Abstract
Diabetes mellitus is a common chronic metabolic disorder. This study aimed to investigate the effects of co-treatment with l-carnitine (LC) and zinc oxide nanoparticles (ZnONPs) on serum levels of sex hormones, oxidative stress, and ovarian histopathology in streptozotocin (STZ)-induced diabetic rats. Female Wistar rats (n = 56, 180–220 g) received a single intraperitoneal (IP) injection of STZ (65 mg/kg). They were randomly assigned into the following groups: diabetic group (Dia), Dia+Met group (100 mg metformin/kg/day), Dia+LC group (200 mg/kg/day), Dia+ZnONPs group (10 mg/kg/day), and Dia+LC+ZnONPs group (200 mg LC/kg/day and 10 mg ZnONPs/kg/day). Control group (Ctl) received the same volume of STZ solvent. After 21 days of treatment, blood serum was centrifuged for sex hormone assays. The right ovary was used for biochemical analysis, and the left ovary was fixed in 10% neutral buffered formalin for histological assessment. The levels of estradiol, progesterone, FSH, and LH significantly increased in the Dia+ZnONPs+LC group (P < 0.001) compared with the Dia group. Co-treatment with LC and ZnONPs reduced malondialdehyde and carbonyl protein and increased glutathione, catalase, and superoxide dismutase activities in ovarian tissue compared with the Dia group (P < 0.05). Moreover, the number of all ovarian follicles significantly increased in this group compared with the Dia group (P < 0.05). The results of this study indicated that co-treatment with LC and ZnONPs could preserve ovarian function by increasing sex hormones levels and antioxidant activity and decreasing lipid peroxidation in diabetic rats. Therefore, this compound supplementation may improve ovulation and fertility in people with diabetes mellitus.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetes mellitus is one of the most common noncommunicable chronic diseases in the world, which is a major public health problem. Type I diabetes (insulin-dependent) and type II diabetes (non-insulin-dependent) are the two main types of diabetes mellitus [1]. Diabetes mellitus causes oxidative stress owing to increased lipid and protein oxidation and decreased antioxidant enzymes, such as catalase (CAT), superoxide dismutase (SOD), and glutathione peroxidase (GPx) [2]. Evidence links diabetes-related oxidative stress to infertility [3]. Diabetic women are at higher risk of developing polycystic ovary syndrome, hirsutism [4], menstrual irregularity [5], and premature menopause [6]. The condition also decreases the number and diameter of ovarian follicles [7, 8] and ovulation rate [9]. On the other hand, it increases the number of atretic follicles [8] and oocyte aneuploidy [9].
Nowadays, there are many medications available to lower high blood glucose levels or prevent and treat diabetes. Metformin is an antihyperglycemic drug that is orally used to control type II diabetes [10]. Nanoparticles are also among the medications that have recently been considered the drug of choice in treatment of diabetes owing to reduced side effects, tissue selectivity, and reduced toxicity [11]. Metal nanoparticles, such as gold, silver, iron, zinc, and oxide nanoparticles, have many medical applications [12]. Zinc nanoparticles (ZnONPs, 1–100 nm) are one of these drugs that have been demonstrated to reduce blood glucose in diabetes [13].
l-carnitine (LC), a small water-soluble molecule, is synthesized in human liver and kidneys [14, 15]. It exerts its antioxidant effects directly as a free radical scavenger or indirectly by affecting antioxidant enzymes [16]. In addition, LC transports long-chain fatty acids into the mitochondria, protects mitochondria against oxidative stress, and reduces apoptosis [17]. LC has been shown to increase insulin sensitivity and improve insulin resistance in patients with type II diabetes [18]. The simultaneous use of LC with ZnONPs as a supplement or drug may improve fertility process in diabetic patients.
Therefore, the current study aimed to investigate the effects of co-treatment with LC and ZnONPs on serum concentrations of estrogen, progesterone, FSH, and LH; the levels of antioxidants such as glutathione (GSH), CAT, and SOD; stress biomarkers, including malondialdehyde (MDA) and protein carbonyl (PC); and ovarian histology in adult streptozotocin (STZ)-induced diabetic rats.
