Abstract
Purpose
In our work, furan, lycopene, and furan + lycopene treatments were applied to non-diabetic and diabetic female rats via gavage.
Methods
Ovarian tissue alterations with histopathology, immunohistochemistry, malondialdehyde levels, oxidative stress parameters such as superoxide dismutase, catalase, glutathione peroxidase, glutathione-S-transferase and harmful effect on ovarian tissue DNA were evaluated in all groups for 28 days.
Results
Furan caused the changes histological, ovarian cell’s DNA structure, malondialdehyde levels, antioxidant enzymes activities as in a statistically significant manner in each group. Useful effect of lycopene was determined both in non-diabetic and diabetic treatment groups against furan according to the used experimental parameters. Although some histopathological alterations were seen in diabetic and non-diabetic/diabetic plus furan-treated group’s ovarians, lycopene restored these variations near to normal levels in furan + lycopene treated groups for in 28 days. Additionally, the results of our immunohistochemical analysis and alterations of the oxidative stress parameters results also supported these findings.
Conclusions
Our result confirms that lycopene has protective effect and significantly altered diabetes and furan-induced toxicity in the rat ovarian tissue.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Furan is classed as a dangerous toxicant by the International Agency for Research on Cancer. It leads to diverse types of cancers in humans [1]. Furan may occur in various kinds of processed foods, during canning process which changes carbohydrates’ structure during jarring process. Moreover, coffee also contains furan naturally [2]. Therefore, there is concern about its harmful effects in animals and humans [3]. It was first reported in foods over 30 years ago [4]. It is also generated during combustion; therefore, it is found in smoke and engine exhaust [5]. A previous study has shown that it is a toxicant agent and has harmful effect on biological system of rats [6, 7]. Due to these known adverse effects of furan, it is significant to show the toxicological effects of furan.
There are protective mechanisms in cell to avoid from oxidative stress via reducing the pro-oxidative disorders by antioxidants. Antioxidants are used in cells for evaluating protective effect on level of oxidative stress by catalase (CAT), glutathione peroxidase (GPx), superoxide dismutase (SOD), and glutathione-S-transferase (GST). Many toxicants are harmful to cell’ membranes, since they produce malondialdehyde (MDA) from lipid peroxidation (LPO) and reactive oxygen species (ROS) [8, 9].
Lycopene is found in fruits together with carotenoid. Many studies demonstrated that lycopene has helped to eliminate the adverse effects of risk factors in the case of heart and cancer diseases [10, 11]. The previous studies have demonstrated that lycopene destroys ROS damage in cell membranes and DNA damage [12, 13], and lycopene has been studied for a long time in the hunt for hydroxyls and superoxides to prove its antioxidant capacity. It also has protective role against lipid peroxidation, caused ROS in cell membranes and DNA damage [14]. Harmful chemicals can change cell’s signaling pathways, and defensive and protective systems of cells in varying physiological and pathological conditions [6, 10].
Diabetes is a metabolic disorder and 2.5–3% of world’s population is struggling with this important illness. Pathophysiological mechanisms of diabetes were shown in many studies [15]. Apoptosis, inflammation, and oxidative stress can often be seen along with high glucose [16, 17]. Free radicals increase in the pancreatic β-cells, as they generate from protein glycosylation and glucose auto-oxidation [18]. Subsequently, oxidative stress can cause pancreatic inflammation and apoptosis in these cells [19, 20].
Comet assay is one of the methods for DNA damage detection in the cell under in vitro and in vivo conditions [6, 7]. This method is also called as single-cell gel electrophoresis and widely used in a variety of cells’ single- and double-strand breaks and alkali-labile sites [21]. This inexpensive method is relatively sensitive and rapid. It is commonly used in genotoxicity testing for widespread applications in human population and environmental monitoring [22, 23].
The consumption of lycopene has shown to support reducing all complications regarding to diabetes mellitus (DM). It was found that it cures the advancement of toxic effect of furan, protects the liver, lung, and kidney against harmful effects, but there are not enough studies about its effects on the ovarian. Hence, in this study, we focused on ovary in experimental non-diabetic and diabetic rats. Currently, protective effect of lycopene against ovary structure and functions is unknown. The purpose of this work is to identify the effect of furan on the ovary of non-diabetic and diabetic female rats, and to show whether these adverse effects can be cured by lycopene and on the tissue damage, whether level of oxidative stress and DNA damage in the rat’ ovary can be decreased.
