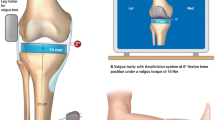
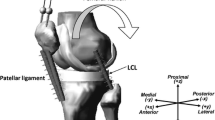
Abstract
Introduction
Despite the existence of diverse total knee implant designs, few data is available on the relationship between the level of implant constraint and the postoperative joint stability in the frontal plane and strain in the collateral ligaments. The current study aimed to document this relation in an ex vivo setting.
Materials and methods
Six fresh-frozen lower limbs underwent imaging for preparation of specimen-specific surgical guides. Specimens were dissected and assessed for joint laxity using the varus–valgus stress tests at fixed knee flexion angles. A handheld dynamometer applied tensile loads at the ankle, thereby resulting in a knee abduction–adduction moment of 10 Nm. Tibiofemoral kinematics were calculated using an optical motion capture system, while extensometers attached to medial collateral (MCL) and lateral collateral ligament (LCL) measured strain. Native joint testing was followed by four TKA designs from a single implant line—cruciate retaining, posterior stabilised, varus–valgus constrained and hinged knee (HK)—and subsequent testing after each implantation. Repeated measures linear mixed-models (p < 0.05) were used to compare preoperative vs. postoperative data on frontal plane laxity and collateral ligament strain.
Results
Increasing implant constraint reduced frontal plane laxity across knee flexion, especially in deep flexion (r2 > 0.76), and MCL strain in extension; however, LCL strain reduction was not consistent. Frontal plane laxity increased with knee flexion angle, but similar trends were inconclusive for ligament strain. HK reduced joint laxity and ligament strain as compared to the native condition consistently across knee flexion angle, with significant reductions in flexion (p < 0.024) and extension (p < 0.001), respectively, thereby elucidating the implant design-induced joint stability. Ligament strain exhibited a strong positive correlation with varus–valgus alignment (r2 = 0.96), notwithstanding knee flexion angle or TKA implant design.
Conclusion
The study demonstrated that increasing the constraint of a TKA resulted in lower frontal plane laxity of the knee. With implant features impacting laxity in the coronal plane, consequentially affecting strain in collateral ligaments, surgeons must consider these factors when deciding a TKA implant, especially for primary TKA.
Level of evidence
V.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Knee instability has been reported as the third most common failure mode following total knee arthroplasty (TKA), having been diagnosed in 7.5% of TKA cases requiring revision surgery [1]. Postoperative joint stability in the frontal plane is driven by the interaction between the implant and the surrounding soft-tissue, and optimised by imposing a constraint on this interaction using implant design features, such as implant conformity, post/cam mechanisms and mechanical linkages [2, 3].
In a cruciate retaining (CR) TKA, the posterior cruciate ligament (PCL) is preserved to promote stability and femoral rollback [2]. In a posterior stabilized (PS) design, however, the PCL is sacrificed and replaced by a post/cam mechanism to influence knee stability [2]. Both CR and PS TKA rely on the collateral ligaments to provide frontal plane stability. In the case of severe bone deformity, ligament insufficiency or revision arthroplasty, the varus–valgus constrained (VVC) design provides additional constraint in the frontal plane using an elevated tibial post which is further reinforced as compared to that in a PS design [4]. The highest level of constraint is provided by a hinged knee (HK) design, where the femoral and tibial components are mechanically connected by an axle [3]. Despite the existence of diverse implant designs, there is a lack of data characterizing the relationship between the level of constraint provided by the implant and the postoperative joint stability in the frontal plane and the strain in collateral ligaments.
The goal of the present study was to characterize the influence of increasing implant constraint, within a single implant line (Legion, Smith and Nephew, Memphis, USA), on the joint laxity in the frontal plane and the strain in the medial collateral ligament (MCL) and the lateral collateral ligament (LCL) of cadaveric knees. It was hypothesized that a hinged TKA offers maximum joint constraint, thereby resulting in least frontal plane laxity and collateral ligament strain as compared to other TKA designs.