Materials and Methods
Chemicals
All chemicals were purchased from Sigma-Aldrich Chemical (St. Louis, MO, USA). Sex hormone kits were provided by East Bio-Pharm (USA), and SOD and CAT activity assay kits were obtained from ZellBio GmbH (Germany).
Animals
All animal experimentation protocols were approved by the Ethics Committee of Mazandaran University of Medical Sciences (IR.MAZUMS.REC.1398.3038).
Adult female Wistar rats (n = 56) weighing 180–220 g were provided from the Laboratory Animal Breeding Center in Mazandaran University of Medical Sciences. The animals were kept in 12 h light and dark conditions at approximately 22 ± 2 °C and had easy access to water and food during the study. After 2 weeks, their estrous cycle was assessed by vaginal smear and hematoxylin and eosin (H&E) staining [19]. Rats in the estrous phase were randomly assigned into the following groups:
-
1-
Control group (Ctl): a single intraperitoneal (IP) injection of 0.2 ml sodium citrate buffer (STZ solvent) was given, followed by 0.2 ml distilled water daily (as solvent of LC and ZnONPs).
-
2-
Diabetic group (Dia): the rats received an IP injection of STZ (65 mg/kg). STZ was freshly dissolved in sodium citrate buffer (pH 4.6), and after 72 h, their blood was taken from tail vein to measure the fasting blood glucose level using a glucometer (Bionime model, Taiwan), and those with a blood glucose level > 300 mg/dl were considered diabetic [20].
-
3-
Dia+Met group: diabetic rats received an IP injection of 100 mg metformin/kg/day for 21 days.
-
4-
Dia+LC group: diabetic rats received IP injection of 200 mg LC/kg/day for 21 days.
-
5-
Dia+ZnONPs group: diabetic rats received IP injection of 10 mg ZnONPs/kg/day for 21 days.
-
6-
Dia+LC+ZnONPs group: diabetic rats received simultaneous IP injection of 200 mg LC/kg and 10 mg ZnONPs/kg/day for 21 days.
Fasting blood glucose levels were measured 1 day after the last injection. The animals were then weighed and anesthetized using a combination of ketamine-xylazine. Then, the ovaries were removed and weighed after removing extra tissue and surrounding fat.
Biochemical Analyses
The right ovary was placed in a microtube containing mannitol and frozen at − 80 °C until the experiment. During the study, the ovaries were homogenized in tris buffer [21]. Thereafter, GSH levels, the activity of antioxidants (CAT, EC 1.11.1.6; SOD, EC 1.15. 1.1), and oxidative stress biomarkers (MDA and PC) were evaluated in all groups. The levels of GSH in ovarian tissue were determined by the method described by Beutler et al. [22]. A total of 1.5 ml EDTA and 1.5 ml of 10% TCA were added to each sample. After centrifugation for 15 min at 3500 rpm, 2.5 ml tris buffer (pH 8.9) and 500 μl TNB were added to the supernatant. The resulting yellow was read at 412 nm using a spectrophotometer. Glutathione was expressed in nmol/mg protein.
CAT activity was measured using ELISA kits (Cat No: ZB-CAT-48A) according to the manufacturer’s instructions. In this study, the CAT activity unit was calculated and expressed as a unit/mg protein based on the amount of sample that catalyzed the decomposition of 1 μM H2O2 into water and O2 in 1 min.
ELISA kits (Cat No: ZB-SOD-48A) were used to measure SOD activity, following the manufacturer’s instructions. The SOD activity was defined and expressed in units/mg protein as the amount of sample that catalyzed the decomposition of 1 μM of O2− to H2O2 and O2 in 1 min.
Measurement of the MDA levels in the ovarian tissue was done using thiobarbituric acid (TBA) method. The reaction of TBA with fatty acids produced a purple complex, and absorption was measured at 532 nm by ELISA. Tissue MDA levels were expressed as μM/mg protein [23].
