Abstract
Purpose
Evaluating early iron supplementation in non-anemic mothers with postpartum depression (PPD).
Methods
This randomized, double-blind, placebo-controlled trial evaluated 70 mothers with PPD. One week after delivery, the mothers were randomly allocated in the iron-treated (50 mg elemental iron/daily) and placebo-treated groups. After 6 weeks, the improvement of PPD symptoms was compared between the groups.
Results
Ferritin significantly increased in the iron-treated group (p < 0.001), but not in the placebo group (p = 0.09). After intervention, ferritin was higher in the iron-treated group (medians: 78.2 vs. 37 mg/dl, p = 0.01). The rate of iron deficiency significantly decreased in the iron-treated group (p = 0.009), but not in the placebo group (p = 0.4). After intervention, the rate of iron deficiency was higher in the placebo group (31.4 vs. 8.5 %, p = 0.01). The Edinburgh Postnatal Depression Scale (EPDS) score significantly decreased in the iron-treated group (p < 0.001), but not in the placebo group (p = 0.13). After intervention, the EPDS score was lower in the iron-treated group (medians 9 vs. 12, p = 0.01). The improvement rate for PPD was significantly higher in the iron-treated group (42.8 vs. 20 %, p = 0.03). After intervention, mothers with continued PPD had lower ferritin than the improved mothers (41.8 vs. 67 mg/dl, p = 0.03). Mothers with continued depression had higher rate of iron deficiency compared to the improved mothers (27.1 vs. 4.5 %, p = 0.02).
Conclusions
Early iron supplementation in mothers with PPD significantly improves the iron stores and causes a significant improvement in PPD with a 42.8 % improvement rate during 6 weeks. Continued PPD might be related to the lower postpartum ferritin levels in untreated mothers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Postpartum depression (PPD) is a non-psychotic depressive episode that begins within or extends into the postpartum period [1]. The diagnosis of PPD is based upon the same symptomology as any other major depressive episode and manifests as crying spells, insomnia, depressed mood, fatigue, anxiety, negative maternal attitudes, and poor concentration [2]. While most countries report a prevalence of 10–15 %, certain countries have reported a PPD prevalence of nearly 60 % [3]. The observed disparities in PPD prevalence appear to be due to the differences among different societies in reporting styles, cultural variables, socioeconomic levels, social support, stress, nutrition, and differences in the perception of mental health and its stigma [3].
Mothers with PPD exhibit fewer positive parenting behaviors, such as playing with their children, breastfeeding, and taking safety precautions [4]. They also perceive more difficulties in the parenting role, which can lead to increased child behavioral issues and cause further parent–child conflict. Additionally, studies have shown that the infants of mothers with PPD continue to experience adverse physical and mental health problems into the school age and adolescent periods [4, 5].
Although the etiology remains unknown, deficiencies in serum trace elements during the postnatal period have been suggested as important contributors to PPD in addition to hormonal and biological changes [6–11]. Iron deficiency is the leading single nutrient deficiency in women throughout the world. Given that many studies have documented the association of iron deficiency anemia and/or low ferritin with PPD [7–11], dietary intervention and iron supplementation subsequently emerged as potential means to reduce the incidence of PPD [12, 13].
Following an extensive search, we encountered a gap in this area of the literature; there are no prospective controlled studies concerning iron supplementation in the postpartum period [14, 15]. One study has assessed the effect of iron supplementation on emotions and cognition in anemic postpartum patients when administered at the 6th postnatal week [9]. In another study, supplementation with a preparation containing several vitamins and minerals, including 60 mg of iron, was compared to placebo and demonstrated a significant decrease in PPD in the intervention group [16]. Given that many women with PPD experience symptoms during the first postpartum month, studies are needed to evaluate the effect of iron supplementation during this crucial period.
It is well documented that iron deficiency progresses through several stages [17–19]. Iron deficiency without anemia represents the first stage of iron deficiency and is usually underestimated and not diagnosed due to normal hematologic indices. Iron deficiency without anemia can have numerous adverse effects on the brain and mental health [17–19]. It is not clear what percentage of women with PPD have iron deficiency without anemia or whether there are any associations between this stage of iron deficiency and PPD.
