Abstract
Introduction
Genitourinary tuberculosis is the fourth most common cause of extrapulmonary tuberculosis, although often underestimated by clinicians due to its rare and non-specific symptoms. One of the disease’s complications is infertility. Although Portugal is one of the European countries with the highest prevalence of tuberculosis, its impact on Portuguese female fertility is unknown. With this study, we intend to evaluate the prevalence of genital tuberculosis, its presenting symptoms, and pregnancy outcomes in infertile women followed in a Portuguese tertiary hospital.
Methods
Retrospective and descriptive study, performed using an electronic database and consultation of clinical files. Studied population: infertile women followed from 2000 until 2019 at the reproductive unit of a Portuguese tertiary hospital, who underwent endometrial biopsy/curettage in the context of their etiological investigation. The diagnosis of genital tuberculosis was based on histological criteria.
Results
Over the 19 years, 2653 endometrial specimens were analyzed. Pathological evaluation was positive for tuberculosis in 19 cases (0.72%). There was a decrease in new diagnoses throughout the observation period.
Conclusion
Despite being one of the European countries with the highest prevalence of tuberculosis, genital TB does not appear to have a significant impact on the etiology of female infertility in Portugal. Nevertheless, it is a diagnosis to be considered in selected patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Worldwide, tuberculosis (TB) remains an important public health problem and the leading cause of death from a single infectious agent [1]. Portugal is one of the European countries with highest rates of TB, with an incidence of 20/100.000 habitants, according to data of 2017 [2]. Among Portuguese women, the incidence was 0.74 per 1000 inhabitants [2].
The Italian anatomist Morgagni was the first to describe a case of inflammation of the female genital tract due to tuberculosis in 1744 [3]. Genital tuberculosis is almost invariably secondary to hematogenous spread from a distant focus, usually the lungs, but transmission through sexual intercourse is possible [4]. The Fallopian tubes are probably the primary target for genital infection, considering they are affected in almost all cases, the endometrium in 50–90%, the ovaries in 20–30%, the cervix in 1–15%, and the vulva and vagina scarcely ever [5].
The available studies reported four major presenting complaints: infertility, abnormal bleeding, pelvic pain, and amenorrhea [6]. At least 11% of patients are asymptomatic and diagnosed incidentally. Systemic symptoms (weight loss, fatigue, mild evening elevation of temperature) tend to be relatively mild. Infertility is the presenting and most common finding in 40–50% of patients in the largest series published [7]. The factors explaining infertility in these patients are: (1) tubal; (2) defective ovarian function; (3) uterine/endometrial. [8]
With this study, we aim to evaluate the prevalence of genital tract tuberculosis, its presenting symptoms, and pregnancy outcomes, among an infertile female population, over 19 years (2000–2019).
Methods
We performed a retrospective and descriptive study of all women seeking treatment for infertility in a tertiary care center in Portugal (Lisbon North University Hospital Center), between 2000 and 2019, who had a uterine curettage or endometrial biopsy during their infertility etiological investigation. We defined infertility as one year of unprotected intercourse without conception. Uterine curettage or endometrial biopsy were performed routinely in all women submitted to hysteroscopy or laparoscopy.
We identified the cases through an electronic records registry searching for all endometrial biopsies associated with the diagnoses coded in ICD 9 (international coding of disease) as "infertility" (code 628) and "tuberculosis" (code 016) at our institution. We excluded all cases that did not originate in the reproductive medicine unit.
We reviewed medical records of all patients with the diagnosis of genital TB. Collected variables included age, country of birth, marital status, presenting complaints, previous or current history of non-genital TB and family history of TB, obstetric history, type and duration of infertility, treatment for infertility, and resulting pregnancies. Data were missing in some cases.
Investigations included complete blood count (CBC), chest X-rays, hysterosalpingography, and pelvic ultrasound. The tubal evaluation was performed either by hysterosalpingography (HSG) or by laparoscopy.