PC content in ovarian tissue was determined by spectrophotometry using the method of Levine et al. [24]. Briefly, EDTA+TCA solution (20%) was added to each microtube containing homogenized ovarian tissue. After centrifugation for 10 min at 6500 rpm, the remaining precipitate was dissolved in 0.1 M NaOH solution; thereafter, DNPH (10 mM) was added to the microtube. After incubation for 30 min at room temperature, 500 μl of 20% EDTA+TCA was added and centrifuged at 6500 rpm for 10 min. The remaining precipitate was dissolved in 1000 μl of ethanol + ethyl acetate solution (50:50, v/v). After double-centrifugation at the previous speed and time, the precipitate was dissolved in 200 μl of 6 M guanidine hydrochloride, and the absorbance of the final solution was measured at 365 nm by spectrophotometer. The PC content was expressed as mM/mg protein.
Hormonal Measurements
Blood samples were taken from the heart; thereafter, the serum was separated and frozen at − 80 °C until testing. Serum levels of estrogen, progesterone, FSH, and LH were measured by ELISA kits (rat estradiol hormone Cat No: CK-E30608, rat progesterone Cat No: CK-E30580, rat follicle-stimulating hormone Cat No: CK-30597, rat luteinizing hormone Cat No: CK-E90904, respectively), according to the manufacturer’s instructions. The absorbance of the specimen and standard solution were read at 450 nm by ELISA (BioTeK-Synergy H1, USA).
Histopathological Study
Left ovarian tissues saved for histopathological studies were fixed in 10% neutral buffered formalin. After molding with paraffin, 5-μm-thick sections were prepared and stained with hematoxylin and eosin. The numbers of primordial, primary, secondary, tertiary, graafian follicles, corpus luteum, and atretic follicles were recorded using light microscopy (NIKON, Japan). The primordial follicle contains a squamous cell layer around the oocyte. The primary follicle contains a layer of cubic cells. The secondary follicle contains 2–5 layers of granulosa cells. A tertiary follicle is a follicle with a multilayered granulosa cell containing a number of small holes, and the graafian follicle has a large hole that occupies most of the follicle volume. An atretic follicle was discovered based on morphological criteria such as pyknotic granulosa cells and degenerated oocyte. Examinations of primordial, primary, and secondary follicles were performed at 40× magnification, and tertiary follicles were assessed at 10× magnification. Each follicle was counted in at least three sections and then expressed in total [25].
Statistical Analysis
Data were analyzed by using Graph Pad Prism 6.01 software. The results were expressed as mean ± standard error of mean (S.E.M.). One-way analysis of variance was performed, and Tukey’s post hoc test was used for multiple comparisons. Differences of P < 0.05 were considered significant.
Results
Effect of LC and ZnONPs on Body Weight, Ovarian Weight, and Serum Glucose Levels in STZ-Induced Diabetic Rats
There were no significant differences in initial weight of rats between the experimental groups and the Ctl group. Nonetheless, the final weights of rats in the experimental groups were significantly lower than that of rats in the Ctl group (P < 0.001). Compared with the Dia group, the final weights of rats were significantly higher in Dia+Met, Dia+LC, and Dia+LC+ZnONPs groups (P < 0.001). Diabetic rats showed reduced ovarian weight compared with the Ctl group, but this decrease was not significant (Table 1).
Increased ovarian weights were observed in all treatment groups; nevertheless, the increase was found to be only significant in the Dia+Met group (P < 0.001). Initial glucose levels were significantly high in all experimental groups (P < 0.001) compared with the Ctl group. The final glucose levels showed significant elevations in Dia group (P < 0.001), but they decreased in Dia+Met, Dia+LC, Dia+ZnONPs, and Dia+LC+ZnONPs groups, which were statistically significant only in Dia+LC+ZnONPs group (P ˂ 0.001, Table 1).
Effect of LC and ZnONPs on the Biomarkers of Oxidative Stress in STZ-Induced Diabetics Rats
The information about GSH in different groups is demonstrated in Fig. 1a. GSH is a non-enzymatic antioxidant, and its low levels provide a basis for increased levels of cellular oxidative stress [26]. In current study, GSH levels decreased in all groups compared with the Ctl group, but the difference were only significant in the Dia (P < 0.001) and Dia+Met (P < 0.05) groups. In the Dia+LC+ZnONPs group, the GSH level was found to be significantly higher than the Dia group (P < 0.05).
