant. Statistical significance was defined as p value < 0.05. All analyses were conducted using SAS version 9.4 (SAS Institute; Cary, NC) and R version 3.5.0.
Results
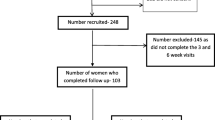
In total, 29,596 women delivered at DUHS during the study period with 25,608 women (86.5%) excluded mainly due to the absence of anemia (n = 18,844, 63.7%) and missing EPDS or PHQ-9 data (n = 5892, 19.9%). Data from 3988 women were analyzed; 368 (9.2%) received intravenous iron therapy, while 3620 (90.8%) did not (Fig. 1).
Patient characteristics and clinical factors are summarized in Table 1. Women who received intravenous iron therapy had lower antenatal hemoglobin levels, comprised of a larger proportion of African American or single or widowed women, more commonly had Medicaid coverage, underwent more repeat cesarean delivery, and more commonly had a history of depression compared to women who did not receive intravenous iron therapy. Of the 434 women with history of depression, 74 (17.1%) received intravenous iron therapy and 21 (28.4%) developed PND, conversely, 360 women (82.9%) did not receive intravenous iron therapy and 97 (26.9%) developed PND. Overall, there were no significant differences in age, body mass index, mode of delivery, utilization of epidural labor analgesia, post-dural puncture headache, preeclampsia, preterm delivery, gestational diabetes, chorioamnionitis, need for intensive care, history of anxiety, autoimmune disease, chronic pain, and tobacco, alcohol, or illicit drug use between the groups.
In unadjusted analysis, women who received intravenous iron therapy had higher incidence of PND (18.5%) compared to those who were not treated (13.4%) (OR 1.46, 95% CI 1.11–1.94, p = 0.008). After adjustment for potential confounders, multivariable analysis (Table 2) showed that intravenous iron was not associated with significant difference in the incidence of PND (aOR 1.21, 95%CI 0.89–1.63, p = 0.232). In addition, history of depression (aOR 2.42, 95%CI 1.91–3.08, p < 0.001), higher gravidity (aOR 1.09, 95%CI 1.02–1.17, p = 0.016), and Medicaid insurance (aOR 1.44, 95%CI 1.16–1.80, p = 0.001) were independently associated with PND. No significant interactions between intravenous iron and any of the variables were detected, including a history of depression (OR 0.77, 95%CI 0.40–1.49, p = 0.438).
Discussion
In this retrospective cohort study, we found no significant association between antenatal intravenous iron therapy and PND incidence after adjustment for potential confounders.
Few studies have investigated the association between antenatal iron therapy and PND incidence, particularly within the US obstetric population and pertaining to use of intravenous iron formulations. Prior studies conducted in Tehran and Saudi Arabia [15, 16] have reported no significant association between oral iron and PND, however, a multitude of socioeconomic and environmental factors are known to influence PND incidence [1, 4,5,6, 17] and may limit the generalizability of these studies to the USA. In this context, our results provide important evidence that antenatal iron therapy for anemia is not associated with significant change in PND incidence in women delivering at DUHS. Furthermore, unlike prior studies that examined oral iron formulations, our study investigated the use of intravenous iron, which have greater efficacy, faster onset, reduced gastrointestinal adverse effects, and are in most cases administered through a single dose, thereby mitigating issues with patient compliance [18].
A recent review reported that postnatal iron therapy was associated with reduced PND incidence, but a similar association was not found when iron was administered in the antenatal period [14]. However, the review was limited by the scarcity of studies investigating antepartum anemia, and meta-analysis was precluded by heterogeneity in methodology within the included studies. Moreover, the review did not differentiate between the use of oral versus intravenous iron formulations. Nonetheless, it is possible that the development of postnatal depressive symptoms exacerbates iron deficiency, either because women with PND are less compliant with iron therapy, or alternatively, because factors that increase PND risk may also predispose to iron deficiency. For instance, a longitudinal study by Tran et al. [17] showed that social factors such as partner violence, childhood abuse, lower socioeconomic status, and coincidental life adversity were predictive of higher EPDS scores, and in turn, reduce the likelihood that these women will be compliant with iron therapy or preventive healthcare. Hence, a reciprocal relationship may exist between postnatal iron deficiency and PND, which may not be apparent in the antenatal period prior to the onset of depressive symptoms.
In addition, the relationship between iron deficiency and PND may occur via the influence of intermediate factors. In a study by Eckerdal et al. [25] postpartum hemorrhage, though not directly associated with PND, resulted in anemia and a negative delivery experience that were in turn associated with increased PND incidence, leading to the suggestion that these intermediate factors may have mediating roles in PND development. It is possible that the absence of these intermediate factors during the antenatal period may have mitigated the risk of developing PND in women with iron-deficiency anemia.
