Abstract
Background
The purpose of this meta-analysis was to compare the blood loss and complications of intra-articular (IA) with intravenous (IV) tranexamic acid (TXA) for total knee arthroplasty (TKA).
Methods
A comprehensive search of studies was conducted to identify related articles in Pubmed, Embase, Cochrane central Register of Controlled Trials, springerLink, OVID and the Research published from January 1980 to September 2016. All studies that compared IA TXA with IV TXA application on TKA were included. Main outcomes of the two methods were collected and analyzed by using Review Manager 5.3.
Results
There were 16 randomized controlled trials with 1308 cases met the criteria. Compared with IV TXA, IA TXA had similar blood volume of drainage, hidden blood loss, transfusion rate and complications (P > 0.05). IA TXA had lower total blood loss than IV TXA, and there was significant difference (P < 0.05). Subgroup analysis of total blood loss based on times of IV TXA administration showed that repeat dose of IV TXA had a higher total blood loss and postoperative hemoglobin drop (P < 0.05) than IA TXA. However, single dose of IV TXA had a similar efficacy on total blood loss and postoperative hemoglobin drop (P > 0.05) when compared with IA TXA.
Conclusions
Both IA TXA and single dose of IV TXA are effective in reducing total blood loss and postoperative hemoglobin drop without increasing complications of DVT or PE. The current meta-analysis suggests that 1.5 g TXA by IA administration or 1 g TXA by IV administration 10 min before tourniquet deflation is effective and safe in patients undergoing TKA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Total knee arthroplasty (TKA) has been the common procedure used to treat osteoarthritis, and the surgical volume is increasing along with the elders increased. Despite the dramatic reduction in complications, TKA is associated with a substantial postoperative blood loss. The estimated blood loss during TKA varies from 500 to 1500 mL, and the blood transfusion rate is ranging between 11 and 50%. Blood transfusion has many potential risks including infections, disease transmission, immunologic reaction and even death [1, 2].
Tranexamic acid (TXA) is a synthetic antifibrinolytic agent which stabilizes fibrin clots, resulting in more stable hemostasis by reducing the effects of plasmin, and as a consequence decreases the rate of fibrinolysis [3]. It can be administrated by IA or IV route. Previous studies reported that TXA takes about 30 min for IA administration and 5–15 min for IV administration to take effect. IV TXA has been recommended by some studies and meta-analysis as preferred method for patients undergoing TKA [4, 5]. However, there is no consensus on the doses and times of IV TXA. Even when to use IV TXA is under debate. Pharmacokinetic studies reported that an 20 mg/kg dose of IV TXA is suitable for TKA because therapeutic levels can be maintained for approximately 8 h after operation, which covers the period of postoperative hyperfibrinolysis [6]. Hourlier [7] reported that single-dose IV TXA was effective as continuous infusion in TKA. A study reported by Iwai [8] suggested that repeat-dose IV TXA reduced total blood loss in TKA. Maniar [9] reported that giving TXA preoperatively would deactivate the fibrinolysis as soon as it starts. Akgul [10] reported that a high, single dose of TXA intravenously given to the patient prior to the TKA significantly reduces the bleeding during the operation and within the postoperative 24 h. Sun [11] reported that the preoperative dose and an additional dose of IV TXA were superior to a single preoperative dose of TXA in reducing blood loss in TKA. Recently, studies reported that IA TXA has many advantages, such as easy administration, inhibits local activation of fibrinolysis and less systemic absorption [12, 13]. The current doses of IA TXA vary from 1 to 3 g and used before tourniquet deflation (when used) or before wound close. Previous study reported that there was no difference in the efficacy of 1.5 versus 3.0 g of IA TXA in reducing perioperative blood loss during TKA [14]. Several clinical studies [15–17] and meta-analysis [18, 19] have shown that both IA and IV TXA could effectively reduce blood loss and transfusion in TKA without increasing the complications.