Effects of LC and ZnONPs on GSH levels (a), catalase activity (b), SOD activity (c), MDA levels (d), and PC content (e) in the STZ-induced diabetic rats. Values are expressed as mean ± S.E.M. Single asterisk (*) indicates P < 0.05, double asterisks (**) indicate P < 0.001 compared with control group; single number sign (#) indicates P < 0.05, double number signs (##) indicate P < 0.001 compared with diabetic group
CAT is an enzymatic antioxidant that reduces oxidative stress. As demonstrated in Fig. 1b, CAT activity significantly decreased (P < 0.05) in the Dia group compared with the Ctl group. In the Dia+ZnONPs, Dia+LC, and Dia+LC+ZnONPs groups, CAT activity significantly increased compared with the Dia group (P < 0.05, P < 0.001, and P < 0.05, respectively).
Another enzymatic antioxidant is SOD, which neutralizes superoxide and hydrogen peroxide inside the cell and reduces oxidative stress within the cell [27]. In current study, SOD activity significantly decreased in the Dia group (10.4 ± 1.2) compared with the Ctl group (23.3 ± 3.2) (P < 0.05) and increased in all experimental groups, which was only statistically significant (P < 0.05) in the Dia+LC+ZnONPs group (Fig. 1c).
MDA, one of the products of lipid peroxidation in cells, showed significantly higher levels in the Dia and Dia+Met groups (P < 0.001 and P < 0.05, respectively). The Dia+ZnONPs, Dia+LC, and Dia+LC+ZnONPs groups had lower MDA levels compared with the Dia group; however, as Fig. 1d demonstrates, the decrease was only statistically significant (P < 0.05) in the Dia+LC+ZnONPs group.
PC is commonly used criteria for the measurement of protein oxidation in cells [24]. Our findings showed significant increase in PC levels in the Dia group compared with the Ctl group (P < 0.001). The Dia+ZnONPs, Dia+Met, Dia+LC, and Dia+LC+ZnONPs groups displayed a statistically significant decrease compared with the Dia group (P < 0.001, Fig. 1e).
Effect of LC and ZnONPs on the Serum Levels of FSH, LH, Progesterone, and Estrogen Hormones in STZ-Induced Diabetic Rats
Data on the effect of LC and ZnONPs on sex hormones levels in diabetic rats are presented in Table 2. FSH levels in Dia group were significantly lower than those of the Ctl group (P < 0.001). Treatment of diabetic rats with LC, ZnONPs, and LC+ZnONPs significantly improved FSH levels (P < 0.001). Serum levels of LH significantly decreased in Dia group compared with the Ctl group, but increased in experimental groups (P < 0.05). Moreover, diabetes significantly decreased estradiol and progesterone levels compared with the Ctl group (P < 0.05). In this study, metformin, LC, ZnONPs, and LC+ZnONPs were found to significantly increase estradiol and progesterone levels, and the latter was observed to be significantly different in Dia+ZnONPs and Dia+LC+ZnONPs groups (P < 0.05).
Effect of LC and ZnONPs on Ovarian Histopathological Changes in STZ-Induced Diabetics Rats
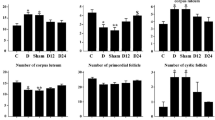
The numbers of preovulatory follicles and corpus luteum in Dia group were lower than the Ctl group (P < 0.05), but the numbers of atretic follicle were higher than the Ctl group (P < 0.001). Treatment with metformin increased the number of follicles and the corpus luteum in the ovaries; however, this increase was not statistically significant. The numbers of primordial, primary, and secondary follicles demonstrated significant increase in Dia+LC group (P < 0.05) compared with the Dia group. Also, ZnONPs treatment caused significant increase in the numbers of primordial and primary follicles (P < 0.05). Compared with the Dia group, the Dia+LC+ZnONPs group was found with significant increase in the numbers of all preovulatory follicles (P < 0.05). Moreover, the number of atretic follicles significantly decreased in the Dia+Met (P < 0.05), Dia+LC, Dia+ZnONPs, and Dia+LC+ZnONPs groups (P < 0.001) compared with the Dia group (Table 3; Fig. 2).