We undertook this study to evaluate the iron storage status in women with PPD and, further, to assess whether early iron supplementation (starting from the second postnatal week) in women with PPD is associated with any improvements in PPD symptoms and iron stores.
Methods
Study population and study design
This randomized, double-blind, placebo-controlled trial evaluated 70 mothers with PPD on day 7 after delivering a healthy term infant at the Vali-Asr Teaching Hospital of Tehran University of Medical Sciences, Tehran, Iran, from November 2013 through September 2014. Postpartum mothers were considered eligible if they were 20–40 years old, had delivered a healthy term infant with adequate weight for gestational age through an uncomplicated elective cesarean section at our institute, did not have any medical illness, and were screened positive for PPD based on the Edinburgh Postnatal Depression Scale (EPDS) [20]. Of the 260 mothers screened using the EPDS, 80 of the women screened positive (EPDS score ≥11) [21] and were considered eligible for the study. Subsequent exclusion criteria were as follows: having postpartum anemia [hemoglobin (Hb) <10.5 mg/dl], thalassemia, antepartum or heavy postpartum hemorrhage, a history of any psychiatric disorder before or during pregnancy, any medical illness upon enrollment, or a family history of psychiatric disorders; not having PPD based on the psychiatric interview; and refusing to provide informed consent to participate in the study. Ten postpartum women were excluded due to these criteria.
The British Committee for Standards in Hematology (BCSH) and the World Health Organization (WHO) define anemia as Hb <10.5 mg/dl in the third gestational trimester and Hb <10 mg/dl in the postpartum period [22, 23]. Given our focus on the early postpartum period, anemia was defined as Hb <10.5 mg/dl for this study [24].
A computerized random number generator was used for sequence generation, which was carried out by M.S. Simple randomization with a 1:1 allocation ratio was used in this study. We used consecutive opaque envelopes to conceal treatment allocation, which was performed by O.E. Importantly, the envelopes were opaque when held to the light, opened sequentially, and opened only after the participant’s name and other details were written on the appropriate envelope. The implementation of assignments was carried out by S.H.
This study utilized double-blinding. M.S performed the blinding, and all of the healthcare providers, participants, and data collectors were blinded to the treatment agents in use (iron vs. placebo).
The intervention, data and specimen collection
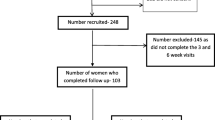
One week after delivery, eligible mothers were contacted by phone and invited to participate in the study. After explaining the procedural details of the experiment, mothers who agreed to enroll were requested to complete the EPDS. The EPDS instrument was used due to its high sensitivity, specificity, and validity in detecting PPD. It has a score that ranges from 0 to 30. Mothers were considered to have possible PPD when the EPDS score was ≥11. The cutoff was set at 11, as studies have shown this cutoff to maximize the sensitivity and specificity in detecting PPD to 100 and 92 %, respectively [21]. Mothers who had an EPDS score ≥11 were interviewed by a psychiatrist using the Structured Clinical Interview for DSM-IV. Based on the DSM-IV criteria, depressive symptoms must be present for at least 2 weeks. Therefore, the mothers’ mental health during the last gestational week and the first postnatal week was also evaluated during the psychiatric interview, allowing for distinction of PPD from other more transient and mild conditions, such as baby blues. Only mothers who had an EPDS score ≥11 and who were diagnosed with PPD based on the psychiatric interview were enrolled in the study. An informed written consent was obtained from all the participants. Upon enrollment, a standardized questionnaire was completed for every participant through interviews and medical records. A blood sample was drawn from the participants to assess Hb, hematocrit (HCT), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), mean cell volume (MCV), serum iron, and ferritin levels. The mothers were then randomly allocated into two groups: (1) the intervention (iron treated) group and (2) the control (placebo treated) group (Fig. 1).
The participants in the intervention group received ferrous sulfate tablets containing 50 mg of elemental iron once daily, while the control group received a placebo once daily. The placebo consisted of cellulose tablets that were identical in shape and color to the ferrous sulfate tablets. After 6 weeks, the participants were visited by the same psychiatrist and asked to repeat the EPDS. Lastly, an additional blood sample was obtained to determine iron stores and hematologic indices.
