Abstract
Introduction
A review of the data supporting robotic systems currently available is presented focussing on precision and reproducibility, radiological outcomes, clinical outcomes, and survivorship.
Materials and methods
Scientific literature published on robotic systems for knee arthroplasty was reviewed using the reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. Inclusion criteria were any study involving robotic-assisted UKA or TKA that reported precision of implant positioning or functional outcomes or range of motion or survivorship, including cadaveric or dry bone studies with a minimum of 6-month follow-up.
Results
Thirty-nine studies were identified for robotic-assisted unicompartmental knee arthroplasty, and 24 studies for robotic-assisted total knee arthroplasty. Those that reported on radiological outcomes or cadaver studies consistently demonstrated improved precision with the use of robotic systems irrespective of the system. PROMS and survival data demonstrated equivalent short-term results. However, many studies reported outcomes inconsistently and few had long-term clinical follow-up or survivorship data.
Conclusions
This review adds to the body of evidence supporting improved precision and reproducibility with robotic assistance in knee arthroplasty. Despite intensive funding of research into robotic knee systems, there remains considerable heterogeneity in exposure and outcome analysis and few quality long-term studies demonstrating translation to better clinical outcomes and implant survivorship.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The expectation is that robotic assistance delivering precision in knee surgery will translate to improved clinical outcomes and long-term implant survivorship. Short-term studies demonstrating evidence of non-inferiority to instrumented techniques [2, 37] is eroding the scepticism about robotic systems. Robots come at significant cost [14] without evidence of long-term improved survivorship. Therefore, it remains prudent to evaluate robotic systems to ascertain that the goal of improving long-term survivorship of implants remains in view.
The current iteration of robotic-assisted knee arthroplasty (RAKA) developed from incremental integration of digital and computerised technologies beginning 3 decades ago, with computer navigation. The evolution of this process has been driven partially by the orthopaedic product market. To fully understand the currently available tools, an overview of a decades’ long history of mergers and acquisition of technologies and implants is helpful (Fig. 1). Some technologies have been integrated, while others have been taken off the market. Essentially, robotic systems build on CAS with task execution under instruction from the operating surgeon, but with higher precision, reproducibility, and less physical effort [28]. Depending on system (Table 1), robots offer active constraint in the execution of the operative plan, thus minimising harm to surrounding soft-tissues [29, 39, 41, 79]. Robots are ergonomically nimbler than humans, enabling more complex cuts and unconstrained implant design. Data collected at each decision step, allow accurate correlation with clinical outcome completing evaluation. The power of RAKA systems is leveraged when the data captured and analysed at each step (Fig. 2) enables targeted evaluation of precisely defined variables that may influence the clinical success of knee arthroplasty surgery. Documentation of alignment [10, 23], soft tissue tensioning [87], patella treatment and implant design, increase the granularity of intra-operative decision making and the tools with which knee arthroplasty can be analysed and improved (Fig. 2).
We examined published literature on robotic systems for outcomes of surgical precision and reproducibility, clinical reports of PROMS, and survivorship. Synthesis of the available data is hindered by lack of long-term studies, heterogeneity of features offered by robotic systems and the reporting of outcomes (Fig. 4).
Ultimately, correlation with improved long-term clinical results and survivorship will justify robotic assistance. It may not be that the robot itself improves survivorship, rather it is the tool most capable of performance analysis, enabling the next generation in improved knee arthroplasty design (Table 1).
Materials and methods
We conducted an evaluation of the current literature based on the PRISMA guidelines [], dividing papers into those assessing outcomes of RA-UKA, and those addressing RA-TKA.
Study selection and screening
A search was conducted independently by two authors (JS and JE) on February 1st, 2021, using four databases (MEDLINE, EMBASE, Pubmed, GOOGLE SCHOLAR). Search terms included [“robotics” (MeSH Terms) OR “robotics” (All Fields) OR “robotic” (All Fields)] OR [“knee” (All Fields) AND “surgery” (All Fields)] OR [“knee surgery” (All Fields)]. The inclusion criteria were any study involving robotic-assisted UKA or TKA that reported precision or accuracy of implant positioning, functional outcome, range of motion or survivorship, including cadaveric or dry bone studies between 2000 and 2020. Studies were initially screened through titles and abstracts. Bibliographies were searched of all major robotic systematic reviews. Studies were excluded if they were review articles, not published in English, outcome data were not extractable or did not have more than 6-month follow-up. Systems that have been subsumed (ACROBOT, CASPAR and PI Galileo) were excluded from the final analysis. Any discrepancies were resolved through discussion with a third reviewer (BF).
UKA search results
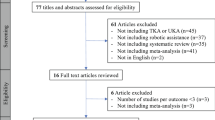
A total of 638 records were identified for screening initially. Of these, 594 were excluded based on listed criteria. A total of 45 full text articles identified for eligibility, after which a further 6 were excluded. Thirty-nine studies with minimum 6-month follow-up were included in the final synthesis (Fig. 3).
Results
Precision, radiographic findings, clinical results and survivorship for R-A UKA are limited to the MAKO and NAVIO systems. Most publications report on the MAKO, including survivorship data out to 5 years [9, 44, 84]. Some included studies considered both lateral R-A UKA, and R-A BiKA (medial UKA + patellofemoral arthroplasty) [8, 9, 81].
R-A UKA
Table 2 summarises 39 publications fulfilling inclusion criteria on outcomes for R-A UKA. Studies documenting radiological, clinical and survival data using Acrobot and Caspar [18, 33, 69, 71] systems have been subsumed by MAKO (Stryker) and Cori (Smith and Nephew), respectively (Fig. 1.), and therefore have not been included in this review. With respect to UKA, the only robotic systems with published data are MAKO and NAVIO. Most studies (26) analysed the MAKO robot, followed by the NAVIO (8). Two studies compare the MAKO and NAVIO robotic platforms to each other [49, 67]. Twenty-three studies declared financial support from the company that owned the robot in the study. Results are summarised in Tables 3 and 4.