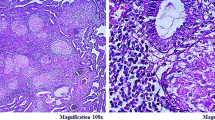
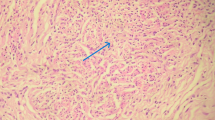
The diagnosis of genital TB was based on a histological study of tissue samples fixed in 10% buffered formalin, dehydrated in upgrading alcohol and xylene, paraffin-embedded, and stained with hematoxylin–eosin. Classic TB histopathological findings were need for diagnosis: epithelioid cell granulomas (small- to medium-sized) in different stages, Langhans’ giant cells, and central caseation associated with chronic inflammation. [9] We also performed a microscopic examination with the Ziehl–Neelson stain to reveal acid-fast bacilli in all cases. PCR testing, interferon-gamma release assays (IGRA), and culture for Mycobacterium tuberculosis were performed in some unselected patients.
We used STATA® software for the descriptive analysis of the data. The ethics committee of the Lisbon North University Hospital Center approved this study. Considering the retrospective characteristics of the study, the directive of informed consent was renounced by the ethics committee.
Results
During the nineteen-year period, 820 uterine curettages and 1833 endometrial biopsies were performed in the infertility investigation in the human reproductive unit of our university hospital. The diagnosis of genital TB was performed in 19 cases (0.72%). Population features are summarized in Table 1. The median age was 30 (16–43) years. Most patients were born in Portugal, and 2 were born in African countries. Most diagnoses occurred at the beginning of our observation period, and there have been no new cases since 2014.
We found two asymptomatic patients that tested positive for hepatitis B and one for HIV. The latter was on antiretroviral therapy, had no detectable viral load, and no signs of immunosuppression.
Only two women had history of extra genital TB (pulmonary). No patient described familial history of tuberculosis.
Most patients with genital TB were asymptomatic (12/19). Pelvic chronic pain was uncommon (2 cases) and abnormal uterine bleeding was reported by 6 patients. Primary infertility was more common than secondary infertility, with 15 women being nulliparous. Tubal factor was present in 13 cases and male factor in one case; the remaining 5 had no additional infertility factor besides the genital TB. In the study population, the average duration of infertility was 6.3 years at the time of the first appointment in the reproductive unit.
Hysterosalpingograms of eleven of the 19 women were available. In all cases, altered tubal patency was described for one or both tubes. Two women were reported to have an irregular uterine shape. It was not possible to confirm whether the other patients undertook this exam.
Sixteen patients were submitted to surgery. Abnormal intraoperative findings like tubal distortion and multiple adhesions of the tubes and ovaries were found in 4 of them. Two had bilateral hydrosalpinxes, and one had miliary inflammation of the peritoneum.
All of them underwent chest radiography, which showed changes in only one case, suggestive of sequelae of previous tuberculosis (fibronodular opacities in the apical and upper lung zones). This particular patient had a personal history of TB.
The most common findings in endometrial specimens were granulomatous endometritis, central necrosis, Langhans cells, and necrotizing epithelioid cells containing granulomas. Acid-fast bacilli with the Ziehl–Neelson stain were seen in only three cases. PCR analysis of tissue specimens was performed in four cases, and all came positive.
Interferon-gamma release assay (IGRA) was performed in five cases being positive in three. Mycobacterium growth on culture was performed in three cases, but none was positive.
After diagnosis, all patients received standard treatment for TB. The follow-up (mean time 2.6 years) showed that one patient had a spontaneous pregnancy ended as a miscarriage, and two patients underwent assisted reproduction techniques shortly after completing treatment, one of them having a successful pregnancy. The remaining patients did not have any pregnancies while under our care.
Discussion
The actual incidence of genital TB cannot be accurately determined because the diagnosis is late and difficult [3]. In developing countries, genital TB is a relatively more common condition. In fact, most of the studies we identified in our review came from India, which is one of the countries with the highest incidence and prevalence of tuberculosis. There, the overall frequency of female genital tuberculosis was 2.4% in 1982, with a steady decline to 0.8% afterwards [10].