The dose for iron supplementation was chosen based on previous studies that documented the significant improvement of serum ferritin levels with 50 mg of iron in women who had iron deficiency without anemia [19, 25]. Furthermore, the timeframe was set at 6 weeks, given that the first postnatal weeks represent a crucial time in forming the mother–infant relationship and improvement of PPD during this time period could significantly reduce its adverse effects. Additionally, it has been shown that 6 weeks of iron supplementation significantly improves iron stores [19].
The primary outcome of our study was the improvement in PPD symptoms (based on both a negative psychiatric interview and the decrease in the EPDS score to <11). The secondary outcomes were the rate of iron deficiency without anemia (serum ferritin <15 mg/dl and Hb ≥10.5 mg/dl) [17–19] and the serum ferritin, iron, and Hb levels of the participants. The normal range for serum ferritin at our institution was 15–250 mg/dl.
This study was approved by the Research Deputy and the Ethics Committee of Tehran University of Medical Sciences on 20/08/2012 (reference number: 61845-18281). Additionally, it was prospectively registered at the Iranian Registry of Clinical Trials (www.irct.ir), which is a Primary Registry in the WHO Registry Network. (Registration Number = IRCT2012082210642N1).
Of note, changes were made to the study design after registration. Namely, the intention of the registered study was to be an open-label study, and the participants were also to be categorized into groups of iron-treated and non-treated individuals. Nevertheless, given that this study evaluates an important psychological factor, the outcomes had the potential to be strongly affected by the placebo effect biasing the results. Therefore, some months after the registration period but before the recruitment of participants, the company that made the drug was contacted to make a placebo for the study that was completely identical to the iron tablets. The study design was then changed to a double-blind placebo-controlled trial.
Statistical analysis
The sample size was calculated presuming an improvement rate of 15 % without treatment and anticipating a 30–45 % increase with treatment, for an overall power of 80 % and an alpha of 0.05. All of the statistical analyses were performed using SPSS statistical software (version 18.0.0: PASW, Chicago, IL). Data were represented by use of median, mean, standard deviation (SD), and percentages. For the normally distributed data, we used a t test for independent samples and a t test for paired samples; for the skewed data, the Mann–Whitney U test and the paired sign test were used. Chi-squared analysis and Fisher’s exact tests were also used. The level of statistical significance was set at p < 0.05.
Results
Descriptive statistics
A total of 260 postpartum mothers who had delivered a healthy term infant with adequate weight for gestational age through uncomplicated elective cesarean section were screened for PPD using the EPDS on the seventh postnatal day. Of these 260 mothers, 80 screened positive for PPD (all had EPDS score ≥11) and were considered initially eligible to participate in the study. Nevertheless, ten mothers were excluded as a result of meeting the following criteria: five did not have PPD based on the psychiatric interview, two had a history of previous psychiatric disorders, and three had experienced a heavy postpartum hemorrhage and/or anemia. Thus, 70 postpartum mothers with PPD at 7 days after delivering a healthy term infant were enrolled. A total of 35 mothers were randomly assigned to the intervention group to receive 50 mg of iron supplementation once daily, and the other 35 mothers were randomly allocated to the control group to receive a placebo once daily.
Upon enrollment, the mean ± standard deviation (SD) for the participants’ age was 31.8 ± 4.8 years, the EPDS score was 13.2 ± 4 (median 12, range 11–30), the Hb was 12.5 ± 1.1 mg/dl, and the ferritin was 37 ± 39.7 mg/dl (median 24.8, range 8–212). Overall, 26 participants (37.1 %) had iron deficiency without anemia. Demographically, 47 participants (67.1 %) had academic educations, 42 (60 %) were unemployed, and 35 (50 %) were primiparous.
No statistically significant differences were observed in the demographics and iron stores upon enrollment between those randomized to the intervention versus the control groups (Table 1).