Considering effectiveness of both IA and IV TXA, studies were performed to compare IA TXA with IV TXA for patients undergoing TKA. Tzatzairis [20] reported that 1 g IA TXA has a similar efficacy with 1 g IV TXA in the treatment of patients undergoing TKA. Seo [21] reported that 1.5 g IA TXA was more effective than 1.5 g IV TXA in terms of reducing total blood loss and transfusion rate. Aggarwal [22] reported that IA TXA was better than IV TXA in reducing blood loss. Despite the evidences from these studies that compared IA TXA with IV TXA, there is still no consistent conclusion on which route, IA or IV is the preferred treatment for TKA [20, 23]. Therefore, a meta-analysis was conducted to investigate the total blood loss, blood transfusion and complications of IA TXA and IV TXA in TKA.
Materials and methods
Search strategy
Studies were indentified through a computerized search in Pubmed, Embase, Cochrane central Register of Controlled Trials, SpringerLink, OVID and the Research using the following terms: total ankle arthroplasty OR total arthroplasty replacement OR TKA OR TKR and tranexamic acid or TXA from January 1980 to September 2016.
Inclusion and exclusion criteria
Studies should be to meet the following inclusion criteria: (1) randomized controlled trials; (2) comparison of IA and IV TXA in patients undergoing total knee arthroplasty; (3) patients older than 18 years; (4) the clinical outcomes included blood loss or postoperative complications; (5) the articles were restricted to English language.
Exclusion criteria: (1) type of studies as “case report,” “review,” “letters,” “talk” and “commentary”; (2) data were duplicated or overlapped.
Literature search result
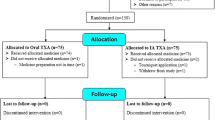
Figure 1 shows details of the identification of studies, their inclusion and exclusion. Three hundred and thirty-two articles were identified through the search, and abstract screen of these 332 articles resulted in exclusion of 76 duplicates and 235 other articles that did not fit the inclusion criteria, leaving 21 articles for full text. Three articles were not RCT, and two articles were not written in English. This eventually resulted in 16 studies included.
Risk of bias assessment
Two authors independently performed the initial screening and data extraction. Any disagreements were resolved by discussion among the authors. Information retrieved from the studies included study design, patient characteristics, sample size, geographical and complications. Literature quality assessment was performed by two researchers. A third researcher was the adjudicator when there was debate between the two researchers. The quality assessment was risk of bias in all included studies. And the evaluation criteria and methods followed the Cochrane Collaboration’s proposal and used their tool RevMan 5.3 to assess risk of bias. The methodological quality of the RCTs was assessed by using the modified Jadad scale, which includes randomization, allocation concealment, blinding, withdrawals and dropouts. High-quality studies were reflected by scores of 4–7.
Statistical analysis
We performed statistical analysis with the RevMan 5.3 provided by Cochrane Collaboration. The assessment for statistics heterogeneity was calculated through χ 2 and I 2 test, with values of 25, 50 and 75% considered low, moderate and high heterogeneity. A random effect model was used when I 2 > 50%. For continuous data, means and standard deviations were pooled to a mean difference (MD) and 95% confidence internal (CI) in the meta-analysis. For binary data, risk ratios (RR) and 95% confidence interval (CI) were assessed (ɑ = 0.05 for the inspection standards). Subgroup analysis based on times of IV TXA administration was performed for total blood loss and postoperative hemoglobin drop to explore a potential source of heterogeneity.
Results
Demographic characteristics
A total of 1308 patients from 16 studies were included. The demographic characteristics were summarized in Table 1. There were 653 patients treated with IA TXA, and 655 patients treated with IV TXA. Two studies were from the USA [24, 25], two from Spain [26, 27], three from Greece [20, 23, 28], three from India [22, 29, 30], two from Iran [8, 31], one from Singapore [32], one from Thailand [33], one from Korea [21] and one from Brazil [34]. The interventions of IA and IV group were collected in Table 2.
Risk of bias
The summary of bias is summarized in Fig. 2. All trials reported random sequence generation, but only six trials reported allocation concealment [24, 26–28, 32, 33], and six trials reported blinding [21, 22, 25, 27, 32, 35]. Two trials had patients lost to follow-up [9, 26].