Discussion
Diabetes mellitus is one of the most common chronic metabolic disorders that is associated with hyperglycemia and several complications, such as retinopathy, hyperlipidemia, and polyneuropathy [18, 28]. In the present study, co-treatment with LC and ZnONPs increased antioxidant levels (GSH, CAT, and SOD), decreased the MDA levels and carbonyl protein content, and increased the number of preovulatory follicles in the ovarian tissue of diabetic rats. It also increased serum levels of FSH, LH, estradiol, and progesterone.
In rodents, a single dose of STZ with partial destruction of pancreas induces type I diabetes [29]. In current study, STZ-induced diabetes decreased body weight and increased blood glucose levels in rats, and the weight loss could be linked to diabetes-induced hyperglycemia. These findings are in agreement with a previously published work of authors [21]. Diabetes could decrease ovarian weight especially after 4 weeks [28]. However, the current study lasted less than this time, and the ovarian weight did not demonstrate a significant decrease.
Metformin as an antidiabetic agent in patients with type II diabetes is also found to reduce insulin dose requirement and LDL cholesterol levels in patients with type I diabetes. It provides a new perspective in therapy of type I diabetes [30, 31]. In current study, metformin improved body and ovarian weight in diabetic rats, and also diminished blood glucose concentrations, although not in a significant manner.
In addition, LC and ZnONPs are reported to reduce blood glucose levels and improve insulin resistance in patients with diabetes [13, 18]. Similarly, present findings suggest an association between co-treatment with LC and ZnONPs and increased body and ovarian weight and reduced blood glucose levels in diabetic rats.
Diabetes-induced oxidative stress in the reproductive system cause adverse effects on fertility [32, 33]. The GSH, a non-enzymatic antioxidant, interacts with ROS and leads to reduced activities of oxidative stress in ovary. CAT and SOD, enzymatic antioxidants, play fundamental roles in oocyte maturation and follicular development. In the present study, diabetes increased MDA and PC levels in ovarian tissue, owing to reduced GSH level and CAT and SOD activity. These results are in agreement with the findings of Ranasinghe et al. who found that diabetes increases lipid peroxidation and decreases plasma CAT and SOD levels in rats [26]. Our results showed that metformin reduced PC levels in diabetic rats.
In patients with type I diabetes, zinc intake can decrease lipid peroxidation and improve glucose homeostasis [34]. ZnONPs are reported to increase the expression levels of SOD, CAT, and GPx mRNA in the testis of diabetic rats [35]. In this study, ZnONPs treatment led to increased CAT activity and decreased PC levels in the ovary of diabetic rats.
Evidence suggests that LC treatment of grass carp ovary cell line increases GSH and decreases MDA levels, thereby increasing cell viability [36]. LC has hypoglycemic and antioxidant effects and exhibits inhibitory effect on protein degradation [37]. Furthermore, LC decreases the formation of free radicals and mitochondrial dysfunction in the hippocampus of diabetic rats [38]. In current study, co-treatment of diabetic rats with LC and ZnONPs resulted in significant decrease in lipid peroxidation and protein oxidation in diabetic rats and improved GSH levels, CAT, and SOD activities.
FSH stimulates granulosa cells to produce estrogen and progesterone. LH stimulates theca cells to synthetize androgen and then aromatase action of granulosa cells that converts androgen to estradiol. Diabetes is known to decrease the FSH level and reduce the conversion of androgen to estradiol [39]. Disruption of hormone secretion and altered hormonal balance can lead to various health consequences and diminish folliculogenesis [40]. Our results showed reduction of FSH, LH, estradiol, and progesterone levels and a decline in number of follicles in diabetic rats. In contrast, it was reported that diabetes does not affect the number of ovarian follicles, but it increases apoptotic cells in antral follicles in the ovaries of rats [41]. Our findings are in accordance with previous studies [42,43,44] that found association between diabetes and histological changes in ovarian structure caused by reduced number of follicles and the corpus luteum and increased atretic follicles. Therefore, imbalance in the hypothalamus-pituitary-ovarian axis, along with increased oxidative stress and reduced antioxidant activity caused by diabetes, influences ovarian histological structure and has a pivotal role in reproductive tract dysfunction [45].
In current study, metformin improved LH and estradiol levels, but had no significant effect on the number of preovulatory follicles, and caused a significant decrease in number of atretic follicles.