Materials and methods
Specimen preparation
Six fresh-frozen right lower limbs (five males, one female, Age: 77 ± 10 years, Height: 172 ± 6 cm, Weight: 71 ± 15 kg) were obtained from the institute body donation program following ethical approval by the local ethics committee (NH019 2016-06-03). None of the specimens had a history of lower limb disorder or prior surgical intervention.
A magnetic resonance imaging scan (Ingenia 3.0 T, Philips Healthcare, Amsterdam, Netherlands) of the knee and a full leg radiograph were obtained for each specimen to design specific cutting blocks for a primary CR TKA (Legion, Smith & Nephew, Memphis, USA) using the Visionaire protocol (Visionaire, Smith & Nephew, Memphis, USA). Rigid marker frames with reflective spheres were attached to the femur and tibia using bicortical bone pins. Computed tomography (CT) scans (slice thickness = 0.6 mm; Siemens Somatom Force, Siemens Healthcare, Erlangen, Germany) obtained for each frozen specimen in full extension were used to identify the location of the markers relative to anatomic landmarks (Mimics 18.0, Materialise, Leuven, Belgium) in order to define a joint coordinate system for the femur and tibia [5].
Each lower limb was thawed for 24 h before resecting it 32 cm proximal and 28 cm distal to the knee joint. Care was taken to preserve the joint capsule, ligaments and tendons while dissecting the surrounding skin and subcutaneous tissue. The femur and tibia were embedded in metal pots using acrylic resin (Struers, Ballerup, Denmark) in a physiologic orientation. While the specimen was placed in complete extension, calibrated axial extensometers (accuracy = 0.5%, hysteresis = 0.07%; MTS, Type 634.12F-24, Eden Prairie, Minnesota, USA) were affixed to the superficial MCL and the LCL along the axis of the ligaments around the mid-portion region using a series of suture loops (2/0 non-absorbable polyester braided suture wire; Cardioxyl, Peters Surgical, Bobigny Cedex, France).
Experimental testing
Specimen laxity was assessed using varus–valgus stress tests at flexion angles of 0°, 30°, 60°, and 90° (Fig. 1). The femoral pot was rigidly fixed, although the tibia was free to move in all three translations and rotations, thereby allowing six degrees of freedom at the joint. The joint was subjected to an adduction–abduction moment of 10 Nm by manually applying a tensile force with a handheld force gauge (resolution = 0.1 N; Series 4, Mark-10, Copiague, USA) at the distal tibia perpendicular to the tibial long axis. This moment has been shown to be below the damage threshold of the MCL and LCL [6, 7]. A six-camera optical motion capture system (capture frequency = 100 Hz; MX40+ , Vicon, Oxford, UK) was used to record the trajectories of the markers attached to the femur and tibia, and synchronise signals from the force gauge and the extensometers (capture frequency = 200 Hz). All trials were performed in triplicate by a single investigator.
Following testing on the native knee, the specimens were implanted with a series of cemented mechanically aligned total knee prostheses (Legion Total Knee System, Smith & Nephew) with an increasing level of constraint (Fig. 2). The first condition involved the implantation of a cemented Legion CR prosthesis using a medial parapatellar approach involving the preservation of the PCL. For the second condition, the femoral component and polyethylene insert were changed to Legion PS (no varus–valgus constraint), after resecting the PCL. The PS polyethylene insert was then changed to a VVC (± 3° varus–valgus constraint, 10° hyperextension, ± 4° rotation). Finally, both the femoral and tibial components were revised to the HK prosthesis (± 2° varus-valgus constraint, 3° hyperextension, no constraint in rotation). A tibial 0° recut was performed to accommodate the hinged tibial component. The aforementioned experimental protocol was replicated following each implanted condition. The thickness of the poly-ethylene insert was selected at the discretion of the surgeon after balancing the ligaments, if required. The cement–implant interface was covered with a film of petroleum jelly to facilitate implant removal without additional bone damage.