Results of meta-analysis
Total blood loss
A total of nine studies provided data of total blood loss (Fig. 3). There was significant difference between the groups with respect to total blood loss (MD −66.12 mL, 95% CI −118.10 to 14.14, I 2 = 76%, P < 0.05). Four studies were assigned to the repeat dose of IV TXA subgroup, and five studies were assigned to the single dose of IV TXA subgroup. The repeat dose of IV TXA subgroup showed a higher total blood loss as compared with the IA group, and there was significant difference (MD −98.57 mL, 95% CI −182.11 to −15.02, I 2 = 90%, P < 0.05). The single dose of IV TXA subgroup showed a higher total blood loss as compared with the IA group, although there was no significant difference (MD −45.62 mL, 95% CI −112.02 to 20.78, I 2 = 0%, P > 0.05) (Fig. 3).
Forest plot of total blood loss showed that there was significant difference between IA TXA group and IV TXA group. Subgroup analysis based on times of IV TXA administration showed that there was significant difference between IA TXA group and repeat dose of IV TXA group. There was no significant difference between IA TXA group and single dose of IV TXA group
Blood volume of drainage
A total of 11 studies provided data of blood volume of drainage. The heterogeneity test indicated moderate statistical evidence of heterogeneity (χ 2 = 36.87, P < 0.01, I 2 = 73%). There was no significant difference between the groups in terms of blood volume of drainage (MD −29.89 mL, 95% CI −68.99 to 9.2, P > 0.05) (Fig. 4).
Hidden blood loss
A total of three studies provided data of hidden blood loss. The heterogeneity test indicated low statistical evidence of heterogeneity (χ 2 = 3.34, P > 0.05, I 2 = 40%). There was no significant difference between the groups with respect to hidden blood loss (MD 48.57 mL, 95% CI −20.5 to 117.64, P > 0.05) (Fig. 5).
Postoperative hemoglobin drop
A total of ten studies provided data of postoperative hemoglobin drop. The pooled standard mean difference in postoperative hemoglobin drop was −0.01 mL (95% Cl −0.38 to 0.35), indicating that there was 0.01 mL less postoperative hemoglobin drop in the IA group as compared to the IV group, but this difference was no statistically significant (χ 2 = 75.47, I 2 = 88%, P > 0.05). Four studies were assigned to the repeat dose of IV TXA subgroup, and six studies were assigned to the single dose of IV TXA subgroup. The repeat dose of IV TXA group showed a greater postoperative hemoglobin drop as compared with the IA group, and there was significant difference (MD −0.43 mL, 95% Cl −0.8 to −0.05, P < 0.05). The single dose of IV TXA group showed a lower postoperative hemoglobin drop as compared with the IA group, although there was no significant difference (MD −0.51,95% Cl −1.02 to 0.01, P = 0.05) (MD 0.29 mL, 95% Cl −0.16 to 0.74, P > 0.05) (Fig. 6).
Forest plot of postoperative hemoglobin showed that there was no significant difference between IA TXA group and IV TXA group. Subgroup analysis based on times of IV TXA administration showed that there was statistical significance between IA TXA group and repeat dose of IV TXA group. There was no significant difference between IA TXA group and single dose of IV TXA group
Blood transfusion rate
A total of 13 studies provided data of blood transfusion rate. The heterogeneity test indicated low statistical evidence of heterogeneity (χ 2 = 5.78, P > 0.01, I 2 = 14%). There was no statistically significant difference between the groups with respect to the blood transfusion rate (RR: 1.17, 95% CI: 0.84–1.93, χ 2 = 14, 15, I 2 = 15%, P > 0.05) (Fig. 7).
Blood units transfused per patient
A total of five studies provided data of blood units transfused per patient. The heterogeneity test indicated low statistical evidence of heterogeneity (χ 2 = 1.434, P > 0.05, I 2 = 25%). There was no significant difference between the two groups in terms of blood units transfused per patient (MD −0.1, 95% CI −0.23 to 0.04, P > 0.05) (Fig. 8).