R-A TKA
A total of 515 abstracts addressing R-A TKA were identified for further screening. After first stage screening, a total of 24 full texts were included based on the listed eligibility criteria (Fig. 4). Most studies were on the MAKO with 11 meeting inclusion criteria, followed by the ROBODOC (6), OMNIbot (3), NAVIO (2) and ROSA with (2) (Tables 5, 6 and 7). At the time of writing, no published results are currently available for the Velys™ system (De Puy, J&J). The longest clinical follow-up was 15 years, reported for the ROBODOC system [42].
MAKO UKA
a. Precision
Twelve studies report on precision and accuracy with R-A UKA. In 2012, Citak et al. [16] performed a cadaveric study analyzing femoral and tibial implant placement errors by comparing preoperatively planned position with postoperatively achieved results between R-A (MAKO) UKA and conventional groups. The root-mean-square error (RMSE) was used to quantify alignment errors, reporting femoral component placement were within 1.9 mm and 3.7° in all directions of the planned implant position for the robotic group, while RMS errors for the manual group were within 5.4 mm and 10.2°. Average RMS errors for tibial component placement were within 1.4 mm and 5.0° in all directions for the robotic group; while, for the manual group, RMS errors were within 5.7 mm and 19°.
In 2014, Mofidi et al. [60] published accuracy results based on a retrospective radiological analysis of 232 knees, comparing postoperative femoral and tibial sagittal and coronal alignments to the equivalent measurements collected intraoperatively by the Mako robot. They reported average coronal and sagittal plane inaccuracy of respectively 2.2° ± 1.7°, and 3.6° ± 3.3°, concluding that inaccuracy observed may be attributed to cementing technique.
Only two studies [5, 51] had a conventional comparison arm and in both a modest benefit was demonstrated in the robotic group with fewer outliers in both planes. The most scientifically rigorous of these was the randomised controlled trial conducted by Bell et al. [5] directly comparing MAKO R-A UKA with instrumented Oxford UKA. They found that the R-A UKA system resulted in a significantly greater proportion of components implanted within 2° of the target femoral component sagittal position (57% versus 26%, p = 0.0008), femoral component coronal position (70% versus 28%, p = 0.0001), femoral axial position (53% versus 31%, p = 0.0163) and tibial component sagittal position (80% versus 22%, p = 0.0001) and tibial axial position (48% versus 19%, p = 0.0009).
MacCallum et al. [51] in their 2016 retrospective radiological analysis of a registry cohort of 177 patients receiving a conventional UKA and 87 patients receiving a R-A UKA determined that coronal tibial baseplate positioning was more accurate with respect to their arbitrary safe zone for R-A UKA than conventional UKA (2.6° ± 1.5°versus 3.9° ± 2.4° p < 0.0001), but sagittal plane alignment was not (4.9° ± 2.8°versus 2.4° ± 1.6°p < 0.0001). Overall precision was delivered by the R-A UKA system, but accuracy of PTS determined by arbitrarily defined “safe zone” of 3°–9° was better with a conventional technique.
Gaudiani et al. [25] performed a radiological assessment of R-A (MAKO) UKA implant positioning reporting on pre- and postoperative reproduction of (sagittal) posterior condylar offset ratio (PCOR), posterior tibial slope (PTS) and (coronal) joint line reproduction and (correction of) mechanical axis (MA). They found precise reproduction of the joint line and PCOR. Both pre-operative PTS and varus were corrected towards neutral. The authors conclude that R-A UKA helps quantify surgical parameters.
In 2018, Kleeblad et al. [45] reported on the feasibility of correcting varus deformity in their series of 200 R-A UKA with a mean pre-operative deformity of 10° (7°–18°). They concluded that 98% of candidates with a preoperative varus deformity between 7 and 18° could be reliably restored to within 7° varus using the MAKO R-A Restoris UKA system.
There were two studies demonstrating comparable precision between NAVIO and MAKO robots [49, 67].
b. PROMS
Eighteen studies reported PROMS with the MAKO R-A UKA (Table 4). All studies with conventional comparison groups reported equivocal or better PROMS, however in no study were they statistically significant at last follow-up. Three studies reported satisfaction rates, 80% [21], 91% [25] and 92% [65]. In their RCT [26] with clinical outcome, Gilmour et al. concluded equivalency between R-A UKA and conventional UKA at 2 years.
c. Survivorship
A total of 27 studies had clinical outcome data on R-A UKA using the MAKO tool. Nineteen studies reported survivorship outcomes on pooled data of 6042 UKA performed using MAKO, the majority of which were medial UKAs, with smaller numbers for lateral compartment or bicompartmental arthroplasty. In their registry study of 2851 MAKO Restoris arthroplasties, St Mart et al. [77] reported comparable short-term (3 year) cumulative percent revision of 2.6% for MAKO R-A UKA, despite increased early infections. Three studies reported 5-year revision rate of 2.2% [8], 3% [44] and 7.7% and 3.4% (for tibial inlay and onlay implants, respectively) [84]. The longest follow-up survival data available for R-A UKA are 5 years.
NAVIO (CORI) UKA
a. Precision
Smith et al. [74] originally validated the accuracy of the NAVIO robotic system for UKA in a saw bone model. The authors reported RMS angular errors were 1.05°–1.52° for the three planes of the femoral implant and 0.66°–1.32° for the three planes of the tibial implant. The mean femoral and tibial cut surface data for all 20 bones showed that there was a slight undercut of 0.14 mm for the femur and 0.21 mm for the tibia, results comparable to those reported for the MAKO system by Citak et al. [16]. The motorized cutting burr used in this platform was originally described in a paper by Jaramaz et al. [34]. The accuracy of the cutting burr was quantified as an average distance from the planned implant position of 0.54 mm (SD 0.23 mm) and an average angular difference of 1.08° (SD 0.53). Herry et al. in 2017 [31] reported improved joint line restitution when utilizing NAVIO robotic assistance with the same implant, although the joint line was distalized in both the conventional and R-A groups.
Two studies compared outlier numbers for NAVIO UKA implants to conventional techniques [59, 62]. In both studies, the conventional technique had over double the outliers in both the coronal and sagittal position of tibial and femoral components.