Materials and methods
Animals and chemicals
Female Wistar–Albino rats (300–320 g) were administrated according to standard protocol for use and care of laboratory animals. Çukurova University Animal Experiments Local Ethics Committee approved our treatment procedure. Rats were feed with standard laboratory chow and water ad libitum at 23 ± 1 °C with periods of light and dark (12 h/12 h). Furan, streptozotocin (STZ), lycopene, and other chemicals were obtained from Sigma-Aldrich. Distilled water was used for dissolving furan and lycopene.
Animal grouping and treatment
Fifty-six Wistar–Albino rats were allocated as eight groups: In control group (group 1), 1 mL of 0.9% NaCl saline solution was injected to rats as orally during 28 days. Lycopene group (group 2) received lycopene at 4 mg/kg b.w. via gavage for 28 days. Furan group (group 3) received 40 mg/kg b.w. furan via gavage. Furan + lycopene group (group 4), 40 mg/kg furan and 4 mg/kg b.w. lycopene were given for 28 days. In diabetic control group (group 5), single dose of STZ was injected to cause diabetes. Diabetic lycopene group (group 6) received single dose of STZ and lycopene at 4 mg/kg b.w. via gavage for 28 days. Diabetic furan group (group 7) received a single dose of STZ and 40 mg/kg b.w. furan via gavage. In diabetic furan + lycopene group (group 8), a single dose of STZ 40 mg/kg furan and 4 mg/kg b.w. lycopene was given for 28 days. Rats were taken under general anesthesia by an intraperitoneal injection of ketamine hydrochloride (60 mg/kg, Ketalar) and xylazine hydrochloride (10 mg/kg). Samples were obtained surgically from control and treatment groups and arranged for light microscopic, biochemical, and DNA damage inquiry for examination. Level of malondialdehyde (MDA) and enzymes activities (CAT, SOD, GPx, GST) of ovary tissue was calculated.
Assessment of diabetes mellitus (DM)
STZ dissolved in cold 0.1 M sodium citrate buffer, pH 4.5 (always prepared fresh for immediate use within 5 min). STZ single-dose injection (55 mg/kg) was given intraperitoneally. The blood glucose concentration was measured after 2 days of STZ injection for diabetes induction confirmation via a glucometer. The blood samples were collected from the tail. Animals whose blood glucose levels were over 300 mg/dl were considered diabetic and used for this study [15].
Measurement of tissue damage
Sodium phosphate buffer (pH 7.2) was used for dissecting the ovary tissues’ washing. The obtained ovarian tissues were fixed in 10% formalin and then passed graded ethanol series, and prepared in paraffin block. Hematoxylin and eosin (H&E) was used for cutting the tissue. Tissue images were obtained from olympus light microscope (Olympus BX51, Tokyo, Japan) with an attached camera using seven slides. Histopathological changes in all groups were gradated as none (–), weak (+), mild (++), moderate (+++), and severe (++++) damage.
Immunohistochemistry
Leica Bond-Max (Leica, Bannockburn, IL, USA automatic) immunostainer and Apaf-1 expression were used for immunohistochemistry. Formalin was used for fixation and samples were transferred to paraffin and sections (5–6 μm) were cut using a microtome (Leica RM2255, Germany) and then dried air at 36–37 °C. Images of tissue were obtained from olympus light microscope (Olympus BX51, Tokyo, Japan).
Assessment of oxidative stress
Measurement of malondialdehyde (MDA) level
Level of MDA was determined using the thiobarbituric acid (TBA) test as described by Ohkawa et al. [24]. MDA and TBA were combined with each other to form a colored complex, and these reactions were calculated spectrophotometrically at 532 nm to measure MDA levels. The specific activity was defined as nmol per mg protein.
Measurement of superoxide dismutase (SOD) activity
Inhibition of autoxidation of pyrogallol demonstrates SOD activity. This reaction was calculated according to the Marklund and Marklund’s method [25]. The activity was calculated at 440 nm for 180 s nmol/mg protein which was used as data expression.
Measurement of catalase (CAT) activity
Method of Aebi [26] was used for CAT activity for ovary tissue according to the rate of decomposition hydrogen peroxide (H2O2) at 240 nm for 60 s μmol/mg protein was used as data expression.