It was demonstrated that LC treatment during in vitro maturation of oocytes in mice results in increased oocyte maturation, fertilization, and embryo development up to the blastocyst stage and decreased the expression levels of apoptosis-related genes [46]. In this study, it was noted that co-treatment with LC and ZnONPs improved the levels of FSH, LH, estradiol, and progesterone, in addition to several effects on ovaries such as increase in number of ovarian follicles and corpus luteum and decrease in atretic follicles counts. It is believed that this compound supplementation could balance the hypothalamus-pituitary-ovarian axis by improving the ovarian steroid hormones. Improvement in these hormones could be associated with increased preovulatory follicle counts.
Conclusion
Co-treatment with LC and ZnONPs has more antidiabetic effects compared with metformin. Administration of this compound supplementation increases body weight and serum levels of sex hormones and improves histopathological appearance of the ovaries in STZ-induced diabetic rats by reducing blood glucose, increasing the levels of enzymatic and non-enzymatic antioxidants, and reducing oxidative stress. Indeed, this compound might improve ovulation and fertility rate in people with diabetes mellitus.
References
Shah A, Afzal M. Prevalence of diabetes and hypertension and association with various risk factors among different Muslim populations of Manipur, India. J Diabetes Metab Disord. 2013;12(1):52.
Oueslati N, Charradi K, Bedhiafi T, Limam F, Aouani E. Protective effect of grape seed and skin extract against diabetes-induced oxidative stress and renal dysfunction in virgin and pregnant rat. Biomed Pharmacother. 2016;83:584–92.
Rashid K, Sil PC. Curcumin ameliorates testicular damage in diabetic rats by suppressing cellular stress-mediated mitochondria and endoplasmic reticulum-dependent apoptotic death. Biochim Biophys Acta. 2015;1852(1):70–82.
Tok EC, Ertunc D, Evruke C, Dilek S. The androgenic profile of women with non-insulin-dependent diabetes mellitus. J Reprod Med. 2004;49(9):746–52.
Yeshaya A, Orvieto R, Dicker D, Karp M, Ben-Rafael Z. Menstrual characteristics of women suffering from insulin-dependent diabetes mellitus. Int J Fertil Menopausal Stud. 1995;40(5):269–73.
Dorman JS, Steenkiste AR, Foley TP, Strotmeyer ES, Burke JP, Kuller LH, et al. Menopause in type 1 diabetic women: is it premature? Diabetes. 2001;50(8):1857–62.
Garris DR. Effects of progressive hyperglycemia on ovarian structure and function in the spontaneously diabetic Chinese hamster. Anat Rec. 1984;210(3):485–9.
Cox N, Meurer K, Carlton C, Tubbs R, Mannis D. Effect of diabetes mellitus during the luteal phase of the oestrous cycle on preovulatory follicular function, ovulation and gonadotrophins in gilts. J Reprod Fertil. 1994;101(1):77–86.
Cheng P-P, Xia J-J, Wang H-L, Chen J-B, Wang F-Y, Zhang Y, et al. Islet transplantation reverses the effects of maternal diabetes on mouse oocytes. Reproduction. 2011;141(4):417–24.
Sanchez-Rangel E, Inzucchi SE. Metformin: clinical use in type 2 diabetes. Diabetologia. 2017;60(9):1586–93.
Di Pietro M, Parborell F, Irusta G, Pascuali N, Bas D, Bianchi MS, et al. Metformin regulates ovarian angiogenesis and follicular development in a female polycystic ovary syndrome rat model. Endocrinology. 2015;156(4):1453–63.
Duan X, Li Y. Physicochemical characteristics of nanoparticles affect circulation, biodistribution, cellular internalization, and trafficking. Small. 2013;9(9–10):1521–32.
Siddiqui SA, Or Rashid MM, Uddin MG, Robel FN, Hossain MS, Haque MA, et al. Biological efficacy of zinc oxide nanoparticles against diabetes: a preliminary study conducted in mice. Biosci Rep. 2020;40(4):BSR20193972.