Batailler et al. [3] in their retrospective case control study compared implant position between conventional and Navio R-A UKA and found a significantly higher rate of outliers in the conventional group with respect to limb alignment, and coronal and sagittal tibial base plate positioning, reporting that up to 35% of conventional tibial base plates as radiographic outliers. In 2021, Negrin et al. [62] published on the radiological accuracy of a group of conventional UKA compared to R-A UKA and based on an arbitrary radiological target. Of the 34 patients admitted to the study, 18 underwent R-A UKA achieving the arbitrary target 87% of the time, compared with 28% in the conventional group.
b. PROMs
A total of eight Canetti et al. [11] studies reported clinical outcome data for R-A UKA using the NAVIO system.
Five studies reported PROMS using the NAVIO robot. All studies reported superior or equivocal results when comparing to a conventional technique, however the differences achieved never reach statistical significance. The longest follow-up was 2 years. Satisfaction was reported in two studies as 74% [3]and 82% [59]. Return to sport was reported by Canetti et al. [11] for lateral R-A UKAs compared with those using conventional instrumentation. Two studies [49, 67] directly compared robotic UKR systems. In both cases the MAKO robots was compared to the NAVIO, neither study finding clinically significant differences in results (KFS and KSS) at 12 [49] or (KOOS) 24 months [67]. Both studies found an increased operating time of about 15 min for the NAVIO compared to the MAKO robot. No differences in complication rates were reported in either study.
c. Survivorship
Four studies reported survivorship results with maximum 2-year follow-up. Two-year revision rate was reported as 0% [11], 5% [3], 0.8% [4] and 4% [59].
MAKO TKA
a. Precision
Sires et al. [72] in 2020 reported in their case series of 33 TKAs that 87.36% of intraoperative measurements of femoral component position came within 3° compared with the findings of the postoperative CT, and a further 71.27% were within 2° compared with post-operative CT measurements. Tibial component placement was recorded within 3° of the postoperative CT findings in 93.11%, and within 2° in 74.14% of measurements. Intra-operatively recorded limb alignment was within 3° of the CT measurement in 93.10% of cases. In series comparing MAKO to conventionally instrumented TKA, Sultan et al. [80] reported improved accuracy and consistent restoration of posterior condylar offset ratio and Insall-Salvati Ratio in a radiographic study with MAKO to conventional TKA and Kayani et al. [7] in a single surgeon series demonstrated improved implant accuracy with the MAKO. Coronal and sagittal alignment for both tibial and femoral components were closer to planned position and the differences between groups were all reported to be statistically significant.
Two cadaveric studies [29, 41] reported that MAKO robotic precision improved PCL protection. Manning et al. [55] demonstrated improved tibiofemoral balancing using the MAKO compared to conventional gap technique TKA. The study did not report the level of training or experience of the surgeon performed the gap-balanced TKAs.
b. PROMs
Most studies reporting PROMs in a TKA population using MAKO did not have data beyond 6-month follow-up and were excluded from the final analysis. Only five studies [52, 54, 56, 57, 73] reporting PROMs after 6 months on the MAKO TKR were identified. Most reported a small trend to improved outcome with the MAKO. Marchand et al. reported improved pain scores at 6 [57] and 12 months [56] compared to conventional TKA, with the difference at 1 year being less pronounced and not statistically significant. Smith et al. [73] reported improved satisfaction at 12-month follow-up compared to conventional TKA, however Mahoney et al. [52] reported no difference in 12-month PROMS including satisfaction at 12-month follow-up.
c. Survivorship
Two studies report survivorship data for MAKO TKA beyond 6-month follow-up. Malkani et al. [54] reported a year revision rate of 2.1% (n = 4) in 188 TKA using the MAKO robot. Smith et al.[73] reported outcomes in 120 MAKO TKA compared with 102 instrumented TKA and has 100% implant survival at 1 year for both groups, although stated some patients had been excluded due to the development of periprosthetic joint infection. Mahoney et al. [52]reported 100% implant retention at 12-month follow-up in 143 RTKA using the MAKO.
ROBODOC TKA
ROBODOC was the first active robotic system developed for orthopaedic surgery and has the longest follow-up data [42].
a. Precision
Six studies compare the accuracy of the ROBODOC to conventional techniques [44,45,46,47,48,49]. All studies used a mechanical alignment philosophy and consistently showed fewer outliers in overall hip-knee-angle alignment as well as femoral and tibial sagittal and coronal implant placement. Rotational alignment was not addressed. The comparison manual instrumentation groups included the NexGen, Duracon and Triathlon implants and equipment and were the same implants as used in the ROBODOC series.
b. PROMs
Six studies reported PROMS in TKA cohorts using the ROBODOC [15, 35, 42, 75, 76, 86]. Follow-up periods range from 1 to 10 years with heterogeneous PROMS. Whilst most studies demonstrate a small benefit in PROMS, satisfaction and range of motion for robotic TKA compared to conventional TKA, these differences never reach statistical significance nor a minimally relevant clinical threshold.
c. Survivorship
Four studies [15, 35, 42, 86] reported survivorship outcomes using the ROBODOC platform comparing to conventional TKA groups using the same prosthesis. One study [42] used the Duracon prosthesis, with the remainder using NexGen implants. Three of the studies [15, 35, 86] showed modest improvements in implant survival rates for the robotic groups compared to the conventional technique groups. The largest study with the longest follow-up reported 98% implant survival record, with over 700 conventional and robotic TKAs in each group at 15-year follow-up [42].
NAVIO (CORI) R-A TKA
a. Precision
Casper et al. [12] assessed the accuracy of the NAVIO robot for TKA in a cadaveric study, using either cutting guides or the hand-held semi-active burr using three different prosthesis (Journey II, Genesis II and Legion). The authors compared the translational, angular, and rotational differences between the planned and achieved positions of the implants. The mean femoral flexion, varus/valgus, and rotational error was − 2.0°, − 0.1°, and − 0.5°, respectively. The mean tibial posterior slope, and varus/valgus error was − 0.2°, and − 0.2°, respectively. Accuracy as measured by the robot was improved with use of the burr compared to cutting guides. Bollars et al. [7] concluded equivalence in their case–control study comparing NAVIO assisted to conventional TKA with a goal of mechanical alignment.
b. and c. PROMs and survivorship
No studies have published clinical outcomes beyond 6 months for the NAVIO (Cori) robot in TKA to date.