Measurement of glutathione peroxidase (GSH-Px) activity
Method of Paglia and Valentine [27] was used for GPx activity and measured as spectrophotometrically. NADPH, reduced glutathione, Tris–HCl, and glutathione reductase were mixtured for reaction. H2O2 was added for the beginning of reaction and GPx activity was calculated as the change in absorbance at 340 nm nmol/mg protein that was used as data expression.
Measurement of glutathione-S-transferase (GST) activity
Enzyme activities of GST of ovary were analyzed by determination of the generation of glutathione and the 1-chloro 2,4-dinitrobenzene conjugate [28]. Increments in absorbance were stated at 340 nm. The enzyme is represented as nanomoles of glutathione 1-chloro 2,4-dinitrobenzene conjugate formed per minute per milligram protein.
Protein estimation
The ovary tissue’s protein concentration was measured according to the method of Lowry et al. [29] and bovine serum albumin was used as a standard. These parameters were measured by a spectrophotometer.
Data analysis
SPSS 20.0 for Windows was used for calculation of values. ANOVA and Tukey were applied result for comparing the experimental groups. p < 0.05 show statistically significant between groups. Standard error of the mean (SEM) was used for results.
Measurement of DNA damage with comet assay
Control and treated cells of ovary were obtained and centrifuged with magnetic stirrer at 500×g for 5 min and then rested for 20 min in PBS. Obtained supernatant from control and treatment groups was stirred with low melting point agarose (0.65%); 75 µl of suspension was immediately layered over slides which were precoated with normal melting point agarose (0.05%) and then quickly covered with a cover slip. The slides were kept at +4 °C for 30 min. Slides leaving from coverslip were transferred into cold lysing solution (2.5 M NaCl, 100 mM EDTA, 10 mM Tris, pH 10, in which 10% DMSO, 1% Triton X-100) for 1 h. Horizontal gel electrophoresis platform was filled with freshly made pH > 13 electrophoresis buffer (300 mM NaOH, 1 mM EDTA) until the liquid level completely covers the slides. To unwinding of the DNA, slides were waited for 20 min [30]. Power supply was turned on to 25 V for 20 min. The slides were lifted gently from the buffer and placed on a drain tray and then washed three times for 5 min with neutralizing buffer (0.4 M Tris–HCl buffer, pH 7.5). Slides were stained with 80 µl of ethidium bromide (10 mg in 50 ml of distilled water) for 5 min and then dipped in chilled distilled water to remove excess stain. The slides were covered with coverslip and scored immediately using BS 200 ProP with software image analysis (BS 200 ProP, BAB Imaging System, Ankara, Turkey). A 40× objective on a fluorescent microscope was used for observations DNA damage. The tail DNA% (100—Head% DNA), tail length, and tail moment of 50 comets were identified and calculated differences between groups [31].
Results
Determined tissue damage in ovary
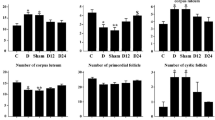
Normal ovary structure with many primordial, primary, secondary, and antral follicles was seen in control group (Fig. 1a). The pathological changes were detected as edema and hemorrhage in diabetic control (Fig. 1b). No change has been detected in lycopene treatment group (Fig. 2a). Lycopene treatment effectively reduced the ovarian tissue damage in diabetic lycopene treatment group (Fig. 2b). Histopathological changing in furan-induced non-diabetics and diabetics’ female rats was observed such as severe hemorrhage, vascular congestion, edema, follicular degeneration, and leukocyte infiltration in the ovary tissue (Fig. 2c, d). Moderate pathological changing was seen in diabetics’ rats taking lycopene plus furan. Hemorrhage, vascular congestion, and edema were seen in this group (Fig. 2e, f). The histopathological alterations in samples of rats were graded for non-diabetic and diabetic furan and/or lycopene, and were determined as scored in Table 1.
Ovary section of control (a) and diabetic control (b) rats showing normal morphology of many different stages of developing follicles. A antrum, GC granular cell, PF primary follicle, ZP zona pellucida, GEp germinal epithelial, TA tunica albuginea, CL corpus luteum, SF secondary follicle, asterisk edema, double arrow hemorrhage ×200
Ovary sections of a lycopene, b diabetic lycopene, c furan, d diabetic furan, e furan + lycopene, f diabetic furan + lycopene treated showing A antrum, GC granular cell, PF primary follicle, SF secondary follicle, GC germinal cell, CL corpus luteum, asterisk edema, double arrow: hemorrhage, filled right side pointing triangle vascular congestion, open right side pointing triangle leukocyte infiltration, and single arrow follicular degeneration × 200
Secretion of Apaf-1 was seen as weakly in the control, diabetic control, lycopene, and diabetic lycopene treatment group (Figs. 3, 4a, b), but furan treatment group has moderate Apaf-1 expression (Fig. 4c). Secretion of Apaf-1 was strongly obtained in the diabetic furan group (Fig. 4d). The non-diabetic and diabetic furan + lycopene group has moderate secretion in terms of Apaf-1 (Fig. 4e, f).