Iron supplementation and postpartum depression
After the intervention, the mean EPDS score was found to be significantly decreased in the iron-treated group (p < 0.001), whereas in the placebo group, there was no statistically significant change (p = 0.13) (Table 2). The EPDS score was also significantly lower in the intervention group than in the placebo group [median (range): 9 (6–19) vs. 12 (8–17), U = 410, Z = −2.39, p = 0.01] (Table 3). Lastly, the improvement rate (percentage of women who had a negative psychiatric interview and an EPDS score <11) was significantly higher in the intervention group than in the placebo group (42.8 vs. 20 %, p = 0.03) (Table 3).
Iron supplementation and iron stores
Following supplementation, the serum ferritin level significantly increased in the iron-treated group (p < 0.001), while in the placebo group, no statistically significant change was observed (p = 0.09) (Table 2). At the end of the study, serum ferritin levels were also significantly higher in the intervention group than in the placebo group [median (range) 78.2 (10.8–200) vs. 37 (4.6–153) mg/dl, U = 395, Z = −2.55, p = 0.01] (Table 3). The rate of iron deficiency significantly decreased in the intervention group (p = 0.009), whereas no significant change was seen in the placebo group (p = 0.4) (Table 2). Lastly, the rate of non-anemic, iron-deficient mothers was significantly higher in the placebo group than in the intervention group (31.4 vs. 8.5 %, p = 0.01), while the serum iron, Hb, HCT, MCV, and MCH levels were not significantly different between the two groups (Table 3).
Iron stores and postpartum depression
We evaluated the association between ferritin levels and depression in the entire study group, including both the iron-treated and placebo-treated groups. Mothers who were still depressed had a significantly lower ferritin level than the mothers whose symptoms were resolved (median ferritin levels were 41.8 vs. 67 mg/dl, respectively, p = 0.03). In addition, mothers with continued experiencing depression had higher rates of decreased ferritin level (serum ferritin < 15 mg/dl) compared to mothers with improved depression (27.1 vs. 4.2 %, p = 0.02). There were no statistically significant differences in the serum iron (p = 0.6) or Hb (p = 0.7) levels between the depressed versus non-depressed mothers.
Discussion
In the current study, iron deficiency without anemia was detected in 37.1 % of mothers with PPD. Early iron supplementation significantly improved the iron stores and decreased the rates of iron deficiency in these mothers. Notably, iron supplementation in mothers with PPD was associated with a significant decrease in EPDS scores and an improvement rate of 42.8 % for PPD. Furthermore, there was a significant association between continued PPD and lower ferritin levels at 7 weeks postpartum.
In this study, iron deficiency without anemia was significantly associated with continued PPD. This aligns with the findings of Shariatpanaahi et al. [26] in a study of non-pregnant females, where decreased ferritin levels before the occurrence of anemia were significantly associated with depression. Similarly, Albacar et al. and Beard et al. assessed the relation between PPD and postpartum ferritin levels at 48 h and 10 weeks following delivery in two separate studies, concluding that a low ferritin level after delivery is significantly associated with PPD and, ultimately, that iron plays an important role in the pathophysiology of PPD [8]. Notably, several other studies have documented the association of early postpartum iron deficiency anemia with the later development of PPD [11, 27]. As noted previously, iron deficiency progresses through several stages. The first stage is defined by a negative iron balance, in which the demand or loss of iron exceeds the body’s ability to absorb iron from the diet. As long as iron stores are present and able to be mobilized, the serum iron level, total iron-binding capacity (TIBC), and Hb level remain within normal limits. During a period of negative balance, only serum ferritin levels decrease, while the other markers remain within normal limits. When iron stores become depleted, the serum iron begins to fall, and, gradually, the TIBC increases. It is only in the final stages of deficiency that the Hb levels decrease [17]. Iron deficiency without anemia is usually underestimated and undiagnosed due to having normal hematologic indices. It is documented that iron deficiency without anemia can have numerous adverse effects, including effects on brain function [17, 18]. The results of this study, in addition to the aforementioned studies [8, 9, 11, 26, 27], provide strong evidence that iron deficiency with or without anemia is significantly associated with the occurrence and continuation of PPD.