OMNIBOT R-A TKA
a. Precision
Two studies [47, 48] examined the accuracy of OMNIbot compared to navigated TKA in cadaveric testing finding that the Omnibot delivered more consistent implant positioning and bone cuts. Koulalis et al. [48], in 2010 reported improved precision of coronal implant placement (0.24° vs 1.16°, p = 0.015) and thickness of bone cuts (0.37 vs 1.41 mm, p = 0.01) when using the OMNIbot, also reporting in 2011 [47] improved accuracy of coronal (0.55° versus 1.1°, p = 0.0041) and sagittal (0.75° versus 2.0°, p < 0.0001) plane bone resection, and cut height (0.56 mm versus 1.6 mm, p < 0.0001).
The OMNIbot has been compared to conventional, patient specific cutting (PSC) guides and computer-assisted surgery in accuracy studies. Suero et al. [78] found the OMNIbot to have fewer outliers than a conventional technique for achieving a neutral mechanical alignment. Clark et al. [17] compared the PRAXIM (forerunner to the OMNIbot) precision to a computer-assisted technique. In a retrospective study of 81 matched patients, R-A TKA was 0.5° closer to planned coronal alignment than CAS TKA when comparing the PRAXIM robot to a Stryker navigation system. 37% of the femoral cuts were within a half degree of the planned cut angle, 63% of axial rotations were within a half degree, and 50% of the tibia slope cuts were within a half degree of the planned value. The precision of the OMNIbot has also been compared to patient specific instrumentation (PSI) guides. Nam et al. [61] found 92.7% of OMNIbot-assisted TKAs were aligned within 3° of the intended target compared to only 70.7% of TKAs with PSC (p = 0.02).
In their postoperative CT analysis of implant position, Figueroa et al. [24] in 2019 concluded that the OMNIbot provided high precision for rotational, coronal femoral and tibial cuts, less so for hip-knee angle and sagittal cuts.
b. PROMs
Two studies have published PROMS in a TKA cohort utilizing the OMNIbot. Martin-Hernandez et al. [58] examined 198 patients in a multi-centred, multi-surgeon performed cohort of TKA (118 robotic versus 80 conventional). The conventional group had shorter operating time (78 min) compared to OMNIbot TKA (83 min). At final 3-year follow-up the OMNIbot had modestly improved clinical outcome measures except for the KSS in which the conventional group demonstrated higher scores (83.8 vs 79.2). Wakelin et al. [85] showed that integration of the soft tissue balancing component of this system (Balancebot) with defined tibiofemoral gap goals could improve clinical outcomes. They reported a statistically significant improvement in KOOS pain score (Δ = 11.2, p = 0.002) when these targets were met.
c. Survivorship
Two studies report on survivorship: Wakelin et al. [85] reported a 99.26% survivorship in 135 consecutive TKA performed using the Omnibot and Martin-Hernandez [58] et al. 100% in 119 robotically assisted TKA using the OMNIbot at 3-year follow-up.
ROSA® R-A TKA (Zimmer Biomet, Warsaw, IN, USA)
a. Accuracy
Two cadaveric studies have assessed the precision of the ROSA for TKA. In their study of 30 cadaveric TKAs Parratte et al. [64] reported over 90% of implant alignment being within 3° of target position and > 90% of bone cuts being within 2 mm of intended target cut thickness. Seidenstein et al. [70] reported similar findings when comparing the ROSA to conventionally performed TKA with 100% versus 75% of cases within 3° target implant position and 93% versus 60% within 2° target. They reported R-A precision of bone resection angles to within 0.6° (standard deviation 0.4°), except for the femur flexion (1.3° ± 1.0°), and within 0.7 mm with standard deviations below 0.7 mm for bone cuts.
b. PROMs and survivorship
Currently there is no published literature on clinical outcomes on the ROSA knee system. Zimmer Biomet is currently in the recruitment phase and aims to enrol 300 participants with early results due in late 2022 or early 2023 aiming to evaluate clinical outcomes.
Discussion
The strength of this study is that it summarises the limited clinical results of R-A KA, across five different robotic systems, confirming potential for robotic assistance to improve surgical precision and patient outcomes. Comparative studies in this review consistently reported statistical equivalence between treatment arms for risk of infection, revision nor manipulation under anaesthesia.
Limitations
Studies documenting robotic assistance are often industry supported and lack in scientific rigour [53].
Studies on R-A UKA were concentrated in North America [4, 9, 16, 20, 22, 25, 27, 30, 40, 44,45,46, 50, 51, 82,83,84]. One of the largest series from Burger et al. [8] reported 5-year survivorship in a single surgeon series on a group that included medial, lateral, patellofemoral and bicompartmental (medial and patellofemoral) knee arthroplasty. Despite calls for improved scientific rigour, variables are rarely optimally matched between comparison groups [68].
The most scientifically rigorous studies have been conducted on the ROBODOC. Studies on this open platform vary in implants, with inconsistent patella resurfacing strategies. Studies supporting the ROBODOC system are geographically limited to South Korea [15, 35, 42, 75, 76, 86].
Patient selection bias limits the conclusions that can be drawn about robotic systems. UKA results appear more sensitive to patient and implant selection than robotic platform used. R-A UKA outcomes are negatively influenced by age < 65, BMI > 30 kg/m2 and inlay tibial prosthesis [68]. These findings were consistent across the series by Burger et al. [8], Pearle et al. [65] and Zuiderbaan et al. [88] and Kleeblad et al. [44]. Blyth et al. [6] noted poorer PROMS in patients with high levels of anxiety and depression pre-operatively. Two studies [66, 88] found no relationship between BMI and revision rate.
Variation in the quantification of precision of implant positioning between studies makes comparison difficult. Accuracy is studies reported here is defined as the difference between planned and executed positioning, without clinical validation [81]. Song et al. [75] in their within-patient randomised case–control study of 30 patients receiving bilateral TKAs determined that despite improved accuracy of surgical results with R-A TKA knees, there was no clinically detectable difference at 12 months. Controversy regarding accuracy is also reflected in the heterogeneity of reports on alignment strategy. Alternative methods for assessing accuracy of implant position include joint line restoration reported by Herry et al. [31], PCOR [25, 80] and femoral component prominence, as reported by Klasan et al. [43] but these were not tabulated here.