Ovary sections of a lycopene, b diabetic lycopene, c furan, d diabetic furan, e furan + lycopene, f diabetic furan + lycopene in female rats’ ovary showing Apaf-1 protein expression with immunohistochemical analysis. Single arrow Apaf-1 expression in diabetic control, diabetic furan, and diabetic furan + lycopene group shows multiple apoptotic cells × 200
Determined of MDA levels and antioxidant enzyme activities of ovary tissue
MDA production significantly increased in the diabetic control when compared to the control group (p < 0.05). Level of MDA increased in the furan-treated non-diabetic and diabetic groups compared to the diabetic control and control group. MDA level decreased in the furan + lycopene-treated group compared to the furan-treated group (p < 0.05) (Fig. 5).
CAT, SOD, GPx, and GST enzymes have lower activities in the diabetic control than the control group (p < 0.05). These enzyme activities statistically decreased in the furan-treated non-diabetic and diabetic groups compared to diabetic control group. Non-diabetic and diabetic furan + lycopene administration increased antioxidant enzymes activities when compared to the non-diabetic and diabetic furan-treated group (p < 0.05) (Fig. 5).
Determined DNA damage in the ovary tissue
The mean tail DNA% and tail length significantly raised in diabetic control and non-diabetic/diabetic + furan treatment groups according to the comet assay results. Used parameters for DNA damage decreased in the non-diabetic/diabetic furan + lycopene and diabetic lycopene groups compared with the non-diabetic/diabetic furan and non-diabetic/diabetic control groups, respectively (Fig. 6). Scores of the DNA damage were showed in Table 2 for the control and non-diabetic/diabetic groups.
DNA damage in rat ovary exposed to furan and/or lycopene a control group, b diabetic control group, c lycopene treatment group, d diabetic lycopene treatment group, e furan treatment group, f diabetic furan treatment group, g furan + lycopene treatment group, and h diabetic furan + lycopene treatment group
Discussion
Furan can be used as an intermediate agent for chemical reactions, since it is a main compound of many chemicals. It is known that little amount of exposure to [2] and metabolized cytotoxic metabolites lead-binding proteins and nucleosides irreversibly [32, 33]. The toxicity of furan is attributed as cis-2-butene-1,4-dialdehyde due to the cause uncoupling of mitochondrial oxidative phosphorylation and cell proliferation [34, 35]. The previous studies have shown that furan induced some histopathological damage in the male rats’ kidney and liver along with the changes in liver and serum enzyme levels at increasing doses [36], and so it has been also classified as a potential human carcinogen. El-Akabawy and El-Sherif [37] have demonstrated that the furan induced oxidative changes in the adult rat testis, but the potential of furan to induce oxidative stress damage in the ovary of rats has not been demonstrated yet. This is the first in vivo assessment of furan and lycopene caused effects in non-diabetic and diabetic rat ovarian associated with changes histopathological, oxidative stress parameters, and DNA damage.
Diabetes mellitus (DM) presents a rapid growing health problem and it is one of the most common causes of vascular disease worldwide [38, 39]. Different alterations have been attributed to the increased production of ROS, which results from reduced activity of SOD and CAT, reduced total glutathione level, and increased activity of GPx [40]. Most studies have used streptozotocin for obtaining experimental diabetes. Many of these studies indicated that DM has affected biomechanical structure or fracture healing with histologic changes in the fracture callus in type 1 diabetes animal models [38, 41]. Other studies have shown that DM induced biochemical alterations, protein and collagen metabolism, and DNA structure of cells [42,43,44,45,46]. In addition to these studies, we have also used diabetic rats to evaluate the effects of lycopene (4 mg/kg bw) and furan (40 mg/kg bw) in diabetic individuals for oxidative stress parameters in this study.
