Abstract
Background
Ovarian adult granulosa cell tumours are low-grade malignant sex cord–stromal neoplasm with a low recurrence rate. Prognostic factors for recurrence include tumor stage, tumor rupture in Stage I neoplasms and the presence of residual tumors after surgery. However, in recurrent tumors, prognostic factors for overall survival (OS) are lacking. In the present paper, we conducted a systematic meta-analysis with the aim to assess prognostic factors for OS in patients with recurrent GCT.
Methods
Electronic databases were searched for all studies assessing prognostic factors in recurrent adult granulosa cell tumor of the ovary. Student T test, Fisher’s exact test and Kaplan–Meier survival analysis with long-rank test were used to assess differences among groups; a p value < 0.05 was considered significant.
Results
Eleven studies analyzing 102 recurrent tumors were included in the systematic review. Tumor stage and localization of recurrent tumors were significantly associated with OS on Kaplan–Meier analysis; Cox regression analysis showed a HR of 0.879 for the stage II, of 3.052 for the stage III, and of 2.734 for stage IV tumor was significantly associated with OS (p = 0.037); observed HRs for abdominal and thoracic locations were of 2.405 and of 4.024, respectively.
Conclusions
In conclusion, the present article emphasizes the prognostic significance of tumor stage > II and extrapelvic anatomic sites of recurrences in patients with recurrent granuolase cell tumors of the ovary.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Granulosa cell tumors (GCT) of ovary originate from the ovarian mesenchyme and sex cords and account for 70% of all sex cord stromal tumors, and 5–8% of all ovarian neoplasms [1]. GCT can manifest in women of all age groups, is usually bilateral and has the ability to secrete [1,2,3].
There are two different histological types of GCT: adult-type GCTs (AGCTs) and juvenile-type GCTs (JGCTs) [1,2,3,4].
JGCTs is associated with abnormally high estrogen secretion and usually manifest with precocious puberty in about 75% of cases, [5]. Extremely rare examples of virilizing, testosterone-producing JGCT are reported in the literature (2–3% of GCTs) and manifest with hirsutism, amenorrhea, deepening of the voice, clitoral hypertrophy and acne [6]. In contrast, AGCTs usually present with menstrual irregularities, amenorrhea and endometrial hyperplasia [7, 8].
The prognosis of JGCT is excellent, with tumour recurrence or metastasis being rare [4]. AGCT, on the other hand, is regarded as a low-grade malignant neoplasm since there is a significant propensity for recurrence or metastasis. Often the recurrent or metastatic tumour manifests itself many years after removal of the primary neoplasm with intervals in excess of 10 or even 20 years being not uncommon [1,2,3]. There is no standard management for recurrent GCT. Various treatment options include surgery with/without chemotherapy and/or radiotherapy [9]. Patients with peritoneal metastases are often considered inoperable with treatment largely directed at palliation of symptoms [9, 10].
To date, widely accepted prognostic factors for recurrence include tumor stage, tumor rupture in Stage I neoplasms and the presence of residual tumors after surgery [11, 12]. Up to date tumor size, morphological pattern of growth, cytological atypia and high mitotic count are not considered independent prognostic factors of patient survival [11, 12]. However, in recurrent tumors, prognostic factors for overall survival (OS) are lacking.
In the present paper, we conducted a systematic meta-analysis with the aim to assess prognostic factors for OS in patients with recurrent GCT.
Materials and methods
This study was planned based on methods of previous systematic reviews [13,14,15]. Two independent authors performed all review stage; disagreements, if any, were resolved by consensus. The PRISMA statement [16] was followed to report this study.
Search strategy and study selection
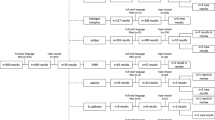
Four electronic databases (i.e., MEDLINE, Scopus, ISI Web of Sciences and Google Scholar) were searched from their inception to May 2021. The following combination of text words was used: granulosa cell tumor AND prognosis. All studies reporting individual clinicopathological and survival data of series of women with GCT were included. Exclusion criteria were: sample size < 5; overlapping patient data; review. Relevant references from eligible studies were also assessed.