Tuberculosis is particularly well studied as cause of female infertility. Published studies about the prevalence of genital TB among infertile women show a prevalence of 2.43 in India and 26% in Pakistan [11, 12]. A study of women registered for in vitro fertilization in north India reported the prevalence of genital TB in patients with tubal factor infertility as 48.5 percent [13].
In Europe, we know that the number of cases has followed the global downward trend, except for countries, such as Sweden and the UK, which had a slight proportional increase that reflects the growing proportion of migrants from developing countries [14]. Most European studies were published more than 20 years ago. We found no western studies specifically relating genital tuberculosis and female infertility. Furthermore, most studies only focus on the relative prevalence of genitourinary TB. For instance, national surveillance data from the Netherlands (1993–2001) stated an incidence of genitourinary TB of 1.7% [15]. A study from Switzerland between 1960 and 1970s reported genitourinary involvement in 4% of all TB cases [16]. Nogales-Ortiz et al. analyzed 78,000 gynecological specimens, in Spain, during 31 years (1946–1977), and found genital tuberculosis in 1.8% [17].
To our knowledge, there are no data in Portugal about genital TB. From the comparison with other European countries, despite their data coming from older studies, we can assume that the prevalence we identified in Portugal (0.72%) is not higher, especially as we studied a high-risk population (infertile women) for this diagnosis. We assume that the decrease in genital tuberculosis accompanied a decrease in the overall incidence of tuberculosis in Portugal. This is supported by the decrease in the number of cases over the years, and the absence of new diagnoses in the last five years of the study. The review of our cases did not allow to define a typical profile of an infertile patient with genital tuberculosis. However, we can conclude that the median age (30 years) of our sample and the symptoms identified are consistent with other studies in infertile women [7]. Other symptoms reported in the literature are ascites, vague abdominal distension, and weight loss, but patients are frequently asymptomatic [18]. Remarkably, the majority of women in our sample did not have immunosuppression or important co-morbidities. In a recent report from the United States of America, 63% of tuberculosis patients were migrants [19]. Despite Portugal being a country of immigration from many low-income countries, mainly Africans, only two women were foreign-born. A possible explanation for this finding is a selection bias since national population may have better access to fertility care (while migrants may have more difficulty to get it).
In what concerns personal and familial history of TB, we also found quite low numbers when compared with an Indian study that reported that 85% of the patients had a positive personal history of TB [20]. Also, an Australian study reported that 10% of the patients had had a positive history of TB contact of some form [21]. However, other studies have shown that personal or familiar background of TB is uncommon and mostly obtained retrospectively after the diagnosis [4].
As in other studies, we found that infertility is mostly primary [10], and a high proportion of tubal factor (13/19). At our center, hysterosalpingography (HSG) is part of the first-line assessment of women with infertility. It is a useful — yet limited—diagnostic tool for genital tuberculosis [16]. Ultrasonography is also a useful imaging technique in these cases [12]. Even when hysterosalpingography or ultrasonography is not suggestive of TB, some TB high-risk countries include a chest X-ray and a uterine curettage in the infertility work-up. However, a negative chest X-ray does not rule out the possibility of genital TB. [12] An endometrial biopsy can provide a diagnosis of genital tract TB with high specificity but a low sensitivity of at most 50% [4]. Taken into account the results of the present study, we conclude that the institution of a routine endometrial biopsy in all infertile women is not reasonable. However, it may be useful in selected cases of infertility, such as with severe tubal factor, in the absence of a history of pelvic inflammatory disease or when association with a TB background. Surgery can also have a role in the diagnosis, allowing a visual inspection and biopsies.
The final diagnosis of genital tuberculosis is challenging and several tests have been proposed. There is no need to perform all tests for every single case of genital TB. The type of tests performed depends upon the location of TB and its clinical presentation, but there are no specific guidelines for the choice. In a recent publication in India, the diagnosis of genital tuberculosis was made by polymerase chain reaction (54.3%), histological findings on biopsy (22.8%), acid-fast bacilli culture (2.8%), and at laparoscopy or hysteroscopy (20%) [22]. Culture with bacteriological identification of M. tuberculosis is considered the gold standard. However, it has a limited yield because of the prolonged period of culture required (2–6 weeks) and the difficulty of the technique. A study showed that only 2.35% of the endometrial samples cultured are positive [23]. In our clinical setting, we did not require a positive bacteriologic diagnosis since demonstrating TB architectural patterns by histological evaluation has been universally accepted as a good method of diagnosis [10].