Histopathological alterations were obtained in the ovary tissue after daily treatment of furan. Bas and Pandır [10] have shown that furan induced lung toxicity in the diabetic rats with severe pathological alterations. Emphysematous changes, hemorrhage, changes in connective tissue of the alveolar septa, edema, and desquamation of the epithelial cell of the terminal bronchiole were observed in the diabetic furan group. Lycopene treatment cured these transformations. Emphysematous and hemorrhage were seen in the diabetic furan + lycopene group in moderate level. Unal et al. [11] demonstrated that histological damages of kidney were more severe in diabetic furan group, particularly extensive inflammatory cell infiltration, glomerular lobulation, glomerular atrophy, tubular degeneration, hemorrhage, and dilatation of Bowmann’s space. Lycopene supplementation was protective against furan caused histopathological changes, too. Administration of furan increased severe hemorrhage, edema, follicular degeneration, and vascular congestion in the ovary tissue of non-diabetic and diabetic treatment group in this study. However, milder pathological changes were seen in lycopene + furan non-diabetic and treated diabetic rats. When the non-diabetic group and the diabetic group were compared, much more pathological changes were observed in diabetic group. In this way, it seems that lycopene ameliorate furan induced toxicity, but exact protection was not seen in rat ovary tissues.
Apoptosis occurs during normal physiological process in cells. External or internal warnings activate apoptotic mechanism. Mitochondrial pathway works and apoptotic protein is forms in case of DNA damage, hyperoxia, and oncogene activation [47, 48]. Apaf-1, the signal protein, was used to show apoptosis in many animal studies [49, 50]. Mouse ovary’s granulosa cells secreted Apaf-1 in apoptosis process [49]. Bas et al. [13] were used Apaf-1 antibody for ischemia/reperfusion (I/R) injury in Wistar rats’ ovary. Their study has shown that the I/R has a harmful effect on testis tissue, but administration of vardenafil reduced these effects. Germ cell was evaluated by apoptosis with the Apaf-1 antibody [50]. Ovarian furan injury with lycopene in non-diabetic and diabetic condition has not been evaluated using the expression of Apaf-1 antibody until now. In this study, Apaf-1 expression was evaluated in control and treatment groups. Apaf-1 expression in the non-diabetic and diabetic furan groups was seen stronger than non-diabetic and diabetic control groups. Non-diabetic and diabetic furan + lycopene groups have lower Apaf-1 expression than non-diabetic and diabetic furan group.
MDA, which occurs during lipid peroxidation, is a signal of oxidative stress and leads to tissue damage [2, 51]. Antioxidant enzymes such as CAT, SOD, GST, and GPx enzymes struggle to prevent the harmful effect of chemicals on ovarian tissue. They are the most important enzymatic systems in cellular membranes for protection of tissues against toxicants [52,53,54]. If antioxidant mechanism of cells is damaged by chemicals’ antioxidant enzyme activities and their gene expressions decrease because of increasing oxidative stress [55]. This study showed a significant reduction in CAT, SOD, GPx, and GST activities and a significant elevation of MDA levels in furan induced ovarian tissue injury. Under these conditions, administered lycopene increased the enzymatic activities and lead to MDA level decrease in rats ovary. The same results were obtained in diabetic groups and also there are more harmful effects in the diabetic groups compared to non-diabetic groups.
Single and double-strand breakage, disruption of deoxyribose, and creation of DNA–protein were seen DNA structure during the oxidative stress [56]. These modifications have been detected with alkaline comet assays in both clinical and occupational exposures [57]. Deoxyguanosine to 8-oxo-7,8-dihydro-2-deoxyguanosine (8-oxodG) occurs in oxidative stress-induced DNA damage [58]. 8-oxodG and 8-hydroxyguanine (8-oxoG) increased in diabetes of cells’ DNA [59] and is indicators of oxidative DNA damage in blood and tissue. MDA is important indicator of oxidative stress in cell membranes of diabetes. Currently, 8-oxodG, alkaline, and modified comet assay are widely used for showing oxidatively damaged DNA in diabetes [60]. Body mass index, serum glucose level, and FPG-sensitive are related with oxidative stress in diabetes [61]. The present study has shown that the relationship between the subsequent DNA damage and diabetes in the ovary of diabetic rat was demonstrated with alkaline comet assay because of being suitable endpoint of detection. This study also indicated that furan has increased the DNA damage, but lycopene has ameliorated this effect on ovary cells under non-diabetic or diabetic conditions and also detected the fitness of the modified comet assay in the present of the oxidative stress-induced DNA damage.