Heterogeneity in PROMS reported makes between-study comparison difficult. Furthermore, their validity remains questionable. Adriani et al. [1] in their systematic review concluded that only the Forgotten Joint Score showed acceptable validity. Only two studies [6, 84] used FJS and it is possible that subtle differences are not being captured.
Improvement in objectivity in reporting of outcomes is expected to follow with technological advances. For example the FDA (United States Food and Drug Administration) approval for collaboration between Canary Medical Inc. and Zimmer Biomet to produce implants capable of real-time measurement of activity, range of motion and in-situ pressure[32].
Inconsistent reporting of survivorship between studies is misleading. Gaudiani et al.[25] reported 99% survivorship in their cohort of 91 robotic UKA, despite conversion of 2 UKA in the cohort to TKA and ‘one device was removed’. These were not included amongst revisions.
Secondary outcomes of faster procedure times, reduced learning curves[19, 38], reduced hospital stay and sterilization costs, need for fewer theatre personnel, reduced infection risk, and ultimately reduced financial burden per case are often touted in publications, but there is limited evidence to support this and can be a complex calculation dependent on an economy of scale[13, 36]. These outcomes were beyond the scope of this analysis.
There is much published on diminishing learning curves with robotic techniques, but few studies analyse the effect of the institutional and surgeon volume on the quality of surgeries performed. Ultimately, it may be that the precision afforded by robotics is more important for the inexperienced low-volume surgeon than the experienced surgeon.
The association between improved precision and improved long-term clinical outcome remains undetermined. Industry is intimately involved in most studies presented and care must be taken with the interpretation of results. Due to the volume of potential confounding variables in many publications, it will only be with diligent recording and analysis that the benefit afforded by R-A KA can be recognised. Fortunately, data capture and analysis are features of robotic systems.
Conclusion
The most important finding from our review is that in cadaveric, radiographic and clinical studies, robotic platforms confirm improved precision and reproducibility compared with conventional techniques, regardless of the robotic platform. Clinical studies demonstrated reduced morbidity associated with increased precision of a robotic-assisted cutting tool [29, 39, 41, 79]. Comparisons conducted between robotic-assistance models have shown equivalence amongst robotic systems. These findings are limited in external validity due to the heterogeneity of the scientific literature.
References
Adriani M, Malahias M, Kahlenberg A-G, Kahlenberg CA, Ast MP, Sculco PK (2020) Determining the validity, reliability, and utility of the forgotten joint score: a systematic review. J Arthroplasty 35(4):1137–1144. https://doi.org/10.1016/j.arth.2019.10.058 (Epub 2019 Nov 5. PMID: 31806559)
Batailler C, Fernandez A, Swan J, Servien E, Haddad FS, Catani F et al (2020) MAKO CT-based robotic arm-assisted system is a reliable procedure for total knee arthroplasty: a systematic review. Knee Surg Sports Traumatol Arthrosc. https://doi.org/10.1007/s00167-020-06283-z
Batailler C, White N, Ranaldi FM, Neyret P, Servien E, Lustig S (2019) Improved implant position and lower revision rate with robotic-assisted unicompartmental knee arthroplasty. Knee Surg Sports Traumatol Arthrosc 27:1232–1240
Battenberg AK, Netravali NA, Lonner JH (2020) A novel handheld robotic-assisted system for unicompartmental knee arthroplasty: surgical technique and early survivorship. J Robot Surg 14:55–60
Bell SW, Anthony I, Jones B, MacLean A, Rowe P, Blyth M (2016) Improved accuracy of component positioning with robotic-assisted unicompartmental knee arthroplasty: data from a prospective, randomized controlled study. J Bone Joint Surg Am 98:627–635
Blyth MJG, Anthony I, Rowe P, Banger MS, MacLean A, Jones B (2017) Robotic arm-assisted versus conventional unicompartmental knee arthroplasty: exploratory secondary analysis of a randomised controlled trial. Bone Joint Res 6:631–639
Bollars P, Boeckxstaens A, Mievis J, Kalaai S, Schotanus MGM, Janssen D (2020) Preliminary experience with an image-free handheld robot for total knee arthroplasty: 77 cases compared with a matched control group. Eur J Orthop Surg Traumatol 30:723–729
Burger JA, Kleeblad LJ, Laas N, Pearle AD (2020) Mid-term survivorship and patient-reported outcomes of robotic-arm assisted partial knee arthroplasty. Bone Joint J 102-B:108–116
Burger JA, Kleeblad LJ, Sierevelt IN, Horstmann WG, van Geenen RCI, van Steenbergen LN et al (2020) A comprehensive evaluation of lateral unicompartmental knee arthroplasty short to mid-term survivorship, and the effect of patient and implant characteristics: an analysis of data from the Dutch arthroplasty register. J Arthroplasty 35:1813–1818
Calliess T, Ettinger M, Savov P, Karkosch R, Windhagen H (2018) Individualized alignment in total knee arthroplasty using image-based robotic assistance: video article. Orthopade 47:871–879
Canetti R, Batailler C, Bankhead C, Neyret P, Servien E, Lustig S (2018) Faster return to sport after robotic-assisted lateral unicompartmental knee arthroplasty: a comparative study. Arch Orthop Trauma Surg 138:1765–1771