All surgical procedures were performed by a single surgeon trained in primary and revision TKA. The joint capsule was closed with interrupted sutures following each change in implant condition.
No significant bone defects occurred. The extensometers remained attached to the collateral ligaments during the entire process.
Data analysis
Joint kinematics for each postoperative condition were compared to those for the native joint to quantify changes in the varus–valgus orientation during the laxity tests. Ligament strain was expressed as the engineering strain calculated from the extensometer data, with the unloaded, fully extended configuration considered as the zero reference.
Repeated measures linear mixed-models were used to compare preoperative and postoperative data (SPSS 23, IBM, Armonk, NY, USA). If statistically significant (p < 0.05), pairwise post hoc tests were conducted with Sidak’s correction. A previously performed power analysis indicated that six specimens are required for a difference in the mean MCL and LCL strain of 3.6% and 5.8%, respectively, to be statistically significant with a power of 0.8 [8].
The specimens with early failure and outliers—evaluated using box-and-whisker, Tukey’s method (< lower quartile—1.5 * interquartile range and > upper quartile + 1.5 * interquartile range)—were excluded from the study.
Results
Comparisons with increasing implant constraints
Increasing joint constraints imposed by the implant design resulted in a reduction in frontal plane laxity, and a consequent increase in joint stability, during the valgus and varus stress tests conducted at all knee flexion angles (Fig. 3a). The reduction in frontal plane laxity along implant constraint was higher for higher knee flexion angles for both the valgus (r2 = 0.66 at 0°, r2 = 0.95 at 90°) and varus (r2 = 0.43 at 0°, r2 = 0.76 at 90°) stress tests, although more prominently observed for the valgus stress test. When averaged over all flexion angles, joint stability increased with every added implant constraint in both the valgus and varus direction, barring an unexpected behaviour of HK in varus (Table 1).
MCL strain plotted against increasing implant constraint revealed a postoperative reduction in extension (0°) for all implant designs compared to the native condition, but only for HK across all knee flexion angles (Fig. 3b); however, the LCL strain did not follow consistent trends along increasing implant constraints over knee flexion. When averaged over all flexion angles, collateral ligament strain reduced with every added implant constraint following PS (Table 1).
Comparison with increasing flexion angle
Irrespective of the TKA implant design, postoperative frontal plane laxity during the valgus and varus stress tests was observed to be lesser than that in the native condition throughout knee flexion (Fig. 4a). Valgus laxity increased with increasing knee flexion, although this was most pronounced in the native condition (Fig. 4a). Postoperative MCL strain followed similar trends in being lesser than that in the native condition throughout flexion, except at 90°; however, this was not observed consistently in postoperative LCL strain across knee flexion (Fig. 4b).
Mean (a) tibiofemoral abduction and (b) collateral ligament strain across six specimens with increasing knee flexion angle for the native condition (dashed grey) and following total knee replacements performed using cruciate retaining (solid blue), posterior stabilised (solid red), varus–valgus constrained (dashed red) and hinged knee (solid green) implant designs
In the case of frontal plane laxity during the valgus stress test, when compared to the native condition, the HK resulted in over 23% reduction in valgus angles across knee flexion, albeit reductions being statistically significant only at 60° (39%, p = 0.007) and 90° (47%, p = 0.024) (Table 2). Correspondingly, the strain in MCL was also found to be lower in HK as compared to the native condition by over 35% across knee flexion, with reductions in extension (67%, p < 0.001) and at 30° flexion (45%, p < 0.001) being statistically significant. While other TKA designs also resulted in a reduction in valgus angle (Fig. 3a) and MCL strain (Fig. 3b) as compared to the native condition, reductions were statistically significant only for the valgus angle following VVC implantation in extension (32%, p < 0.045), and for MCL strain following CR in extension (49%, p = 0.001) and 30° flexion (32%, p = 0.017), following PS in extension (44%, p = 0.003) and following VVC in extension (55%, p < 0.01).