Conclusion
Lycopene administration reversed the histopathologic changes that occured because of the ovarian damage. Furan + lycopene group has significantly higher (p < 0.05) in point of antioxidant enzymes activities compared to furan treatment in the ovarian tissue. MDA level of the ovarian was significantly lowered in furan + lycopene group than other treatment groups (p < 0.05). In this study, we detected that administration of lycopene ameliorated the ovarian tissue from the toxic effect of non-diabetic and diabetic furan. While our results have shown that protective effect of lycopene on furan induced ovarian toxicity, large prospective randomized controlled studies are necessary.
References
IARC (International Agency for Research on Cancer) (1995a) IARC monographs on the evaluation of carcinogenic risks to humans, dry cleaning, some chlorinated solvents and other industrial chemicals, vol 63. France, Lyon, pp 3194–3407
Moro S, Chipman JK, Wegener JW, Hamberger C, Dekant W, Mally A (2012) Furan in heat-treated foods: formation, exposure, toxicity, and aspects of risk assessment. Mol Nutr Food Res 56:1197–1211
Hamadeh HK, Jayadev S, Gaillard ET, Huang Q, Stoll R, Blanchard K (2004) Integration of clinical and gene expression endpoints to explore furan-mediated hepatotoxicity. Mutat Res 549:169–183
Maga JA, Katz I (1979) Furans in foods. Crit Rev Food Sci Nutr 11:355–400
IARC (International Agency for Research on Cancer) (1995b) Dry cleaning, furan. In: IARC monographs on the evaluation of carcinogenic risks to humans, some chlorinated solvents and other industrial chemicals, vol 63. France, Lyon, pp 393–407
Pandır D (2015) Assesment of the DNA damage in human sperm and lymphocytes exposed to the carcinogen food contaminant furan with comet assay. Braz Arch Biol Technol 58:773–780
Pandır D (2015) Protective effect of (-)-epigallocatechin-3-gallate on capsaicin-induced DNA damage and oxidative stress in human erythrocytes and leucocytes in vitro. Cytotechnology 67(2):367–377
Cooke GM, Taylor M, Bourque C, Curran I, Gurofsky S, Gill S (2014) Effects of furan on male rat reproduction parameters in a 90-day gavage study. Reprod Toxicol 46:85–90
Farokhi F, Farkhad NK, Togmechi A, Soltani K (2012) Preventive effects of Prangos ferulacea (L.) Lindle on liver damage of diabetic rats induced by alloxan. Avicenna J Phytomed 2:63–71
Bas H, Pandır D (2016) Protective effects of lycopene on furan-treated diabetic and non-diabetic rat lung. Biomed Environ Sci 29:143–147
Unal B, Pandir D, Bas H (2016) Lycopene protects the diabetic rat kidney against oxidative stress-mediated oxidative damage induced by furan. Braz Arc Biol Technol 59:e16150794 (January–December 1–12)
Atessahin A, Karahan I, Türk G, Gür S, Yılmaz S, Ceribası AO (2006) Protective role of lycopene on cisplatin-induced changes in sperm characteristics, testicular damage and oxidative stress in rats. Reprod Toxicol 21:42–47
Bas H, Kara O, Kara M, Pandır D (2013) Protective effect of vardenafil on ischemia-reperfusion injury in rat ovary. Turk J Med Sci 43(5):684–689
Leonard SS, Xia C, Jiang BH, Stinefelt B, Klandorf H, Harris GK, Shi X (2003) Resveratrol scavenges reactive oxygen species and effects radical-induced cellular responses. BBRC 309:1017–1026
Schmatz R, Mazzanti CM, Spanevello R, Stefanello N, Gutierres J, Corrêa M (2009) Resveratrol prevents memory deficits and the increase in acetylcholinesterase activity in streptozotocin-induced diabetic rats. Eur J Pharmacol 610:42–48
Babu PVA, Liu D, Gilbert ER (2013) Recent advances in understanding the anti-diabetic actions of dietary flavonoids. J Nutr Biochem 24:1777–1789
Yin W, Li B, Li X, Yu F, Cai Q, Zhang Z, Cheng M, Gao H (2015) Anti-inflammatory effects of grape seed procyanidin B2 on a diabetic pancreas. Food Funct 6:3065–3071
Elosta T, Ghous N, Ahmed N (2012) Natural products as anti-glycation agents: possible therapeutic potential for diabetic complications. Curr Diabetes Rev 8:92–108