Casper M, Mitra R, Khare R, Jaramaz B, Hamlin B, McGinley B et al (2018) Accuracy assessment of a novel image-free handheld robot for Total Knee Arthroplasty in a cadaveric study. Comput Assist Surg (Abingdon) 23:14–20
Chawla H, Ghomrawi HM, van der List JP, Eggman AA, Zuiderbaan HA, Pearle AD (2017) Establishing age-specific cost-effective annual revision rates for unicompartmental knee arthroplasty: a meta-analysis. J Arthroplasty 32:326–335
Chen KK, Kim KY, Vigdorchik JM, Meere PA, Bosco JA, Iorio R (2019) Cost-effectiveness analysis of robotic arthroplasty—book chapter. In: Lonner J (ed) Robotics in knee and hip arthroplasty. Springer, Cham. https://doi.org/10.1007/978-3-030-16593-2_7
Cho KJ, Seon JK, Jang WY, Park CG, Song EK (2019) Robotic versus conventional primary total knee arthroplasty: clinical and radiological long-term results with a minimum follow-up of ten years. Int Orthop 43:1345–1354
Citak M, Suero EM, Citak M, Dunbar NJ, Branch SH, Conditt MA et al (2013) Unicompartmental knee arthroplasty: is robotic technology more accurate than conventional technique? Knee 20:268–271
Clark TC, Schmidt FH (2013) Robot-assisted navigation versus computer-assisted navigation in primary total knee arthroplasty: efficiency and accuracy. ISRN Orthop. https://doi.org/10.1155/2013/794827
Cobb J, Henckel J, Gomes P, Harris S, Jakopec M, Rodriguez F et al (2006) Hands-on robotic unicompartmental knee replacement: a prospective, randomised controlled study of the acrobot system. J Bone Joint Surg Br 88:188–197
Collins K, Agius PA, Fraval A, Petterwood J (2021) Initial experience with the NAVIO robotic-assisted total knee replacement-coronal alignment accuracy and the learning curve. J Knee Surg. https://doi.org/10.1055/s-0040-1722693
Deese JM, Gratto-Cox G, Carter DA, Sasser TM Jr, Brown KL (2018) Patient reported and clinical outcomes of robotic-arm assisted unicondylar knee arthroplasty: minimum two year follow-up. J Orthop 15:847–853
Dretakis K, Igoumenou VG (2019) Outcomes of robotic-arm-assisted medial unicompartmental knee arthroplasty: minimum 3 year follow-up. Eur J Orthop Surg Traumatol 29:1305–1311
Dunbar NJ, Roche MW, Park BH, Branch SH, Conditt MA, Banks SA (2012) Accuracy of dynamic tactile-guided unicompartmental knee arthroplasty. J Arthroplasty 27:803-808 e801
Ettinger M, Tucking LR, Savov P (2020) Kinematic alignment in total knee arthroplasty with image-based and image-independent robotic support. Orthopade 49:604–610
Figueroa F, Wakelin E, Twiggs J, Fritsch B (2019) Comparison between navigated reported position and postoperative computed tomography to evaluate accuracy in a robotic navigation system in total knee arthroplasty. Knee 26:869–875
Gaudiani MA, Nwachukwu BU, Baviskar JV, Sharma M, Ranawat AS (2017) Optimization of sagittal and coronal planes with robotic-assisted unicompartmental knee arthroplasty. Knee 24:837–843
Gilmour A, MacLean AD, Rowe PJ, Banger MS, Donnelly I, Jones BG et al (2018) Robotic-arm-assisted vs conventional unicompartmental knee arthroplasty. The 2 year clinical outcomes of a randomized controlled trial. J Arthroplasty 33:S109–S115
Gladnick BP, Nam D, Khamaisy S, Paul S, Pearle AD (2015) Onlay tibial implants appear to provide superior clinical results in robotic unicompartmental knee arthroplasty. HSS J 11:43–49
Hampp EL, Chughtai M, Scholl LY, Sodhi N, Bhowmik-Stoker M, Jacofsky DJ et al (2019) Robotic-arm assisted total knee arthroplasty demonstrated greater accuracy and precision to plan compared with manual techniques. J Knee Surg 32:239–250
Hampp EL, Sodhi N, Scholl L, Deren ME, Yenna Z, Westrich G et al (2019) Less iatrogenic soft-tissue damage utilizing robotic-assisted total knee arthroplasty when compared with a manual approach: a blinded assessment. Bone Joint Res 8:495–501
Hansen DC, Kusuma SK, Palmer RM, Harris KB (2014) Robotic guidance does not improve component position or short-term outcome in medial unicompartmental knee arthroplasty. J Arthroplasty 29:1784–1789
Herry Y, Batailler C, Lording T, Servien E, Neyret P, Lustig S (2017) Improved joint-line restitution in unicompartmental knee arthroplasty using a robotic-assisted surgical technique. Int Orthop 41:2265–2271
https://canarymedical.com (2021)
Jakopec M, Harris SJ, Baena FR, Gomes P, Cobb J, Davies BL (2001) The first clinical application of a “hands-on” robotic knee surgery system. Comput Aided Surg 6:329–339
Jaramaz B, Nikou C (2012) Precision freehand sculpting for unicondylar knee replacement: design and experimental validation. Biomed Tech (Berl) 57:293–299
Jeon SW, Kim KI, Song SJ (2019) Robot-assisted total knee arthroplasty does not improve long-term clinical and radiologic outcomes. J Arthroplasty 34:1656–1661
Jess H, Lonner YAF (2018) Pros and cons: a balanced view of robotics in knee arthroplasty. J Arthroplasty 33:2007–2013
Kayani B, Konan S, Ayuob A, Onochie E, Al-Jabri T, Haddad FS (2019) Robotic technology in total knee arthroplasty: a systematic review. EFORT Open Rev 4:611–617
Kayani B, Konan S, Huq SS, Tahmassebi J, Haddad FS (2019) Robotic-arm assisted total knee arthroplasty has a learning curve of seven cases for integration into the surgical workflow but no learning curve effect for accuracy of implant positioning. Knee Surg Sports Traumatol Arthrosc 27:1132–1141
Kayani B, Konan S, Pietrzak JRT, Haddad FS (2018) Iatrogenic bone and soft tissue trauma in robotic-arm assisted total knee arthroplasty compared with conventional jig-based total knee arthroplasty: a prospective cohort study and validation of a new classification system. J Arthroplasty 33:2496–2501