Even after the mandatory anti-tuberculous treatment, fertility is quite low, in special when fibrosis was already present. In a cohort of 68 Indian women with treated genital TB, nine patients conceived, but eight had spontaneous abortions. [24]. Some studies suggest that tubal surgery has low efficacy because of genital TB reactivation and a high incidence of ectopic pregnancies [16]. IVF treatments seem to be the best option for these cases [16]. Soussis and colleagues reported the use of in vitro fertilization in genital TB with a 28.6% success rate [25]. Our patients showed a low pregnancy rate.
Our study has limitations related to its retrospective nature, including lack of some data about follow-up. However, given the rarity of this disease in Europe, retrospective studies can keep an important value in this particular field. Also, we based the diagnosis just on the endometrial biopsies pathology, which could have led to an undervaluation of the number of women with the disease. However, the endometrium is affected in 90% of genital tuberculosis cases [12]. The generalization of the prevalence found for the Portuguese female population as a whole is not possible since most cases of genital tuberculosis occur postmenopausal.
Nevertheless, this was, to our knowledge, the first Portuguese study on the topic, and it took place in a hospital with a reference fertility center using a stable approach in the investigation of infertility etiology.
In conclusion, tuberculosis is not as much a disease of the past as we might think. Although the overall incidence of genital tuberculosis was less than 1%, it is still a cause of infertility that should not be overlooked, especially if we are dealing with patients with risk factors. This article allows us to draw an XXI century picture of genital tuberculosis in reproductive health in Portugal.
References
WHO Regional Office for Europe/European Centre for Disease Prevention and Control. Tuberculosis surveillance and monitoring in Europe 2019 – 2017 data. Europe WRO for, editor. Copenhagen; 2019.
World Health Organization. Global tuberculosis report. Ginebra-Suiza; 2018. 2018. https://www.who.int/tb/publications/global_report/en/
Kulchavenya E (2014) Urogenital tuberculosis: definition and classification. Ther Adv Infect Dis 2(6):117–122
Chow TWP, Lim BK, Vallipuram S (2002) The masquerades of female pelvic tuberculosis: case reports and review of literature on clinical presentations and diagnosis. J Obstet Gynaecol Res 28(4):203–210
Qureshi RN, Samad S, Hamid R, Lakha SF (2001) Female genital tuberculosis revisted. J Pak Med Assoc 51(1):16–18
Pesut D, Stojsić J (2007) Female genital tuberculosis–a disease seen again in Europe. Vojnosanit Pregl 64(12):855–858
Sutherland AM (1985) Gynaecological tuberculosis: analysis of a personal series of 710 cases. Aust New Zeal J Obstet Gynaecol 25(3):203–207
Sharma JB, Sharma E, Sharma S, Dharmendra S (2018) Female genital tuberculosis: revisited. Indian J Med Res 148(Suppl):S71–S83. https://doi.org/10.4103/ijmr.IJMR_648_18
Mondal SK (2013) Histopathologic analysis of female genital tuberculosis: a fifteen-year retrospective study of 110 cases in eastern India. Turk Patoloji Derg 29(1):41–45. https://doi.org/10.5146/tjpath.2013.01146 (PMID: 23354795)
Agarwal J, Gupta JK. Female genital tuberculosis--a retrospective clinico-pathologic study of 501 cases. [published correction appears in Indian J Pathol Microbiol 1994 Apr;37(2):238]. Indian J Pathol Microbiol. 1993;36(4):389–397.