Coskun O, Kanter M, Korkmaz A, Oter S (2005) Quercetin: a flavonoid antioxidant, prevents and protects streptozotocin-induced oxidative stress and b-cell damage in rat pancreas. Pharmacol Res 51:117–123
Rashid K, Sil PC (2015) Curcumin enhances recovery of pancreatic islets from cellular stress induced inflammation and apoptosis in diabetic rats. Toxicol Appl Pharmacol 282:297–310
Olive PL, Banáth JP (2006) The comet assay: a method to measure DNA damage in individual cells. Nat Protoc 1:23–29
Forchhammer L, Ersson C, Loft S, Moller L, Godschalk RW, Van Schooten FJ (2012) Inter-laboratory variation in DNA damage using a standard comet assay protocol. Mutagenesis 27:65–672
Amaeze NH, Schnell S, Sozeri O, Otitoloju AA, Egonmwan RI, Arlt VM, Bury NR (2015) Cytotoxic and genotoxic responses of the RTgill-W1 fish cells in combination with the yeast oestrogen screen to determine the sediment quality of Lagos lagoon. Niger Mutagen 30:117–127
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Marklund S, Marklund G (1974) Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem 474:469–474
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Paglia DE, Valentine WN (1967) Studies on the quantitative and qualitative characterization of glutathione peroxidase. J Lab Clin Med 70:158–169
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione-S-transferases: the first enzymatic step in mercapturic acid formation. J Biol Chem 249:7130–7139
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 19:265
Ozkan D, Yuzbasıoglu D, Unal F, Yılmaz S, Aksoy H (2009) Evaluation of the cytogenetic damage induced by the organophosphorous insecticide acephate. Cytotechnology 59:73–80
Behravan J, Mosafa F, Soudmand N, Taghiabadi E, Razavi BM, Karimi G (2011) Protective effects of aqueous and ethanolic extracts of Portulaca oleracea L. aerial parts on H2O2-induced DNA damage in lymphocytes by comet assay. Acupunct Meridian Stud 4:193–197
Burka LT, Washburn KD, Irwin RD (1991) Disposition of [14C] furan in the male F344 rat. J Toxicol Environ Health 34:245–257
Crews C, Castle LA (2007) Review of the occurrence, formation and analysis of furan in heat-processed foods. Trends Food Sci Technol 18:344–345
Mugford CA, Carfagna MA, Kedderis GL (1997) Furan-mediated uncoupling of hepatic phosphorylation in Fisher-344 rats: an early event in cell death. Toxicol Appl Pharm 144:1–11
Kedderis GL, Ploch SA (1999) The biochemical toxicology of furan. Chem Ind Inst Toxicol 1:1–8
Selmanoglu G, Karacaoglu E, Kılıc A, Kockaya EA, Akay MT (2012) Toxicity of food contaminant furan on liver and kidney of growing male rats. Environ Toxicol 27:613–622
El-Akabawy G, El-Sherif NM (2016) Protective role of garlic oil against oxidative damage induced by furan exposure from weaning through adulthood in adult rat testis. Acta Histochem 118:456–463
Letchuman GR, WanNazaimoon WM, WanMohamad WB, Chandran LR, Tee GH, Reddy GK, Stehno-Bittel L, Hamade S, Enwemeka CS (2001) The biomechanical integrity of bone in experimental diabetes. Diabetes Res Clin Pract 54:1–8
Shaw JE, Sicree RA, Zimmet PZ (2010) Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract 87:4–14
Shi Y, Vanhoutte PM (2008) Oxidative stress and COX cause hyper-responsiveness in vascular smooth muscle of the femoral artery from diabetic rats. Br J Pharmacol 154:639–651
Rerup CC (1970) Drugs producing diabetes through damage of the insulin secreting cells. Pharmacol Rev 22:485–518
Black CT, Hennessey PJ, Ford EG, Andrassy RJ (1989) Protein glycosylation and collagen metabolism in normal and diabetic rats. J Surg Res 47:200–202
Chang K, Uitto J, Rowold EA, Grant GA, Kilo C, Williamson JR (1980) Increased collagen cross-linkages in experimental diabetes: reversal by beta-aminopropionitrile and d-penicillamine. Diabetes 29:778–781