In comparison to prior case–control studies investigating the association between iron therapy and PND, our study analyzed a large cohort of women with antenatal anemia, and obtained detailed data from the EMR which permitted analysis and adjustment for a large number of potential confounding variables specific to women delivering at DUHS. Furthermore, our study examined the use of intravenous iron therapy, which is more efficacious than oral formulations and avoids the issue of treatment non-compliance. Finally, our definition of PND based on EPDS and PHQ-9 scores ≥ 10 is consistent with commonly used PND screening practices and validation studies [19, 22, 23].
However, our study has limitations inherent to retrospective analyses. Although we have adjusted for the effects of known PND risk factors, the retrospective design precluded the analysis of other potential confounders such as poor social support, unplanned pregnancy, and pain intensity [4,5,6]. Given the significant differences in certain characteristics between the two groups, it is possible that our results may be influenced by confounding variables that were unknown or unaccounted for. Moreover, extracting clinical data from the EMR using ICD-9 codes was shown to have high specificity but relatively poor sensitivity [26], which may have affected the data regarding important confounding variables and in turn increases the risk of residual confounding.
Another limitation is the possibility of classification error; all women who received intravenous iron had laboratory diagnosis of iron-deficiency anemia prior to treatment, but a proportion of women who did not receive intravenous iron may have non-iron deficiency anemia. However, since iron deficiency is responsible for up to 75% of antenatal anemia cases [10], the confounding effects of non-iron deficiency anemia on PND are likely to be minimal. Also, evaluation of serum iron levels following intravenous iron therapy was not routinely performed at DUHS, although the use of quantitative investigations such as serum ferritin levels in pregnant women is controversial due to the lack of obstetric-specific ferritin thresholds and potential influence of pregnancy-related changes in acute phase protein levels and iron utilization [27].
Our findings may be limited by selection bias. It is likely that women who were referred for and received intravenous iron therapy had more significant iron-deficiency anemia compared to those who did not, given that the study period spans across the implementation of the PAC program, and may therefore be at greater risk of developing PND. Although multivariable analysis was utilized to adjust for possible confounders, there is always the possibility of unknown confounders that we could not measure and account for in our analysis. In addition, the exclusion of 20% of women due to the absence of postnatal EPDS or PHQ-9 scores may result in selection bias since women with PND may not be compliant with postnatal care and PND screening. It is common practice for pregnant women to receive oral supplements containing iron, and additional oral iron supplementation may be given to those with mild iron deficiency or non-iron deficiency anemias. Information regarding the use of oral supplements is not routinely recorded in the EMR, since it is usually taken over the counter rather than by prescription, and hence cannot be accounted for in our analysis. Finally, we note that only 9.2% of anemic women received intravenous iron therapy, likely because standardized management of antenatal anemia with intravenous iron therapy was only initiated in 2014 and slowly expanded to include all cases of antenatal anemia during the study period.
To conclude, our results suggest that antenatal intravenous iron therapy in anemic women delivering at DUHS is not associated with significant change in PND incidence within 12 months after childbirth. Future research should focus on validating our findings in other US obstetric populations and investigating the potential role of intermediate risk factors on PND development.
Availability of data and materials
De-identified data are available upon reasonable request.
Code availability
Not applicable.
References
Kang SY, Kim H-B, Sunwoo S (2020) Association between anemia and maternal depression: a systematic review and meta-analysis. J Psychiatr Res 122:88–96
Grigoriadis S, VonderPorten EH, Mamisashvili L et al (2013) The impact of maternal depression during pregnancy on perinatal outcomes: a systematic review and meta-analysis. J Clin Psychiatry 74:e321–e341
Dennis CL, McQueen K (2009) The relationship between infant-feeding outcomes and postpartum depression: a qualitative systematic review. Pediatrics 123:e736–e751
Chandrasekaran N, De Souza LR, Urquia ML et al (2018) Is anemia an independent risk factor for postpartum depression in women who have a cesarean section? - A prospective observational study. BMC Pregnancy Childbirth 18:400
Evagorou O, Arvaniti A, Samakouri M (2016) Cross-cultural approach of postpartum depression: manifestation, practices applied, risk factors and therapeutic interventions. Psychiatr Q 87:129–154
Osborne LM, Monk C (2013) Perinatal depression–the fourth inflammatory morbidity of pregnancy?: Theory and literature review. Psychoneuroendocrinology 38:1929–1952