Superficial or deep infection of wound
All studies provided data of superficial or deep infection. Digas [28] reported one case of superficial infection in IA group. Drosos [23] reported one case of superficial infection in IV group. One case of superficial infection and one case of deep infection in IA group while four cases of superficial infection in the IV group reported by May [24]. Tzatzairis [20] reported three cases of superficial infection in the IA group and two cases of superficial infection and one case of deep infection in the IV group. There was no statistically difference between the groups in terms of superficial and deep infection (RR 0.81, χ 2 = 1.23, I 2 = 0%, P > 0.05) (Fig. 9).
Thromboembolic complications
All studies provided data of thromboembolic complications. One study reported by Digas [28] documented one DVT case of the IA group. May [24] reported one case of DVT and one case of PE in IA group, while two PE cases and two DVT cases in IV group. Seo [21] reported three cases of DVT in IA group. Another study reported by Zekcer [35] documented one case of DVT in IA group. Patel [25] reported one case in the IA group and two cases in the IV group have clinical suspicion of DVT and the duplex Doppler study of each of these patients was negative. There was no significant difference between the groups with respect to thromboembolic complications (RR = 0.50, χ 2 = 2.01, I 2 = 0%, P > 0.05) (Fig. 10).
Discussion
The purpose of this meta-analysis was to compare the outcome of IA and IV TXA in the treatment of TKA. There was no significant differences in blood volume of drainage, hidden blood loss, blood transfusion rate, blood units transfused per patient, superficial and deep infection and thromboembolic complications such as DVT or PE when compared IA TXA with IV TXA in primary TKA. Subgroup analysis showed that repeat dose of IV TXA had a higher total blood loss and a higher postoperative hemoglobin drop as compared with the IA group. In addition, IA TXA had a similar efficacy with single dose of IV TXA in terms of reducing total blood loss and postoperative hemoglobin drop.
Application of tourniquet during TKA may avoid massive blood loss, however, when it is released at the end of the surgery, fibrinolytic activity and bleeding increase [34, 36]. In order to avoid anemia, blood transfusion may be required depending on the amount of blood lost. As blood transfusion increases the risk of postoperative infections, costs, the length of hospital stay and morbidity, pharmacological strategies are recommended to reduce bleeding [37, 38].
Pharmacologic agents such as TXA, an antifibrinolytic drug, have been employed to reduce bleeding and prevent the need for transfusion. Despite the concern about the greater risk of venous thromboembolism, a significant risk in the orthopedic surgeries population was not reported by studies [21, 39]. Many studies have reported that by administrating TXA in TKA, no matter by IA or by IV route, the thromboembolic episodes are not increasing [9, 20, 31]. However, the route of application of TXA in TKA varies across clinical trials [40] and consensus on the most appropriate route is lacking. Then, this meta-analysis of comparing IA TXA with IV TXA in TKA was considered.
Some studies consider that TXA application through IA route can avoid local activation of fibrinolysis and increase local thrombus formation, resulting in less bleeding and less systemic absorption as compared with TXA application through IV route [25, 41]. Our meta-analysis showed that IA TXA reduced the total blood loss by a mean volume of −66.12 mL when compared with IV TXA and this result had significant difference (P > 0.05). From Table 2, we noticed that some studies used repeat dose of IV TXA and the others used single dose of IV TXA. In order to observe which route, repeat dose of IV TXA or single dose of IV TXA, caused the higher total blood loss, subgroup analysis was performed. Subgroup analysis showed that repeat dose of IV TXA had a higher total blood loss than IA TXA and there was significant difference (P < 0.05). This result was inconsistent with previous studies that repeat dose IV TXA further decreases blood loss in TKA [8, 21]. Combined with the result of subgroup analysis that repeat dose of IV TXA has a higher postoperative hemoglobin drop (MD −0.43, P < 0.05) than IA group, our meta-analysis suggested that IA TXA was superior to repeat dose of IV TXA in terms of reducing total blood loss and decreasing postoperative hemoglobin drop. In addition, our subgroup analysis also suggested that IA TXA and single dose of IV TXA have a similar effect on total blood loss and postoperative hemoglobin drop and just be efficient for patients undergoing TKA. Previous studies reported that TXA can effectively reduce the blood volume of drainage by about 50%, and it has no effect on reducing hidden blood loss, which is not mediated by fibrinolysis in the initial phase [42]. Our meta-analysis results suggested that IA TXA and IV TXA have no significant difference in reducing blood volume of drainage and hidden blood loss. Although IV TXA group has a higher total blood loss, there was no significant difference in reducing blood units transfused per patient and transfusion rate when compared with IA group. These results may be attributed to the fact that the transfusion criteria were inconsistent among the studies. Many studies suggested that TXA has anti-inflammatory effects and IA application of TXA in TKA did not increase the infection [43, 44]. Our analysis showed that 6 of 659 patients (0.91%) in the IA group and 8 of 627 patients (1.28%) in the IV group had superficial or deep infection, suggested that IA TXA did not increase the risk of infection compared with IV TXA group.