Flow diagram of the study selection process is reported in Fig. 1.
Data extraction
Main data extracted for analysis were follow-up time after recurrence, status at the last follow-up and each individual clinicopathological factor of recurrent cases (out of which patient age, tumor stage and localization of recurrent tumor were suitable for meta-analysis). Further data extracted were: country, period of enrollment, total sample size and number of recurrent cases, time to recurrence, total follow-up duration.
Risk of bias assessment
The risk of bias was assessed based on the QUADAS-2 [17], (Fig. 2). The four domains assessed were: patient selection (i.e., were inclusion criteria and period of recruitment reported?); index test (i.e., were clinicopathological variables clearly reported?); reference standard (i.e., were survival data clearly reported?); flow (were data reported for all eligible patients?). The risk of bias was categorized as “low”, “unclear” or “high” following previously described criteria [16].
Data analysis
Kaplan–Meier survival analysis with Log-rank test was used to assess the impact of clinicopathological variables on OS; Kaplan–Meier curves were used to graphically report the results. Cox regression survival analysis was used to calculate hazard ratio (HR) with 95% confidence interval for each clinicopathological variable. A p value < 0.05 was considered significant.
Statistical analyses were carried out using Statistical Package for Social Science (SPSS) 18.0 package (SPSS Inc., Chicago, IL, USA).
Results
Study selection and characteristics
Eleven studies [18,19,20,21,22,23,24,25,26,27,28] with 102 recurrent GCT were included in the systematic review. The process of study selection is summarized in Supplementary Fig. 1. Characteristics of the included studies are listed in Table 1.
Risk of bias assessment
For the “patient selection” domain, one study was considered at unclear risk of bias (period of recruitment not specified), while the other studies were considered at low risk. For the “index test” domain, all studies were considered at low risk. For the “reference standard” domain, one studies were considered at high risk (no clear report of survival data), while the other studies were considered at low risk. For the “flow” domain, one study was considered at high risk (inconsistent data reporting) and the remaining studies at low risk.
Survival analysis
The two studies at high risk of bias were excluded from the meta-analysis. A further study [c] was excluded because it reported the total follow-up duration of patients with GCT but not the follow-up time after recurrence.
Patient age was not significantly associated with OS (p = 0.098) on Kaplan–Meier analysis (Fig. 3); Cox regression analysis showed a HR of 0.460 (95% CI 0.188–1.125; p = 0.089) for the age range 40–49, of 0.690 (95% CI 0.289–1.644; p = 0.402) for the age range 50–59, and or 1.398 (95% CI 0.568–3.437; p = 0.466) for an age ≥ 60, using an age < 40 as reference.
Tumor stage was significantly associated with OS (p = 0.007) on Kaplan–Meier analysis (Fig. 4); Cox regression analysis showed a HR of 0.879 (95% CI 0.200–3.856; p = 0.864) for the stage II, of 3.052 (95% CI 1.498–6.221; p = 0.002) for the stage III, and of 2.734 (95% CI 0.768–9.742; p = 0.121) for stage IV, using stage I as reference.
Localization of recurrent tumor was significantly associated with OS (p = 0.037) on Kaplan–Meier analysis (Fig. 5); Cox regression analysis showed a HR of 2.405 (95% CI 1.078–5.365; p = 0.032) for an abdominal localization, and of 4.024 (95% CI 1.054–15.369; p = 0.042) for a thoracic localization, using pelvic localization as a reference.
Discussion
Ovarian adult granulosa cell tumours are regarded as low-grade malignant sex cord–stromal neoplasms with a low recurrence rate and long overall survival, generally detected in early stages without extra-pelvic metastasis [1,2,3]. Recurrent or metastatic GCT can manifests many years after initial surgery and the 10-year survival for GCT ranges from 60 to 90% [1,2,3].
To date, different studies attempted to individuate the most relevant prognostic factors that might predict tumor recurrences and metastases to select the most appropriate candidates for adjuvant therapy after surgical removal.