In the case of frontal plane laxity during the varus stress test, when compared to the native condition, the VVC and HK resulted in over 40% reduction in varus angles during knee flexion, although reductions were significant only at 90° (46%, p = 0.017 following VVC and 40%, p = 0.049 following HK). In contrast, corresponding reductions in LCL strains following VVC and HK were not statistically significant. Moreover, postoperative alterations in varus angles and LCL strains following other implantations were not statistically significant.
Ligament strain vs. joint laxity
The strain in the MCL or LCL plotted with respect to the knee abduction or adduction during the stress tests, respectively, performed at all flexion angles for all implant designs, revealed a strong positive correlation with a nearly unit linear relationship (slope = 0.98, r2 = 0.96) (Fig. 5).
Discussion
The most important finding of this cadaveric study was that increasing joint constraint—from a ligament retaining CR condition, to a ligament sacrificing PS condition, to an imposed restriction VVC condition, through to an entirely artificial HK joint—gradually decreased frontal plane laxity, thereby indicating improved joint stability, as compared to the intact native condition, as hypothesized (Fig. 3a, Table 1). The prominence of this phenomenon in valgus over varus reflects the contemporary TKA designs largely driven towards medial stability. However, for all the native and postoperative conditions, joint laxity was higher in flexion than in complete extension, with results for valgus stress test more prominent than the varus counterpart (Fig. 3a); this contrasts the idea of medial stability-driven TKA designs indicating that this stability is not potentially reflected throughout the range of knee flexion.
In comparison to the native knee, all postoperative conditions exhibited reduction in frontal plane laxity (Fig. 4a); however, the changes in joint laxity due to different levels of constraint were not all statistically significant, possibly owing to intact collateral ligaments throughout the experiment. Although more physiologically replicative of the in vivo condition, intact collateral ligaments disallowed the quantification of the independent contribution of the implant design to the joint constraint [9]. Based on these findings, it could be concluded that addition of constraints in prosthetic design might not be particularly useful in the case of intact, balanced collateral ligaments.
Manning et al. reported differences in frontal plane laxity before and after a single-radius CR TKA using navigation [10]. In contrast to the findings in the current study, no increase in frontal plane laxity with increasing knee flexion was reported in the native knee; moreover, a significant increase in frontal plane laxity post-TKA was reported for higher flexion angles. The results of the current study suggest that joint laxity following multi-radius CR TKA is lower as compared to the native condition, which conforms with those of Hunt et al., who used the same type of single-radius CR implant [11]. This signifies the importance of interpreting and comparing these data with caution owing to different study setups, implant designs and surgical techniques for joint balancing, which determine the tightness of the joint.
Collateral ligament strain was positively correlated to joint alignment in the frontal plane with a nearly unit linear relationship (Fig. 5). This information helps in correlating the dynamic joint alignment, and therefore joint space distraction, to collateral ligament strain, especially important during passive tests for joint laxity performed intraoperatively. Postoperative collateral ligament strains revealed that the implant constraints exhibited greater effects on the MCL than the LCL, especially in extension (Fig. 3b, 4b), which corroborates with postoperative joint laxity findings reflecting contemporary TKA implants designed primarily for medial stability. Moreover, while changes in MCL strain following TKA conformed with the valgus alignment of the knee across knee flexion, changes in postoperative LCL strain were not reflective of the varus alignment (Fig. 4), probably owing to the contribution of other ligamentous stabilisers on the lateral aspect of the joint. While this was in agreement with the common guidelines for implant selection in the literature [12], it differed from an in vitro study which compared the collateral ligament strains following PS TKA, probably owing to methodological variations in measuring strain across knee flexion [13].