The fatal complication of anastaltic was thrombosis and pulmonary embolism. Theoretically, TXA administration through the IV route carries a higher risk of DVT and PE. And a growing number of studies recommend IA TXA to minimize the adverse effects [27, 29]. Our meta-analysis did not show an increased risk of DVT or PE either by using IV or IA TXA. In our meta-analysis, one study reported by Digas [28] documented one DVT case of the IA group. May [24] reported one case of DVT and one case of PE in IA group, while two PE cases and two DVT cases in IV group. Seo [21] reported three cases of DVT in IA group. Another study reported by Zekcer [35] documented one case of DVT in IA group. Patel [25] reported that one case in the IA group and two cases in the IV group have clinical suspicion of DVT and the duplex Doppler study of these patients was negative. Nonetheless, uncertainty still remains because of the different methods of DVT and PE screening and seven included studies failed to report the method of DVT screen. In addition, the small numbers of patients involved and high-risk patients such as those with a history of PTE or DVT and those with cerebrovascular or heart disease excluded also prevented us from drawing an accurate conclusion. Considering the above factors, the conclusion of DVT and PE requires further confirmation. The current meta-analysis suggested that 1.5 g TXA by IA administration or 1 g IV TXA 10 min before tourniquet inflation is effective and safe in patients undergoing TKA.
This study has some limitations. Firstly, all trials included in our analysis excluded high-risk patients, such as patients with previous DVT events, cardiovascular disease and renal dysfunction. Secondly, the dose and timing administration of TXA application in TKA were different. Finally, there was significant heterogeneity among studies when total blood loss and postoperative hemoglobin drop were evaluated.
In conclusion, IA TXA has similar clinically effectiveness compared to IV TXA in blood volume of drainage, hidden blood loss, transfusion rate, blood units transfused per patient and wound infection. In addition, results from subgroup analysis evaluating effect of the times of TXA administration through the IV route suggested that single dose of IV TXA is effective than repeat dose of IV TXA with respect to total blood loss and postoperative hemoglobin drop. Both IA TXA and single dose of IV TXA are effective in reducing total blood loss and postoperative hemoglobin drop without increasing complications of DVT or PE. Further studies with high quality are required to confirm the effectiveness and safety of IA and IV TXA for primary TKA.