The most widely reported clinicopathological factors related to prognosis in GCT include: tumour stage, tumor rupture, size, mitotic activity, nuclear atypia more than mild, histological pattern, patient age, p53 immunohistochemistry, Ki-67 proliferative index and DNA ploidy analysis [11, 12, 18, 20].
Unfortunately, literature data regarding the above-mentioned parameters are conflicting and no single prognostic factor is sufficiently reliable for predicting GCT prognosis.
To date, following many studies, tumors stage represents the only universally accepted prognostic factor in GCT and advanced-stage (II–IV) tumors are believed to benefit from adjuvant therapy. Capsular rupture in low-stage tumors may also represent an important adverse prognostic factor in GCT; although not extensively investigated, few studies showed a significant relation between capsular rupture and tumor recurrences [11, 12, 18].
Different cut off limits of tumor diameter for tumor recurrence have been also reported [11, 12, 18].
The prognostic impact in stage I GCTs of mitotic activity and Ki-67 proliferative index has also been demonstrated by several studies. However, the available results need to be furtherly validated since they suffer from significant variations in methodology between studies [11, 20].
The prognostic significance of lymphovascular invasion (LVSI) has also been taken into account by some authors; in detail, a moderate/prominent LVSI has been related to worse survival in GCT patients. However, its role as independent prognostic factor is still not clear and precise criteria to define lymphatic and blood vessel invasion are still lacking [11, 12, 18, 20].
Despite many studies have investigated the prognostic predictors of GCTs, limited and conflicting results are currently available in the literature regarding the biologic behavior and prognosis of recurrent tumors. Therefore, the main aim of the present study was to assess the most relevant prognostic factors capable of affecting OS in patients with recurrent GCTs.
Starting from 11 studies analyzing 102 recurrent GCTs [18,19,20,21,22,23,24,25,26,27,28], we observed that tumor stage and tumor location represented reliable prognostic predictors for OS. In detail, advanced tumor stages of II or more were significantly associated with worse prognosis both on Kaplan–Meier analysis and Cox regression analysis, with observed HRs of 0.879, 3.052 and 2.734 for stage II, III and IV, respectively.
The anatomic location of tumor recurrences was also significantly related to OS on survival analysis; in fact, worse prognoses were recorded for patients showing tumor recurrences outside the pelvis with observed HR values of 2.405 and 4.024 for abdominal and thoracic localizations, respectively.
This study showed that a tumor stage > II and a localization outside pelvis were significant unfavorable prognostic factors in recurrent GCT; on the other hand, patient age was not significantly associated with OS.
In our study, the median time until recurrence was 42 months and this observation is in line with previous reports. As distant metastases are not infrequent in recurrent tumors, patients at high-risk for recurrence should receive adjuvant systemic chemotherapy. There is no consensus regarding the adequate management and no standard treatment for recurrent GCT, and multiple approaches including surgery, chemotherapy, radiotherapy, and hormone therapies have been proposed [10].
The controversies over the extend of surgery are still matter of debate, with many gynecological oncologists in favour of omitting lymphadenectomy procedure, in particular in tumors without unfavourable clinical-pathological prognostic characteristics and since the high risk of complications and morbidity, and others considering as a valid option a multivisceral surgical debulking in addition to lymphadenectomy [10, 21, 27, 28].
The first-line standard chemotherapy has been suggested to be BEP, which is a combination of bleomycin, etoposide, and cisplatin; CAP, which comprises of cyclophosphamide, adriamycin, and cisplatin, is also used [29, 30].
Etiological relationship between ovarian stimulation and granulosa cell tumorigenesis has also been speculated and a proportion of these tumors express steroid hormone receptors [31]. Therefore, hormonal treatment using gestagen or GnRH agonist may be expected to exert anti-tumor activity for AGCTs [31]. However, the number of reports regarding this issue is still limited and further investigations are needed to verify the role of gestagen and GnRH in treatment of AGCTs.