In our study of mothers with PPD, we found that 50 mg of iron supplementation starting at the second postnatal week and continuing for 6 weeks was associated with a significant decrease in the EPDS score and a significantly higher improvement rate for PPD when compared to placebo. In an Italian study in which mothers received a preparation of several minerals including 60 mg of iron for 30 days that was started on the third postnatal day, the EPDS score significantly decreased in the intervention group by the end of the study [16]. In another investigation, mothers received a preparation containing almost 40 mg of iron for 6 months that was compared to placebo. At the end of the study, an improvement of 25 % in the depression and stress scales of the intervention group was observed [9].
Iron is an essential element for the proper function of different regions of the brain. Iron deficiency can rapidly deplete brain iron concentrations, although dietary repletion is able to normalize them [28]. The effect of iron deficiency on depression can be explained through several possible mechanisms. First, iron is a component of a number of enzymes and proteins, such as oxidative enzymes and respiratory chain proteins, and is also essential for oxidative energy production [7]. Cytochrome C is an oxidative protein, and a decrease in its levels is believed to be associated with the pathogenesis of depression [29]. It has been shown that iron deficiency is associated with a dramatic reduction in cytochrome C levels in the hippocampus of rat brains [30]. In addition, intra-neuronal iron metabolism involves the synthesis, packaging, uptake, and degradation of various neurotransmitters, namely dopamine, serotonin, and gamma-aminobutyric (GABA) [28]. Dopaminergic tracts and dopamine receptors, which play an important role in depression, appear to be consistently sensitive to regional brain iron deficiency. Therefore, iron deficiency is believed to be associated with a decrease in dopamine D1 and D2 receptors and decreased functioning of dopamine in the brain [28, 31–33]. Other studies have demonstrated significantly lower densities of serotonin transporters in the striatum of iron-deficient rats than in iron-sufficient rats, as well as a lesser capability of taking up 3H-serotonin in the synaptosomes [33, 34]. GABA is an additional neurotransmitter that is believed to play a role in depression. Depression is often associated with decreased GABAergic function, with low GABA function proposed to be an inherited biological marker of vulnerability to depression [35]. Iron deficiency has been identified to significantly decrease the GABA concentrations within the hippocampus, caudate putamen, and globus pallidus [36]. In addition to the aforementioned effects, there are also secondary effects of iron on peroxide reduction, amino acid metabolism, and fatty acid elongation and desaturation, all of which have implications for the potential mechanisms of action on neuronal functioning [28].
The main strength of this study was its randomization and placebo-controlled design. Assessing for the first time the effect of early iron treatment (without other minerals) on the resolution of PPD and involving non-anemic mothers were additional strengths of this study. The main limitation of the study was that serum TIBC, and transferrin saturation were not measured; assessing their values could have helped to better understand the iron status of the participants. A lack of assessment of inflammatory markers was another limitation; due to the effects of inflammation on ferritin levels and depressive symptoms, assessing inflammatory markers could have resulted in a better understanding of the underlying pathophysiology of the observed results. Because the primary purpose of the study was assessing the effects of iron supplementation on PPD improvement rates and EPDS scores, other important parameters such as breastfeeding, sleep patterns, baby colic and the side effects of iron supplementation were not clearly recorded and compared between the study groups, although they were investigated during the medical and psychiatric interviews. Evaluating these factors could increase the understanding of the mechanisms behind the success of the intervention and could aid in better clinical decision making.
Conclusions
Iron deficiency is common in mothers with PPD who, by appearance, have normal hemoglobin and serum iron levels after delivery. Early iron supplementation in these mothers significantly improves the status of iron stores and causes a significant reduction in EPDS scores, as well as a significant increase in the improvement rate for PPD. In addition, continued PPD might be related to the low postpartum ferritin levels in untreated mothers. Based on these results, iron stores should be evaluated in all mothers with PPD regardless of anemia and hematologic indices, and when detecting iron deficiency without anemia, daily iron supplementation should be considered for the early improvement in PPD and iron stores. However, it is important to note that these results need to be confirmed by further studies, as both PPD prevalence and ferritin levels are affected by socioeconomic, nutritional, and cultural characteristics present within different societies. Additional studies are also required to evaluate how inflammation and inflammatory markers after delivery could affect ferritin levels and PPD symptoms. Furthermore, prospective studies are required to evaluate the possible side effects of iron supplementation in these mothers, as well as to assess the clinical effects of iron supplementation on infants’ colic, breastfeeding, sleep patterns, and other maternal and neonatal outcomes.