Postoperative frontal plane alignment following VVC and HK implantation recorded during the laxity tests at various knee flexion angles were similar to the prescribed allowable limits of ± 3° and ± 2° respectively (Table 2). The HK implant—the design offering the most constrained joint—significantly reduced the joint laxity from the native condition in both the valgus and varus directions, as hypothesized (Figs. 3a, 4a). This was prominent in high flexion (Table 2), whereby laxity in the native joint tends to be higher as compared to extension, but joint laxity following HK remains similar to that in extension owing to the implant design induced frontal plane stability. For similar reasons, collateral ligament strains following HK were similar across knee flexion (Fig. 4b). When plotted against the applied knee abduction–adduction moment, the comparable trends of joint laxity and ligament strains following HK in extension and 90° flexion clearly elucidate the joint stability induced by the implant design, unlike the native condition where the joint laxity increases considerably from extension to flexion (Fig. 6a). Most importantly, the HK design provided a seemingly constant, more predictable joint stability across the entire range of knee flexion.
Becker et al. recently highlighted the possible implications of alterations in soft tissue tension on the neurosensory system which can be an explanation for pain [14]. There is still a significant lack of knowledge in the understanding of the neuromuscular function of the knee and the effect of different tension patterns of the soft tissue on the outcome after TKA. Many studies have focused on correct balancing of the knee during TKA, but a balanced knee also can end up in poor function due to significantly abnormal soft tissue tension. Colyn et al. observed improved clinical outcome scores and stability when a VVC design was favoured over less constrained implants in the setting of high-grade varus deformities to compensate for the expected lateral laxity without performing extensive release of the MCL to achieve a balanced knee [15]. Delport et al. reported different strains in the collateral ligaments before and after implantation of a TKA [14]. They concluded that this could be seen as a sign of stability by the surgeon, but the patient might experience stiffness due to the increase in soft tissue strain above the threshold of 0.7% which might trigger the afferent fibres to fire, which is why they consequently recommended against overstuffing. Although implantation of a hinged TKA did not significantly decrease collateral ligament strain in our study, it is possible that these designs can lead to increased stability and range of motion in cases of arthrofibrosis, wherein a decrease in soft tissue tension is typically achieved by resection of collateral ligaments. Further research is warranted on this topic.
Although this study was a first of its kind in comparing effects of increasing implant constraint on joint biomechanics during stress tests, it had certain limitations. Since the soft tissue envelope of the knee remained intact during experimental testing, the isolated impact of implant constraints on knee stability, without the contribution of surrounding ligamentous stabilisers, could not be studied. Owing to the increasing levels of joint constraint provided by each chronological design feature, randomization in implant sequence was not possible. Moreover, all implants in this study were selected from a single manufacturer, and results might differ for similar studies conducted with those from another manufacturer due to the nuances in implant design. Stress tests were performed with the abduction–adduction moment about the knee applied using manual varus–valgus loads at the ankle, as opposed to using automated devices or robotic manipulators [16], primarily to closely replicate laxity tests performed clinically and intraoperatively. The use of extensometers limited ligament strain measurement to relative values with respect to a reference, instead of true collateral ligament strains which take into account the pre-existing ligament strain owing to its attachment to the bone [17]. Moreover, experimental difficulties in using extensometers included loss of experimental data of MCL strain for one specimen at 60° during the valgus stress test. It is, therefore, recommended to employ non-invasive methods, such as digital image correlation, to quantify ligament strain. Collateral ligament strains were measured superficially on individual ligaments, thereby neglecting the harmonious contribution of other ligamentous structures, such as the posterior capsule, to overall knee stability. Although MCL strain was measured only in the superficial layer, the deep MCL is known to not substantially contribute to valgus stability [18]. The LCL was the only ligament measured on the lateral side, and we acknowledge that it is not representative of all lateral ligament structures in the knee. Moreover, non-ligamentous stabilisers, such as the iliotibial band and tendons of the popliteus and the hamstrings were also neglected and should be considered for an exhaustive inclusion. Last, a limited number of cadavers were used for the study although in vitro studies in the literature have resorted to similar number of specimens [19,20,21].