Akpolat M, Gulle K, Topcu-Tarladacalisir Y, Safi Oz Z, Bakkal BH, Arasli M, et al. Protection by L-carnitine against radiation-induced ileal mucosal injury in the rat: pattern of oxidative stress, apoptosis and cytokines. Int J Radiat Biol. 2013;89(9):732–40.
Turker Y, Naziroglu M, Gumral N, Celik O, Saygin M, Comlekci S, et al. Selenium and L-carnitine reduce oxidative stress in the heart of rat induced by 2.45-GHz radiation from wireless devices. Biol Trace Elem Res. 2011;143(3):1640–50.
Celik F, Kose M, Yilmazer M, Koken GN, Arioz DT, Kanat PM. Plasma L-carnitine levels of obese and non-obese polycystic ovary syndrome patients. J Obstet Gynaecol. 2017;37(4):476–9.
Mendez-Cuesta LA, Marquez-Valadez B, Perez-De la Cruz V, Maldonado PD, Santana RA, Escobar-Briones C, et al. Early changes in oxidative stress markers in a rat model of acute stress: effect of l-carnitine on the striatum. Basic Clin Pharmacol Toxicol. 2011;109(2):123–9.
Bene J, Hadzsiev K, Melegh B. Role of carnitine and its derivatives in the development and management of type 2 diabetes. Nutr Diabetes. 2018;8(1):8.
Marcondes F, Bianchi F, Tanno A. Determination of the estrous cycle phases of rats: some helpful considerations. Braz J Biol. 2002;62(4A):609–14.
Mohammadi M, Zare Z. Effects of treadmill exercise on cognitive functions and anxiety-related behaviors in ovariectomized diabetic rats. Physiol Behav. 2020;224:113021.
Rezaei N, Mardanshahi T, Shafaroudi MM, Abedian S, Mohammadi H, Zare Z. Effects of L-carnitine on the follicle-stimulating hormone, luteinizing hormone, testosterone, and testicular tissue oxidative stress levels in streptozotocin-induced diabetic rats. J Evid Based Integr Med. 2018;23:2515690x18796053.
Beutler E, Duron O, Kelly B. Improvement method for the determination of glutathione in tissue homogenate. J Lab Clin Med. 1963;61:882–8.
Kei S. Serum lipid peroxide in cerebrovascular disorders determined by a new colorimetric method. Clin Chim Acta. 1978;90(1):37–43.
Levine RL, Garland D, Oliver CN, Amici A, Climent I, Lenz AG, et al. Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol. 1990;186:464–78.
Khedr NF. Fish oil and wheat-germ oil supplementation restores ovarian function in streptozotocin-diabetic rats. Reprod Fertil Dev. 2017;29(9):1689–98.
Ranasinghe P, Pigera S, Galappatthy P, Katulanda P, Constantine GR. Zinc and diabetes mellitus: understanding molecular mechanisms and clinical implications. Daru. 2015;23:44.
Rani AJ, Mythili SV. Study on total antioxidant status in relation to oxidative stress in type 2 diabetes mellitus. J Clin Diagn Res. 2014;8(3):108–10.
Garris DR, Garris BL. Cytolipotoxicity-induced involution of the female reproductive tract following expression of obese (ob/ob) and diabetes (db/db) genotype mutations: progressive, hyperlipidemic transformation into adipocytic tissues. Reprod Toxicol. 2004;18(1):81–91.
Wu J, Yan LJ. Streptozotocin-induced type 1 diabetes in rodents as a model for studying mitochondrial mechanisms of diabetic β cell glucotoxicity. Diabetes Metab Syndr Obes. 2015;8:181–8.
Vella S, Buetow L, Royle P, Livingstone S, Colhoun HM, Petrie JR. The use of metformin in type 1 diabetes: a systematic review of efficacy. Diabetologia. 2010;53(5):809–20.
Livingstone R, Boyle JG, Petrie JR. A new perspective on metformin therapy in type 1 diabetes. Diabetologia. 2017;60(9):1594–600.
Faure P, Benhamou P, Perard A, Halimi S, Roussel A. Lipid peroxidation in insulin-dependent diabetic patients with early retina degenerative lesions: effects of an oral zinc supplementation. Eur J Clin Nutr. 1995;49(4):282–8.