References
Tan T, Eslami M, Rybin D, Doros G, Zhang WW, Farber A (2015) Blood transfusion is associated with increased risk of perioperative complications and prolonged hospital duration of stay among patients undergoing amputation. Surgery 158(6):1609–1616
Zhang L, Liao Q, Zhang T, Dai M, Zhao Y (2016) Blood transfusion is an independent risk factor for postoperative serious infectious complications after pancreaticoduodenectomy. World J Surg 40(10):2507–2512
Alshryda S, Mason J, Vaghela M, Sarda P, Nargol A, Maheswaran S, Tulloch C, Anand S, Logishetty R, Stothart B, Hungin AP (2013) Topical (intra-articular) tranexamic acid reduces blood loss and transfusion rates following total knee replacement: A randomized controlled trial (TRANX-K). J Bone Jt Surg Am 95(21):1961–1968
Jiang X, Ma XL, Ma JX (2016) Efficiency and safety of intravenous tranexamic acid in simultaneous bilateral total knee arthroplasty: a systematic review and meta-analysis. Orthop Surg 8(3):285–293
Motififard M, Tahririan MA, Saneie M, Badiei S, Nemati A (2015) Low dose perioperative intravenous tranexamic acid in patients undergoing total knee arthroplasty: a double-blind randomized placebo controlled clinical trial. J Blood Transfus 2015:948304
Eriksson O, Kjellman H, Pilbrant A, Schannong M (1974) Pharmacokinetics of tranexamic acid after intravenous administration to normal volunteers. Eur J Clin Pharmacol 7(5):375–380
Hourlier H, Reina N, Fennema P (2015) Single dose intravenous tranexamic acid as effective as continuous infusion in primary total knee arthroplasty: a randomised clinical trial. Arch Orthop Trauma Surg 135(4):465–471
Iwai T, Tsuji S, Tomita T, Sugamoto K, Hideki Y, Hamada M (2013) Repeat-dose intravenous tranexamic acid further decreases blood loss in total knee arthroplasty. Int Orthop 37(3):441–445
Maniar RN, Kumar G, Singhi T, Nayak RM, Maniar PR (2012) Most effective regimen of tranexamic acid in knee arthroplasty: a prospective randomized controlled study in 240 patients. Clin Orthop Relat Res 470(9):2605–2612
Akgul T, Buget M, Salduz A, Edipoglu IS, Ekinci M, Kucukay S, Sen C (2016) Efficacy of preoperative administration of single high dose intravenous tranexamic acid in reducing blood loss in total knee arthroplasty: a prospective clinical study. Acta Orthop Traumatol Turc 50(4):429–431
Sun Q, Yu X, Wu J, Ge W, Cai M, Li S (2016) Efficacy of a single dose and an additional dose of tranexamic acid in reduction of blood loss in total knee arthroplasty. J Arthroplasty. doi: 10.1016/j.arth.2016.10.003
Lostak J, Gallo J, Spicka J, Langova K (2016) Intra-Articular application of tranexamic acid significantly reduces blood loss and transfusion requirement in primary total knee arthroplasty. Acta Chir Orthop Traumatol Cech 83(4):254–262
Spanyer J, Patel J, Emberton E, Smith LS, Malkani AL (2016) Topical tranexamic acid in total knee arthroplasty patients with increased thromboembolic risk. J Knee Surg. doi: 10.1055/s-0036-1593371
Wong J, Abrishami A, El BH, Mahomed NN, Roderick DJ, Gandhi R, Syed KA, Muhammad OHS, De Silva Y, Chung F (2010) Topical application of tranexamic acid reduces postoperative blood loss in total knee arthroplasty: a randomized, controlled trial. J Bone Jt Surg Am 92(15):2503–2513
Keyhani S, Esmailiejah AA, Abbasian MR, Safdari F (2016) Which route of tranexamic acid administration is more effective to reduce blood loss following total knee arthroplasty? Arch Bone Jt Surg 4(1):65–69
Pitta M, Zawadsky M, Verstraete R, Rubinstein A (2016) Intravenous administration of tranexamic acid effectively reduces blood loss in primary total knee arthroplasty in a 610-patient consecutive case series. Transfusion 56(2):466–471
Sabatini L, Atzori F, Revello S, Scotti L, Debiasi F, Masse A (2014) Intravenous use of tranexamic acid reduces postoperative blood loss in total knee arthroplasty. Arch Orthop Trauma Surg 134(11):1609–1614