Change history
23 September 2022
This article has been retracted. Please see the Retraction Notice for more detail: https://doi.org/10.1007/s00394-022-03009-4
References
Lanes A, Kuk JL, Tamim H (2011) Prevalence and characteristics of postpartum depression symptomatology among Canadian women: a cross-sectional study. BMC Public Health 11:302. doi:10.1186/1471-2458-11-302
Andrews-Fike C (1999) A review of postpartum depression. Prim Care Companion J Clin Psychiatry 1:9–14
Halbreich U, Karkun S (2006) Cross-cultural and social diversity of prevalence of postpartum depression and depressive symptoms. J Affect Disord 91:97–111. doi:10.1016/j.jad.2005.12.051
Tahirkheli NN, Cherry AS, Tackett AP, McCaffree MA, Gillaspy SR (2014) Postpartum depression on the neonatal intensive care unit: current perspectives. Int J Women’s Health 6:975–987. doi:10.2147/IJWH.S54666
Sinclair D, Murray L (1998) Effects of postnatal depression on children’s adjustment to school. Teacher’s reports. Br J Psychiatry J Ment Sci 172:58–63
Skalkidou A, Hellgren C, Comasco E, Sylvén E, Poromaa IS (2012) Biological aspects of postpartum depression. Women’s Health 8:659–671. doi:10.2217/whe.12.55
Etebary S, Nikseresht S, Sadeghipour HR, Zarrindast MR (2010) Postpartum depression and role of serum trace elements. Iran J Psychiatry 5:40–46
Albacar G, Sans T, Martin-Santos R et al (2011) An association between plasma ferritin concentrations measured 48 h after delivery and postpartum depression. J Affect Disord 131:136–142. doi:10.1016/j.jad.2010.11.006
Beard JL, Hendricks MK, Perez EM et al (2005) Maternal iron deficiency anemia affects postpartum emotions and cognition. J Nutr 135:267–272
Milman N (2011) Postpartum anemia I: definition, prevalence, causes, and consequences. Ann Hematol 90:1247–1253. doi:10.1007/s00277-011-1279-z
Alharbi AA, Abdulghani HM (2014) Risk factors associated with postpartum depression in the Saudi population. Neuropsychiatr Dis Treat 10:311–316. doi:10.2147/NDT.S57556
Aubuchon-Endsley NL, Thomas DG, Kennedy TS, Grant SL, Valtr T (2012) Interactive relations among maternal depressive symptomatology, nutrition, and parenting. Women Health 52:197–213. doi:10.1080/03630242.2012.662933
Bodnar LM, Wisner KL (2005) Nutrition and depression: implications for improving mental health among childbearing-aged women. Biol Psychiatry 58:679–685
Milman N (2012) Oral iron prophylaxis in pregnancy: not too little and not too much! J Pregnancy 2012:514345. doi:10.1155/2012/514345
Miller BJ, Murray L, Beckmann MM, Kent T, Macfarlane B (2013) Dietary supplements for preventing postnatal depression. Cochrane Database Syst Rev 10:CD009104. doi:10.1002/14651858.CD009104.pub2
Paoletti AM, Orru MM, Marotto MF et al (2013) Observational study on the efficacy of the supplementation with a preparation with several minerals and vitamins in improving mood and behaviour of healthy puerperal women. Gynecol Endocrinol 29:779–783. doi:10.3109/09513590.2013.801447
Longo Dan L, Fauci Anthony S, Kasper Dennis L, Hauser Stephen L, Larry Jameson J, Loscalzo Joseph (2012) Harrison’s principles of internal medicine, vol 1, 18th edn. McGraw-Hill, New York
Erdman JW, Macdonald IA, Zeisel SH (2012) Present knowledge in nutrition, 10th edn. Wiley-Blackwell, Hoboken
Tt Brownlie, Utermohlen V, Hinton PS, Giordano C, Haas JD (2005) Marginal iron deficiency without anemia impairs aerobic adaptation among previously untrained women. Am J Clin Nutr 75:734–742