Conclusions
The study demonstrated that increasing the constraint of a TKA resulted in lower frontal plane laxity of the knee. With implant features impacting laxity in the coronal plane, consequentially affecting strain in collateral ligaments, surgeons must consider these factors when deciding a TKA implant, especially for primary TKA.
References
Sharkey PF, Lichstein PM, Shen C, Tokarski AT, Parvizi J (2014) Why are total knee arthroplasties failing today—has anything changed after 10 years? J Arthroplasty 29:1774–1778. https://doi.org/10.1016/j.arth.2013.07.024
Song SJ, Park CH, Bae DK (2019) What to know for selecting cruciate-retaining or posterior-stabilized total knee arthroplasty. Clin Orthop Surg 11(2):142–150. https://doi.org/10.4055/cios.2019.11.2.142
Crawford DA, Lombardi AV Jr (2021) Ligament balancing and constraint in revision total knee arthroplasty. J Knee Surg 34(13):1382–1387. https://doi.org/10.1055/s-0041-1735162
Castagnini F, Bordini B, Cosentino M, Ancarani C, Lucchini S, Bracci G, Traina F (2022) Constraint in complex primary total knee arthroplasty: rotating hinge versus condylar constrained implants. Arch Orthop Trauma Surg. https://doi.org/10.1007/s00402-021-04322-z
Grood E, Suntay W (1983) A joint coordinate system for the clinical description of three-dimensional motions: application to the knee. J Biomech Eng 150:136–144. https://doi.org/10.1115/1.3138397
LaPrade RF, Bernhardson AS, Griffith CJ, MacAlena JA, Wijdicks CA (2010) Correlation of valgus stress radiographs with medial knee ligament injuries: an in vitro biomechanical study. Am J Sports Med 38:330–338. https://doi.org/10.1177/0363546509349347
LaPrade RF, Heikes C, Bakker AJ, Jakobsen RB (2008) The reproducibility and repeatability of varus stress radiographs in the assessment of isolated fibular collateral ligament and grade-III posterolateral knee injuries. An in vitro biomechanical study. J Bone Joint Surg Am 90:2069–2076. https://doi.org/10.2106/JBJS.G.00979
Delport H, Labey L, Innocenti B, De Corte R, Vander Sloten J, Bellemans J (2015) Restoration of constitutional alignment in TKA leads to more physiological strains in the collateral ligaments. Knee Surg Sports Traumatol Arthrosc 23:2159–2169. https://doi.org/10.1007/s00167-014-2971-z
Ghosh KM, Manning WA, Blain AP, Rushton SP, Longstaff LM, Amis AA, Deehan DJ (2016) Influence of increasing construct constraint in the presence of posterolateral deficiency at knee replacement: a biomechanical study. J Orthop Res 34(3):427–434. https://doi.org/10.1002/jor.23026
Manning WA, Ghosh K, Blain A, Longstaff L, Deehan DJ (2017) Tibiofemoral forces for the native and post-arthroplasty knee: relationship to maximal laxity through a functional arc of motion. Knee Surg Sports Traumatol Arthrosc 25:1669–1677. https://doi.org/10.1007/s00167-016-4093-2
Hunt NC, Ghosh KM, Blain AP, Athwal KK, Rushton SP, Amis AA, Longstaff LM, Deehan DJ (2014) How does laxity after single radius total knee arthroplasty compare with the native knee? J Orthop Res 32:1208–1213. https://doi.org/10.1002/jor.22645
Cheung A, Yan CH, Chan PK, Chiu KY (2020) The medial collateral ligament in primary total knee arthroplasty: anatomy, biomechanics, and injury. J Am Acad Orthop Surg 28(12):e510–e516. https://doi.org/10.5435/JAAOS-D-19-00355
Delport H, Labey L, De Corte R, Innocenti B, Vander Sloten J, Bellemans J (2013) Collateral ligament strains during knee joint laxity evaluation before and after TKA. Clin Biomech (Bristol, Avon) 28(7):777–782. https://doi.org/10.1016/j.clinbiomech.2013.06.006