Khamaisy S, Zuiderbaan HA, van der List JP, Nam D, Pearle AD (2016) Medial unicompartmental knee arthroplasty improves congruence and restores joint space width of the lateral compartment. Knee 23:501–505
Khlopas A, Chughtai M, Hampp EL, Scholl LY, Prieto M, Chang TC et al (2017) Robotic-arm assisted total knee arthroplasty demonstrated soft tissue protection. Surg Technol Int 30:441–446
Kim YH, Yoon SH, Park JW (2020) Does robotic-assisted TKA result in better outcome scores or long-term survivorship than conventional TKA? a randomized, controlled trial. Clin Orthop Relat Res 478:266–275
Klasan A, Carter M, Holland S, Young SW (2020) Low femoral component prominence negatively influences early revision rate in robotic unicompartmental knee arthroplasty. Knee Surg Sports Traumatol Arthrosc 28:3906–3911
Kleeblad LJ, Borus TA, Coon TM, Dounchis J, Nguyen JT, Pearle AD (2018) Midterm survivorship and patient satisfaction of robotic-arm-assisted medial unicompartmental knee arthroplasty: a multicenter study. J Arthroplasty 33:1719–1726
Kleeblad LJ, van der List JP, Pearle AD, Fragomen AT, Rozbruch SR (2018) Predicting the feasibility of correcting mechanical axis in large Varus deformities with unicompartmental knee arthroplasty. J Arthroplasty 33:372–378
Kleeblad LJ, van der List JP, Zuiderbaan HA, Pearle AD (2017) Regional femoral and tibial radiolucency in cemented unicompartmental knee arthroplasty and the relationship to functional outcomes. J Arthroplasty 32:3345–3351
Koulalis D, O’Loughlin PF, Plaskos C, Kendoff D, Cross MB, Pearle AD (2011) Sequential versus automated cutting guides in computer-assisted total knee arthroplasty. Knee 18:436–442
Koulalis D, O’Loughlin PF, Plaskos C, Kendoff D, Pearle AD (2010) Adjustable cutting blocks for computer-navigated total knee arthroplasty: a cadaver study. J Arthroplasty 25:807–811
Leelasestaporn C, Tarnpichprasert T, Arirachakaran A, Kongtharvonskul J (2020) Comparison of 1 year outcomes between MAKO versus NAVIO robot-assisted medial UKA: nonrandomized, prospective, comparative study. Knee Surg Relat Res 32:13
Lonner JH, John TK, Conditt MA (2010) Robotic arm-assisted UKA improves tibial component alignment: a pilot study. Clin Orthop Relat Res 468:141–146
MacCallum KP, Danoff JR, Geller JA (2016) Tibial baseplate positioning in robotic-assisted and conventional unicompartmental knee arthroplasty. Eur J Orthop Surg Traumatol 26:93–98
Mahoney O, Kinsey T, Sodhi N, Mont MA, Chen AF, Orozco F et al (2020) Improved component placement accuracy with robotic-arm assisted total knee arthroplasty. J Knee Surg. https://doi.org/10.1055/s-0040-1715571
Malchau H, Graves SE, Porter M, Harris WH, Troelsen A (2015) The next critical role of orthopedic registries. Acta Orthop 86:3–4
Malkani AL, Roche MW, Kolisek FR, Gustke KA, Hozack WJ, Sodhi N et al (2020) New technology for total knee arthroplasty provides excellent patient-reported outcomes: a minimum two-year analysis. Surg Technol Int 36:276–280
Manning W, Ghosh M, Wilson I, Hide G, Longstaff L, Deehan D (2020) Improved mediolateral load distribution without adverse laxity pattern in robot-assisted knee arthroplasty compared to a standard manual measured resection technique. Knee Surg Sports Traumatol Arthrosc 28:2835–2845
Marchand RC, Sodhi N, Anis HK, Ehiorobo J, Newman JM, Taylor K et al (2019) One-year patient outcomes for robotic-arm-assisted versus manual total knee arthroplasty. J Knee Surg 32:1063–1068
Marchand RC, Sodhi N, Khlopas A, Sultan AA, Harwin SF, Malkani AL et al (2017) Patient satisfaction outcomes after robotic arm-assisted total knee arthroplasty: a short-term evaluation. J Knee Surg 30:849–853
Martín-Hernández C, Sanz-Sainz M, Revenga-Giertych C, Hernández-Vaquero D, Fernández-Carreira JM, Albareda-Albareda J, Castillo-Palacios A, Ranera-Garcia M (2018) Navigated versus conventional total knee arthroplasty: a prospective study at three years follow-up. Rev Esp Cir Ortop Traumatol 62(4):282–292. https://doi.org/10.1016/j.recot.2018.01.001 (Epub ahead of print. PMID: 29605558)
Mergenthaler G, Batailler C, Lording T, Servien E, Lustig S (2021) Is robotic-assisted unicompartmental knee arthroplasty a safe procedure? A case control study. Knee Surg Sports Traumatol Arthrosc 29:931–938
Mofidi A, Plate JF, Lu B, Conditt MA, Lang JE, Poehling GG et al (2014) Assessment of accuracy of robotically assisted unicompartmental arthroplasty. Knee Surg Sports Traumatol Arthrosc 22:1918–1925
Nam D, Maher PA, Rebolledo BJ, Nawabi DH, McLawhorn AS, Pearle AD (2013) Patient specific cutting guides versus an imageless, computer-assisted surgery system in total knee arthroplasty. Knee 20:263–267
Negrin R, Duboy J, Iniguez M, Reyes NO, Barahona M, Ferrer G et al (2021) Robotic-assisted vs conventional surgery in medial unicompartmental knee arthroplasty: a clinical and radiological study. Knee Surg Relat Res 33:5
Pailhe R (2021) Total knee arthroplasty: latest robotics implantation techniques. Orthop Traumatol Surg Res. https://doi.org/10.1016/j.otsr.2020.102780
Parratte S, Price AJ, Jeys LM, Jackson WF, Clarke HD (2019) Accuracy of a new robotically assisted technique for total knee arthroplasty: a cadaveric study. J Arthroplasty 34:2799–2803
Pearle AD, van der List JP, Lee L, Coon TM, Borus TA, Roche MW (2017) Survivorship and patient satisfaction of robotic-assisted medial unicompartmental knee arthroplasty at a minimum two-year follow-up. Knee 24:419–428
Plate JF, Augart MA, Seyler TM, Bracey DN, Hoggard A, Akbar M et al (2017) Obesity has no effect on outcomes following unicompartmental knee arthroplasty. Knee Surg Sports Traumatol Arthrosc 25:645–651