Spanheimer RG (1989) Collagen production in bone and cartilage after short-term exposure to streptozotocin. Matrix 9:172–174
Spanheimer RG (1988) Direct inhibition of collagen production in vitro by diabetic rat serum. Metabolism 37:479–485
Spanheimer RG, Umpierrez GE, Stumpf V (1988) Decreased collagen production in diabetic rats. Diabetes 37:371–485
Ferraro E, Corvaro M, Cecconi F (2003) Physiological and pathological roles of Apaf-1 and the apoptosome. J Cell Mol Med 7(1):21–34
Hajra KM, Liu JR (2004) Apoptosome dysfunction in human cancer. Apoptosis 9(6):691–704
Robles R, Tao XJ, Trbovich AM, Maravel DV, Nahum R, Perez GI (1999) Localization regulation and possible consequences of apoptotic protease-activating factor-1 (Apaf-1) expression in granulosa cells of the mouse ovary. Endocrinology 140(6):2641–2644
Erol B, Tokgoz H, Hanci V, Bektas S, Akduman B, Yencilek F (2009) Vardenafil reduces testicular damage following ischemia/reperfusion injury in rats. Kaohsiung J Med Sci 25(7):374–380
Celik I, Suzek H (2009) Effects of subacute exposure of dichlorvos at sublethal dosages on erythrocyte and tissue antioxidant defense systems and lipid peroxidation in rats. Ecotoxicol Environ Saf 7:905–958
Gupta RK, Schuh RA, Fiskum G, Flaws JA (2006) Methoxychlor causes mitochondrial dysfunction and oxidative damage in the mouse ovary. Toxicol Appl Pharmacol 216:436–445
Sasaki M, Joh T (2007) Oxidative stress and ischemia-reperfusion injury in gastrointestinal tract and antioxidant protective agents. J Clin Biochem Nutr 40:1–12
Kara M, Daglioglu YK, Kuyucu Y, Tuli A, Tap A (2012) The effect of edaravone on ischemia–reperfusion injury in rat ovary. Eur J Obstet Gynecol Reprod Biol 162:197–202
Rodriguez C, Mayo JC, Sainz RM, Antolin I, Herrera F, Martin V, Reiter RJ (2004) Regulation of antioxidant enzymes: a significant role for melatonin. J Pineal Res 36:1–9
Evans MD, Cooke MS (2004) Factors contributing to the outcome of oxidative damage to nucleic acid. Bioassays 26:533–542
Balasubramanyam M, Adaikalakoteswari A, Sameermahmood Z, Mohan V (2010) Biomarkers of oxidative stress: methods and measures of oxidative DNA damage (COMET assay) and telomere shortening. Methods Mol Biol 610:245–261
Blasiak J, Arabski M, Krupa R, Wozniak K, Zadrozny M, Kasznicki J, Zurawska M, Drzewoski J (2004) DNA damage and repair in type 2 diabetes mellitus. Mutat Res 554:297–304
Park KS, Kim JH, Kim MS, Kim JM, Kim SK, Choi JY (2001) Effects of insulin and antioxidant on plasma 8-hydroxyguanine and tissue 8-hydroxydeoxyguanosine in streptozotocin-induced diabetic rats. Diabetes 50:2837–2841
Xu GW, Yao QH, Weng QF, Su BL, Zhang X, Xiong JH (2004) Study of urinary 8-hydroxydeoxyguanosine as a biomarker of oxidative DNA damage in diabetic nephropathy patients. J Pharm Biomed Anal 36:101–104
Collins AR, Dusinska M, Gedik CM, Stetina R (1996) Oxidative damage to DNA: do we have a reliable biomarker? Environ Health Perspect 104:465–469
Author information
Authors and Affiliations
Contributions
DP: manuscript writing, SU: manuscript editing, and DP: manuscript editing.
Corresponding author
Ethics declarations
Funding
This work was supported by the Bozok University Scientific Research Projects Unit by the code 6601-FBE/16-38.
Conflict of interest
All authors declare that there is no conflict of interest.
Ethical approval
Female Wistar–Albino rats (300–320 g) were administrated according to standard protocol for use and care of laboratory animals. Çukurova University Animal Experiments Local Ethics Committee approved our treatment procedure (11/1).
Rights and permissions
About this article
Cite this article
Uçar, S., Pandir, D. Furan induced ovarian damage in non-diabetic and diabetic rats and cellular protective role of lycopene. Arch Gynecol Obstet 296, 1027–1037 (2017). https://doi.org/10.1007/s00404-017-4521-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-017-4521-7