Anderson RA, Roussel A-M, Zouari N, Mahjoub S, Matheau J-M, Kerkeni A. Potential antioxidant effects of zinc and chromium supplementation in people with type 2 diabetes mellitus. J Am Coll Nutr. 2001;20(3):212–8.
Faure P, Corticelli P, Richard MJ, Arnaud J, Coudray C, Halimi S, et al. Lipid peroxidation and trace element status in diabetic ketotic patients: influence of insulin therapy. Clin Chem. 1993;39(5):789–93.
Afifi M, Almaghrabi OA, Kadasa NM. Ameliorative effect of zinc oxide nanoparticles on antioxidants and sperm characteristics in streptozotocin-induced diabetic rat testes. Biomed Res Int. 2015;2015:153573.
Wang Q, Ju X, Chen Y, Dong X, Luo S, Liu H, et al. Effects of L-carnitine against H2O2-induced oxidative stress in grass carp ovary cells (Ctenopharyngodon idellus). Fish Physiol Biochem. 2016;42(3):845–57.
Samir SM, Abbas AM, Safwat SM, Elserougy HG. Effect of L-carnitine on diabetes-induced changes of skeletal muscles in rats. J Basic Clin Physiol Pharmacol. 2018;29(1):47–59.
Hino K, Nishikawa M, Sato E, Inoue M. L-carnitine inhibits hypoglycemia-induced brain damage in the rat. Brain Res. 2005;1053(1–2):77–87.
Dupont J, Scaramuzzi RJ. Insulin signalling and glucose transport in the ovary and ovarian function during the ovarian cycle. Biochem J. 2016;473(11):1483–501.
Nasiadek M, Danilewicz M, Sitarek K, Swiatkowska E, Darago A, Stragierowicz J, et al. The effect of repeated cadmium oral exposure on the level of sex hormones, estrous cyclicity, and endometrium morphometry in female rats. Environ Sci Pollut Res Int. 2018;25(28):28025–38.
Yildiz M, Sandikci M. Changes in rat ovary with experimentally induced diabetes and the effects of lycopene on those changes. Romanian J Morphol Embryol. 2016;57(2):703–13.
Pala HG, Pala EE, Artunc Ulkumen B, Aktug H, Yavasoglu A, Korkmaz HA, et al. The protective effect of granulocyte colony-stimulating factor on endometrium and ovary in a rat model of diabetes mellitus. Gynecol Obstet Investig. 2014;78(2):94–100.
Wu Y, Li Y, Liao X, Wang Z, Li R, Zou S, et al. Diabetes induces abnormal ovarian function via triggering apoptosis of granulosa cells and suppressing ovarian angiogenesis. Int J Biol Sci. 2017;13(10):1297–308.
Erfani Majd N, Azizian H, Tabandeh MR, Shahriari A. Effect of Abelmoschus esculentus powder on ovarian histology, expression of apoptotic genes and oxidative stress in diabetic rats fed with high fat diet. Iran J Pharm Res. 2019;18(1):369–82.
Garris DR, Garris BL. Diabetes (db/db) mutation-induced ovarian involution: progressive hypercytolipidemia. Exp Biol Med (Maywood). 2003;228(9):1040–50.
Zare Z, Abouhamzeh B, Masteri Farahani R, Salehi M, Mohammadi M. Supplementation of L-carnitine during in vitro maturation of mouse oocytes affects expression of genes involved in oocyte and embryo competence: an experimental study. Int J Reprod Biomed. 2017;15(12):779–86.
Acknowledgments
The authors thank the Laboratory Animal House of the university (Project number: 97-3038).
Funding
The Immunogenic Research Center (IRC) of Sari Medical Faculty and Chancellor for Research and Technology of Mazandaran University of Medical Sciences, Mazandaran, Iran, financially supported this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Majidi, F.Z., Rezaei, N., Zare, Z. et al. The Protective Effects of l-Carnitine and Zinc Oxide Nanoparticles Against Diabetic Injury on Sex Steroid Hormones Levels, Oxidative Stress, and Ovarian Histopathological Changes in Rat. Reprod. Sci. 28, 888–896 (2021). https://doi.org/10.1007/s43032-020-00317-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43032-020-00317-0