Tripathy SN, Tripathy SN (2002) Infertility and pregnancy outcome in female genital tuberculosis. Int J Gynaecol Obstet 76(2):159–163. https://doi.org/10.1016/s0020-7292(01)00525-2
Grace GA, Devaleenal DBNM (2017) Genital tuberculosis in females. Indian J Med Res 145(4):425–436
Singh N, Sumana G, Mittal S (2008) Genital tuberculosis: a leading cause for infertility in women seeking assisted conception in North India. Arch Gynecol Obstet 278(4):325–327. https://doi.org/10.1007/s00404-008-0590-y
Falzon D, van Cauteren D (2008) Demographic features and trends in tuberculosis cases in the European Region, 1995–2005. Euro Surveill
Te Beek LAM, Van Der Werf MJ, Richter C, Borgdorff MW (2006) Extrapulmonary tuberculosis by nationality, the Netherlands, 1993–2001. Emerg Infect Dis 12(9):1375–1382. https://doi.org/10.3201/eid1209.050553
Kocher C, Weber R, Friedl A (2011) A case study of female genital tuberculosis in a Western European setting. Infection 39(1):59–63
Nogales-Ortiz F, Tarancon I, Nogales FF. The pathology of female genital tuberculosis. A 31-year study of 1436 cases. Obstet Gynecol. 1979;53(4):422–428.
Nissapatorn V, Kuppusamy I, Rohela M, Anuar AK, Fong MY (2004) Extrapulmonary tuberculosis in Peninsular Malaysia: retrospective study of 195 cases. Southeast Asian J Trop Med Public Health 35(Suppl 2):39–45
Adam J. Langer, Shareen A. Iqbal, Robert Pratt CAT, La’Toya D. Lane. Reported Tuberculosis in the United States (2015) Rep Tuberc United States, 2015 Centers Dis Control Prev Natl Cent HIV/AIDS. Viral Hepatitis, STD, TB Prev Div Tuberc Elimin, p 2016
Parikh FR, Nadkarni SG, Kamat SA, Naik N, Soonawala SB, Parikh RM (1997) Genital tuberculosis–a major pelvic factor causing infertility in Indian women. Fertil Steril 67(3):497–500. https://doi.org/10.1016/s0015-0282(97)80076-3
Csordas SE, Monheit BM (1982) Gynaecological tuberculosis in Victoria: a 20-year survey. Aust N Z J Obstet Gynaecol 22(2):86–89. https://doi.org/10.1111/j.1479-828x.1982.tb01410.x
Sharma JB, Pushparaj M, Roy KK et al (2008) Hysterosalpingographic findings in infertile women with genital tuberculosis. Int J Gynaecol Obstet 101(2):150–155. https://doi.org/10.1016/j.ijgo.2007.11.006
Sharma JB, Roy KK, Pushparaj M, Kumar S, Malhotra N, Mittal S (2008) Laparoscopic findings in female genital tuberculosis. Arch Gynecol Obstet 278(4):359–364. https://doi.org/10.1007/s00404-008-0586-7
Mondal SK, Dutta TK (2009) A ten year clinicopathological study of female genital tuberculosis and impact on fertility. JNMA J Nepal Med Assoc 48(173):52–57
Soussis I, Trew G, Matalliotakis I, Margara R, Winston RM (1998) In vitro fertilization treatment in genital tuberculosis. J Assist Reprod Genet 15(6):378–380. https://doi.org/10.1023/a:1022533016670
Funding
This study has no funding.
Author information
Authors and Affiliations
Contributions
CR-C: Conception of the work AND Design of the work AND Acquisition of data AND Analysis of data AND Interpretation of data. JM: Acquisition of data AND Analysis of data. CCJ: Revising the work critically for important intellectual content And Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interests.
Consent statement
Not applicable.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors. The Ethical Committee of North Lisbon Hospital Center approve this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Reis-de-Carvalho, C., Monteiro, J. & Calhaz-Jorge, C. Genital tuberculosis role in female infertility in Portugal. Arch Gynecol Obstet 304, 809–814 (2021). https://doi.org/10.1007/s00404-020-05956-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-020-05956-x