In conclusion, the present article emphasizes the prognostic significance of tumor stage and anatomic site of recurrences in patients with relapsed GCT. However, scientific articles on this topic are still limited and multicentric studies on larger series are still needed to elucidate the prognostic impact of clinicopathological factors in GCTs.
Availability of data and material
All presented data are available from corresponding author upon reasonable request.
Code availability
Not applicable.
References
Lim D, Oliva E (2018) Ovarian sex cord-stromal tumours: an update in recent molecular advances. Pathology 50(2):178–189
Pectasides D, Pectasides E, Psyrri A (2008) Granulosa cell tumor of the ovary. Cancer Treat Rev 34:1–12
Stuart GC, Dawson LM (2003) Update on granulosa cell tumours of the ovary. Curr Opin Obstet Gynecol 15:33–37
Young RH, Dickersin GR, Scully RE (1984) Juvenile granulosa cell tumor of the ovary. A clinicopathological analysis of 125 cases. Am J Surg Pathol 8:575–596
Ndhlovu E, Liu L, Dai J, Dong X, Zhang W, Chen B (2021) Retrospective analysis of clinicopathological characteristics of 19 ovarian juvenile granulosa cell tumor cases. J Obstet Gynaecol Res. https://doi.org/10.1111/jog.14805
Bús D, Buzogány M, Nagy G, Vajda G (2017) Rare virilizing granulosa cell tumor in an adolescent. Mol Clin Oncol 6(1):88–90
Kitamura S, Abiko K, Matsumura N, Nakai H, Akimoto Y, Tanimoto H, Konishi I (2017) Adult granulosa cell tumors of the ovary: a retrospective study of 30 cases with respect to the expression of steroid synthesis enzymes. J Gynecol Oncol 28(4):31
Szewczuk W, Szewczuk O, Czajkowski K, Grala B, Semczuk A (2020) Ovarian adult-type granulosa cell tumor concomitant with simple endometrial hyperplasia: a case study with selected immunohistochemistry. J Int Med Res 48(4):300060519886984
Bergamini A, Ferrandina G, Candotti G, Taccagni G, Scarfone G, Bocciolone L, Cassani C, Marinaccio M, Pignata S, Candiani M, Mangili G (2021) Stage I juvenile granulosa cell tumors of the ovary: a multicentre analysis from the MITO-9 study. Eur J Surg Oncol. https://doi.org/10.1016/j.ejso.2021.02.003
Yasukawa M, Matsuo K, Matsuzaki S, Dainty LA, Sugarbaker PH (2021) Management of recurrent granulosa cell tumor of the ovary: Contemporary literature review and a proposal of hyperthermic intraperitoneal chemotherapy as novel therapeutic option. J Obstet Gynaecol Res 47(1):44–51
Miller K, McCluggage WG (2008) Prognostic factors in ovarian adult granulosa cell tumour. J Clin Pathol 61(8):881–884
Villella J, Herrmann FR, Kaul S, Lele S, Marchetti D, Natiella J, Odunsi K, Mhawech-Fauceglia P (2007) Clinical and pathological predictive factors in women with adult-type granulosa cell tumor of the ovary. Int J Gynecol Pathol 26:154–159
Santoro A, Angelico G, Travaglino A, Raffone A, Arciuolo D, D’Alessandris N, Inzani F, Zannoni GF (2021) Clinico-pathological significance of TCGA classification and SWI/SNF proteins expression in undifferentiated/dedifferentiated endometrial carcinoma: a possible prognostic risk stratification. Gynecol Oncol 161:629–635
Travaglino A, Raffone A, Santoro A, Gencarelli A, Angelico G, Spadola S, Marzullo L, Zullo F, Insabato L, Zannoni GF (2021) Prognostic significance of atypical mitotic figures in smooth muscle tumors of uncertain malignant potential (STUMP) of the uterus and uterine adnexa. APMIS 129:165–169
Raffone A, Travaglino A, Santoro A, Esposito I, Angelico G, Spadola S, Zannoni GF (2020) Accuracy of one-step nucleic acid amplification in detecting lymph node metastases in endometrial cancer. Pathol Oncol Res 26:2049–2056
Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M et al (2015) Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 4:1
Whiting PF, Rutjes AW, Westwood ME et al (2011) QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 155:529–536
Fujimoto T, Sakuragi N, Okuyama K et al (2001) Histopathological prognostic factors of adult granulosa cell tumors of the ovary. Acta Obstet Gynecol Scand 80:1069–1074
Lauszus FF, Petersen AC, Greisen J, Jakobsen A (2001) Granulosa cell tumor of the ovary: a population-based study of 37 women with stage I disease. Gynecol Oncol 81:456–460
Kusamura S, Derchain S, Alvarenga M, Gomes CP, Syrjänen KJ, Andrade LA (2003) Expression of p53, c-erbB-2, Ki-67, and CD34 in granulosa cell tumor of the ovary. Int J Gynecol Cancer 13:450–457
Uygun K, Aydiner A, Saip P et al (2003) Clinical parameters and treatment results in recurrent granulosa cell tumor of the ovary. Gynecol Oncol 88:400–403
Auranen A, Sundström J, Ijäs J, Grénman S (2007) Prognostic factors of ovarian granulosa cell tumor: a study of 35 patients and review of the literature. Int J Gynecol Cancer 17:1011–1018
Rha SE, Oh SN, Jung SE, Lee YJ, Lee AW, Byun JY (2008) Recurrent ovarian granulosa cell tumors: clinical and imaging features. Abdom Imaging 33:119–125
Lee YK, Park NH, Kim JW, Song YS, Kang SB, Lee HP (2008) Characteristics of recurrence in adult-type granulosa cell tumor. Int J Gynecol Cancer 18:642–647
Pectasides D, Papaxoinis G, Fountzilas G et al (2008) Adult granulosa cell tumors of the ovary: a clinicopathological study of 34 patients by the Hellenic Cooperative Oncology Group (HeCOG). Anticancer Res 28:1421–1427
Ayhan A, Salman MC, Velipasaoglu M, Sakinci M, Yuce K (2009) Prognostic factors in adult granulosa cell tumors of the ovary: a retrospective analysis of 80 cases. J Gynecol Oncol 20:158–163
Ud Din N, Kayani N (2014) Recurrence of adult granulosa cell tumor of the ovary: experience at a tertiary care center. Ann Diagn Pathol 18:125–128
Ertas IE, Gungorduk K, Taskin S et al (2014) Prognostic predictors and spread patterns in adult ovarian granulosa cell tumors: a multicenter long-term follow-up study of 108 patients. Int J Clin Oncol 19:912–920
Meisel JL, Hyman DM, Jotwani A et al (2015) The role of systemic chemotherapy in the management of granulosa cell tumors. Gynecol Oncol 136:505–511
Zhao D, Zhang Y, Ou Z, Zhang R, Zheng S, Li B (2020) Characteristics and treatment results of recurrence in adult-type granulosa cell tumor of ovary. J Ovarian Res 13:19
Ciucci A, Ferrandina G, Mascilini F, Filippetti F, Scambia G, Zannoni GF, Gallo D (2018) Estrogen receptor β: potential target for therapy in adult granulosa cell tumors? Gynecol Oncol 150:158–165
Funding
The present research received no fundings.
Author information
Authors and Affiliations
Contributions
Conceptualization, A.S., G.A., A.T.; methodology, F.I., G.S.; software, M.V.; validation, N.D., V.G.; formal analysis, P.T.; investigation, P.S., GF.Z.; resources, GF.Z.; data curation, P.S., F.I.; writing—original draft preparation, A.T.; writing—review and editing, A.S., G.S.; visualization, G.A., D.A.; supervision, GF.Z.; project administration, GF.Z. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Inzani, F., Santoro, A., Travaglino, A. et al. Prognostic predictors in recurrent adult granulosa cell tumors of the ovary: a systematic review and meta-analysis. Arch Gynecol Obstet 306, 315–321 (2022). https://doi.org/10.1007/s00404-021-06305-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-021-06305-2