Catala P, Suso-Ribera C, Marin D, Penacoba C (2021) Predicting postpartum post-traumatic stress and depressive symptoms in low-risk women from distal and proximal factors: a biopsychosocial prospective study using structural equation modeling. Arch Gynecol Obstet 303:1415–1423
Hassdenteufel K, Feisst M, Brusniak K et al (2020) Reduction in physical activity significantly increases depression and anxiety in the perinatal period: a longitudinal study based on a self-report digital assessment tool. Arch Gynecol Obstet 302:53–64
Guidelines WHO (2012) Daily iron and folic acid supplementation in pregnant women. World Health Organization, Geneva
Massot C, Vanderpas J (2003) A survey of iron deficiency anaemia during pregnancy in Belgium: analysis of routine hospital laboratory data in Mons. Acta Clin Belg 58:169–177
Beard JL, Connor JR (2003) Iron status and neural functioning. Annu Rev Nutr 23:41–58
Sheikh M, Hantoushzadeh S, Shariat M, Farahani Z, Ebrahiminasab O (2017) The efficacy of early iron supplementation on postpartum depression, a randomized double-blind placebo-controlled trial. Eur J Nutr 56:901–908
Kim J, Wessling-Resnick M (2014) Iron and mechanisms of emotional behavior. J Nutr Biochem 25:1101–1107
Wassef A, Nguyen QD, St-Andre M (2019) Anaemia and depletion of iron stores as risk factors for postpartum depression: a literature review. J Psychosom Obstet Gynaecol 40:19–28
Ezzeddin N, Zavoshy R, Noroozi M, Sarichloo ME, Jahanihashemi H (2016) The association between postpartum depression and pica during pregnancy. Global J Health Sci 8:120
Alharbi AA, Abdulghani HM (2014) Risk factors associated with postpartum depression in the Saudi population. Neuropsychiatr Dis Treat 10:311–316
Tran TD, Biggs BA, Tran T et al (2013) Psychological and social factors associated with late pregnancy iron deficiency anaemia in rural Viet Nam: a population-based prospective study. PLoS ONE 8:e78162
Shi Q, Leng W, Wazir R et al (2015) Intravenous iron sucrose versus oral iron in the treatment of pregnancy with iron deficiency anaemia: a systematic review. Gynecol Obstet Invest 80:170–178
Phillips J, Charles M, Sharpe L, Matthey S (2009) Validation of the subscales of the Edinburgh Postnatal Depression Scale in a sample of women with unsettled infants. J Affect Disord 118:101–112
Smith-Nielsen J, Matthey S, Lange T, Vaever MS (2018) Validation of the Edinburgh Postnatal Depression Scale against both DSM-5 and ICD-10 diagnostic criteria for depression. BMC Psychiatry 18:393
Egger M, Davey Smith G, Altman D (2008) Systematic reviews in health care: meta-analysis in context. Wiley, Hoboken
Gibson J, McKenzie-McHarg K, Shakespeare J, Price J, Gray R (2009) A systematic review of studies validating the Edinburgh Postnatal Depression Scale in antepartum and postpartum women. Acta Psychiatr Scand 119:350–364
Manea L, Gilbody S, McMillan D (2012) Optimal cut-off score for diagnosing depression with the Patient Health Questionnaire (PHQ-9): a meta-analysis. CMAJ 184:E191–E196
Guinn NR, Cooter ML, Maisonave Y et al (2020) How do I develop a process to effectively treat parturients with iron deficiency anemia? Transfusion 60:2476–2481
Eckerdal P, Kollia N, Lofblad J et al (2016) Delineating the association between heavy postpartum haemorrhage and postpartum depression. PLoS ONE 11:e0144274
Quan H, Li B, Saunders LD et al (2008) Assessing validity of ICD-9-CM and ICD-10 administrative data in recording clinical conditions in a unique dually coded database. Health Serv Res 43:1424–1441
Pavord S, Daru J, Prasannan N et al (2020) UK guidelines on the management of iron deficiency in pregnancy. Br J Haematol 188:819–830
Acknowledgements
We would like the acknowledge the important contributions of Dr. John Williams and Dr. Huiman Barnhart for reviewing and appraising the final draft of this manuscript. We would also like to acknowledge Dr. Rebecca Schroeder and Mr Ashok Bhatta for their assistance with data extraction from the electronic medical records.
Funding
This study was funded by department funds. This research did not receive funding from the public, commercial, or not-for-profit sectors. No funding was received from the National Institutes of Health, Wellcome Trust, or the Howard Hughes Medical Institute.
Author information
Authors and Affiliations
Contributions
HST: protocol development, data collection, manuscript writing. NRG: protocol development, data collection, manuscript writing. MEF: data collection, data analysis, manuscript writing. ASH: protocol development, data collection, manuscript writing, overall supervision.
Corresponding author
Ethics declarations
Conflict of interest
The authors report no potential conflicts of interest or competing interests.
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki, and approval was granted by Duke University Healthcare System Institutional Review Board (Pro00103540).
Consent to participate
Exempt from informed patient consent requirements as all patient data were fully de-identified in accordance with the Health Insurance Portability and Accountability Act (HIPAA).
Consent for publication
No individual data is included in this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tan, H.S., Guinn, N.R., Fuller, M.E. et al. The association between intravenous iron for antenatal anemia and postnatal depression: a retrospective cohort study. Arch Gynecol Obstet 306, 1477–1484 (2022). https://doi.org/10.1007/s00404-022-06417-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-022-06417-3