Porcelli P, Marmotti A, Bellato E, Colombero D, Ferrero G, Agati G et al (2020) Comparing different approaches in robotic-assisted surgery for unicompartmental knee arthroplasty: outcomes at a short-term follow-up of MAKO versus NAVIO system. J Biol Regul Homeost Agents 34:393–404 (Congress of the Italian Orthopaedic Research Society)
Robinson PG, Clement ND, Hamilton D, Blyth MJG, Haddad FS, Patton JT (2019) A systematic review of robotic-assisted unicompartmental knee arthroplasty: prosthesis design and type should be reported. Bone Joint J 101-B:838–847
Rodriguez F, Harris S, Jakopec M, Barrett A, Gomes P, Henckel J et al (2005) Robotic clinical trials of uni-condylar arthroplasty. Int J Med Robot 1:20–28
Seidenstein A, Birmingham M, Foran J, Ogden S (2021) Better accuracy and reproducibility of a new robotically-assisted system for total knee arthroplasty compared to conventional instrumentation: a cadaveric study. Knee Surg Sports Traumatol Arthrosc 29:859–866
Siebert WMS, Kober R, Heeckt PF (2002) Technique and first clinical results of robot-assisted total knee replacement. Knee 9:173–180
Sires JD, Wilson CJ (2020) CT validation of intraoperative implant position and knee alignment as determined by the MAKO total knee arthroplasty system. J Knee Surg. https://doi.org/10.1055/s-0040-1701447
Smith AF, Eccles CJ, Bhimani SJ, Denehy KM, Bhimani RB, Smith LS et al (2019) Improved patient satisfaction following robotic-assisted total knee arthroplasty. J Knee Surg. https://doi.org/10.1055/s-0039-1700837
Smith JR, Riches PE, Rowe PJ (2014) Accuracy of a freehand sculpting tool for unicondylar knee replacement. Int J Med Robot 10:162–169
Song EK, Seon JK, Park SJ, Jung WB, Park HW, Lee GW (2011) Simultaneous bilateral total knee arthroplasty with robotic and conventional techniques: a prospective, randomized study. Knee Surg Sports Traumatol Arthrosc 19:1069–1076
Song EK, Seon JK, Yim JH, Netravali NA, Bargar WL (2013) Robotic-assisted TKA reduces postoperative alignment outliers and improves gap balance compared to conventional TKA. Clin Orthop Relat Res 471:118–126
St Mart JP, de Steiger RN, Cuthbert A, Donnelly W (2020) The three-year survivorship of robotically assisted versus non-robotically assisted unicompartmental knee arthroplasty. Bone Joint J 102-B:319–328
Suero EM, Plaskos C, Dixon PL, Pearle AD (2012) Adjustable cutting blocks improve alignment and surgical time in computer-assisted total knee replacement. Knee Surg Sports Traumatol Arthrosc 20:1736–1741
Sultan AA, Piuzzi N, Khlopas A, Chughtai M, Sodhi N, Mont MA (2017) Utilization of robotic-arm assisted total knee arthroplasty for soft tissue protection. Expert Rev Med Devices 14:925–927
Sultan AA, Samuel LT, Khlopas A, Sodhi N, Bhowmik-Stoker M, Chen A et al (2019) Robotic-arm assisted total knee arthroplasty more accurately restored the posterior condylar offset ratio and the Insall-Salvati index compared to the manual technique; a cohort-matched study. Surg Technol Int 34:409–413
Tamam C, Plate JF, Augart M, Poehling GG, Jinnah RH (2015) Retrospective clinical and radiological outcomes after robotic assisted bicompartmental knee arthroplasty. Adv Orthop. https://doi.org/10.1155/2015/747309
van der List JP, Chawla H, Villa JC, Pearle AD (2016) Different optimal alignment but equivalent functional outcomes in medial and lateral unicompartmental knee arthroplasty. Knee 23:987–995
van der List JP, Chawla H, Villa JC, Pearle AD (2017) The role of patient characteristics on the choice of unicompartmental versus total knee arthroplasty in patients with medial osteoarthritis. J Arthroplasty 32:761–766
van der List JP, Kleeblad LJ, Zuiderbaan HA, Pearle AD (2017) Mid-term outcomes of metal-backed unicompartmental knee arthroplasty show superiority to all-polyethylene unicompartmental and total knee arthroplasty. HSS J 13:232–240
Wakelin EA, Shalhoub S, Lawrence JM, Keggi JM, DeClaire JH, Randall AL et al (2021) Improved total knee arthroplasty pain outcome when joint gap targets are achieved throughout flexion. Knee Surg Sports Traumatol Arthrosc. https://doi.org/10.1007/s00167-021-06482-2
Yang HY, Seon JK, Shin YJ, Lim HA, Song EK (2017) Robotic total knee arthroplasty with a cruciate-retaining implant: a 10 year follow-up study. Clin Orthop Surg 9:169–176
Yim JH, Song EK, Khan MS, Sun ZH, Seon JK (2013) A comparison of classical and anatomical total knee alignment methods in robotic total knee arthroplasty: classical and anatomical knee alignment methods in TKA. J Arthroplasty 28:932–937
Zuiderbaan HA, van der List JP, Chawla H, Khamaisy S, Thein R, Pearle AD (2016) Predictors of subjective outcome after medial unicompartmental knee arthroplasty. J Arthroplasty 31:1453–1458
Funding
Corin group, Arthrex gmbh, and Zimmer biomet.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Ethical approval
As this review is based on published and publicly reported literature, no specific ethical approval for this review is required.
Informed consent
As this is a secondary review study is based on primary research, no new informed consent has been obtained. All included studies had informed consent a priori.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Elliott, J., Shatrov, J., Fritsch, B. et al. Robotic-assisted knee arthroplasty: an evolution in progress. A concise review of the available systems and the data supporting them. Arch Orthop Trauma Surg 141, 2099–2117 (2021). https://doi.org/10.1007/s00402-021-04134-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00402-021-04134-1