Cox JL, Holden JM, Sagovsky R (1987) Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry J Ment Sci 150:782–786
Garcia-Esteve L, Ascaso C, Ojuel J, Navarro P (2003) Validation of the Edinburgh Postnatal Depression Scale (EPDS) in Spanish mothers. J Affect Disord 75:71–76. doi:10.1016/S0165-0327(02)00020-4
Pavord S, Myers B, Robinson S, Allard S, Strong J, Oppenheimer C (2012) UK guidelines on the management of iron deficiency in pregnancy. Br J Haematol 156:588–600. doi:10.1111/j.1365-2141.2011.09012.x
Breymann C, Bian XM, Blanco-Capito LR, Chong C, Mahmud G, Rehman R (2011) Expert recommendations for the diagnosis and treatment of iron-deficiency anemia during pregnancy and the postpartum period in the Asia-Pacific region. J Perinat Med 39:113–121. doi:10.1515/JPM.2010.132
Ashton Acton (2013) Anemia: New insights for the healthcare professional, 2013th edn. ScholarlyEditions, Atlanta
Heath AL, Skeaff CM, O’Brien SM, Williams SM, Gibson RS (2001) Can dietary treatment of non-anemic iron deficiency improve iron status? J Am Coll Nutr 20:477–484
Vahdat Shariatpanaahi M, Vahdat Shariatpanaahi Z, Moshtaaghi M, Shahbaazi SH, Abadi A (2007) The relationship between depression and serum ferritin level. Eur J Clin Nutr 61:532–535. doi:10.1038/sj.ejcn.1602542
Corwin EJ, Murray-Kolb LE, Beard JL (2003) Low hemoglobin level is a risk factor for postpartum depression. J Nutr 133:4139–4142
Beard JL, Connor JR (2003) Iron status and neural functioning. Annu Rev Nutr 23:41–58. doi:10.1146/annurev.nutr.23.020102.075739
Mu J, Xie P, Yang ZS et al (2007) Neurogenesis and major depression: implications from proteomic analyses of hippocampal proteins in a rat depression model. Neurosci Lett 416:252–256. doi:10.1016/j.neulet.2007.01.067
Siddappa AJ, Rao RB, Wobken JD, Leibold EA, Connor JR, Georgieff MK (2002) Developmental changes in the expression of iron regulatory proteins and iron transport proteins in the perinatal rat brain. J Neurosci Res 68:761–775
Nelson C, Erikson K, Pinero DJ, Beard JL (1997) In vivo dopamine metabolism is altered in iron-deficient anemic rats. J Nutr 127:2282–2288
Erikson KM, Jones BC, Hess EJ, Zhang Q, Beard JL (2001) Iron deficiency decreases dopamine D1 and D2 receptors in rat brain. Pharmacol Biochem Behav 69:409–418
Erikson KM, Jones BC, Beard JL (2000) Iron deficiency alters dopamine transporter functioning in rat striatum. J Nutr 130:2831–2837
Morse A, Beard JL, Jones B (1999) Sex and genetics are important cofactors in assessing the impact of iron deficiency on the developing mouse brain. Nutr Neurosci 2:323–335
Kalueff AV, Nutt DJ (2007) Role of GABA in anxiety and depression. Depress Anxiety 24:495–517. doi:10.1002/da.20262
Erikson KM, Shihabi ZK, Aschner JL, Aschner M (2002) Manganese accumulates in iron-deficient rat brain regions in a heterogeneous fashion and is associated with neurochemical alterations. Biol Trace Elem Res 87:143–156
Acknowledgments
This research was funded by the Research Deputy of the Tehran University of Medical Sciences.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
About this article
Cite this article
Sheikh, M., Hantoushzadeh, S., Shariat, M. et al. RETRACTED ARTICLE: The efficacy of early iron supplementation on postpartum depression, a randomized double-blind placebo-controlled trial. Eur J Nutr 56, 901–908 (2017). https://doi.org/10.1007/s00394-015-1140-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-015-1140-6