Becker R, Hirschmann MT, Karlsson J (2017) The role of ligament tension and sensomotoric system in total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc 25:1663–1665. https://doi.org/10.1007/s00167-017-4581-z
Colyn W, Neirynck J, Vanlommel E, Bruckers L, Bellemans J (2022) Primary constrained-condylar-knee designs outperform posterior-stabilized and cruciate-retaining designs in high-grade varus osteoarthritic knees during short-term follow-up: a pilot study. Arch Orthop Trauma Surg. https://doi.org/10.1007/s00402-022-04447-9
Heyse TJ, Tucker SM, Rajak Y, Kia M, Lipman JD, Imhauser CW, Westrich GH (2015) Frontal plane stability following UKA in a biomechanical study. Arch Orthop Trauma Surg 135:857–865. https://doi.org/10.1007/s00402-015-2198-6
Lujan TJ, Dalton MS, Thompson BM, Ellis BJ, Weiss JA (2007) Effect of ACL deficiency on MCL strains and joint kinematics. J Biomech Eng 129:386. https://doi.org/10.1115/1.2720915
Robinson JR, Bull AMJ, Thomas RRDW, Amis AA (2006) The role of the medial collateral ligament and posteromedial capsule in controlling knee laxity. Am J Sports Med 34:1815–1823. https://doi.org/10.1177/0363546506289433
Barsoum WK, Lee HH, Murray TG, Colbrunn R, Klika AK, Butler S, Van Den Bogert AJ (2011) Robotic testing of proximal tibio-fibular joint kinematics for measuring instability following total knee arthroplasty. J Orthop Res 29:47–52. https://doi.org/10.1002/jor.21207
Heyse TJ, Slane J, Peersman G, Dirckx M, van de Vyver A, Dworschak P, Fuchs-Winkelmann S, Scheys L (2017) Kinematics of a bicruciate-retaining total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc 25(6):1784–1791. https://doi.org/10.1007/s00167-016-4414-5
Victor J, Labey L, Wong P, Innocenti B, Bellemans J (2010) The influence of muscle load on tibiofemoral knee kinematics. J Orthop Res 28:419–428. https://doi.org/10.1002/jor.21019
Acknowledgements
The authors gratefully acknowledge the contributions of Mr. Kristof Reyniers and Mr. Jo Verbinnen in preparing the specimens.
Funding
Smith and Nephew provided financial support and contributed to the study design and data collection.
Author information
Authors and Affiliations
Contributions
PB was involved in the study design, data acquisition, data interpretation, manuscript preparation and manuscript review. DSS was involved in the data analysis, data interpretation, manuscript preparation and manuscript review. OT was involved in the study design, data acquisition, data analysis, data interpretation and manuscript review. JS was involved in the study design, data acquisition and manuscript review. RDC was involved in data interpretation and manuscript review. LS was involved in the study design, data interpretation and manuscript review. HV was involved in the study design, data interpretation and manuscript review. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The author’s institution (IORT) receives research funding from Smith and Nephew. RD is an employee of Smith and Nephew. No other conflicts of interest.
Ethical approval
Ethical approval obtained for the cadaver study from the local research ethics committee (NH019, dated 2016-06-03).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Berger, P., Shah, D.S., Taylan, O. et al. Impact of increasing total knee replacement constraint within a single implant line on coronal stability: an ex vivo investigation. Arch Orthop Trauma Surg 143, 2165–2173 (2023). https://doi.org/10.1007/s00402-022-04534-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00402-022-04534-x