Alshryda S, Sukeik M, Sarda P, Blenkinsopp J, Haddad FS, Mason JM (2014) A systematic review and meta-analysis of the topical administration of tranexamic acid in total hip and knee replacement. Bone Jt J 96-B(8):1005–1015
Yang ZG, Chen WP, Wu LD (2012) Effectiveness and safety of tranexamic acid in reducing blood loss in total knee arthroplasty: A meta-analysis. J Bone Jt Surg Am 94(13):1153–1159
Tzatzairis TK, Drosos GI, Kotsios SE, Ververidis AN, Vogiatzaki TD, Kazakos KI (2016) Intravenous vs topical tranexamic acid in total knee arthroplasty without tourniquet application: A randomized controlled study. J Arthroplasty 31(11):2465–2470
Seo JG, Moon YW, Park SH, Kim SM, Ko KR (2013) The comparative efficacies of intra-articular and IV tranexamic acid for reducing blood loss during total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc 21(8):1869–1874
Aggarwal AK, Singh N, Sudesh P (2016) Topical vs intravenous tranexamic acid in reducing blood loss after bilateral total knee arthroplasty: A prospective study. J Arthroplasty 31(7):1442–1448
Drosos GI, Ververidis A, Valkanis C, Tripsianis G, Stavroulakis E, Vogiatzaki T, Kazakos K (2016) A randomized comparative study of topical versus intravenous tranexamic acid administration in enhanced recovery after surgery (ERAS) total knee replacement. J Orthop 13(3):127–131
May JH, Rieser GR, Williams CG, Markert RJ, Bauman RD, Lawless MW (2016) The assessment of blood loss during total knee arthroplasty when comparing intravenous vs intracapsular administration of tranexamic acid. J Arthroplasty 31(11):2452–2457
Patel JN, Spanyer JM, Smith LS, Huang J, Yakkanti MR, Malkani AL (2014) Comparison of intravenous versus topical tranexamic acid in total knee arthroplasty: a prospective randomized study. J Arthroplasty 29(8):1528–1531
Aguilera X, Martinez-Zapata MJ, Hinarejos P, Jordan M, Leal J, Gonzalez JC, Monllau JC, Celaya F, Rodriguez-Arias A, Fernandez JA, Pelfort X, Puig-Verdie L (2015) Topical and intravenous tranexamic acid reduce blood loss compared to routine hemostasis in total knee arthroplasty: A multicenter, randomized, controlled trial. Arch Orthop Trauma Surg 135(7):1017–1025
Gomez-Barrena E, Ortega-Andreu M, Padilla-Eguiluz NG, Perez-Chrzanowska H, Figueredo-Zalve R (2014) Topical intra-articular compared with intravenous tranexamic acid to reduce blood loss in primary total knee replacement: a double-blind, randomized, controlled, noninferiority clinical trial. J Bone Jt Surg Am 96(23):1937–1944
Digas G, Koutsogiannis I, Meletiadis G, Antonopoulou E, Karamoulas V, Bikos C (2015) Intra-articular injection of tranexamic acid reduce blood loss in cemented total knee arthroplasty. Eur J Orthop Surg Traumatol 25(7):1181–1188
Martin JG, Cassatt KB, Kincaid-Cinnamon KA, Westendorf DS, Garton AS, Lemke JH (2014) Topical administration of tranexamic acid in primary total hip and total knee arthroplasty. J Arthroplasty 29(5):889–894
Soni A, Saini R, Gulati A, Paul R, Bhatty S, Rajoli SR (2014) Comparison between intravenous and intra-articular regimens of tranexamic acid in reducing blood loss during total knee arthroplasty. J Arthroplasty 29(8):1525–1527
Sarzaeem MM, Razi M, Kazemian G, Moghaddam ME, Rasi AM, Karimi M (2014) Comparing efficacy of three methods of tranexamic acid administration in reducing hemoglobin drop following total knee arthroplasty. J Arthroplasty 29(8):1521–1524
Chen JY, Chin PL, Moo IH, Pang HN, Tay DK, Chia SL, Lo NN, Yeo SJ (2016) Intravenous versus intra-articular tranexamic acid in total knee arthroplasty: a double-blinded randomised controlled noninferiority trial. Knee 23(1):152–156
Pinsornsak P, Rojanavijitkul S, Chumchuen S (2016) Peri-articular tranexamic acid injection in total knee arthroplasty: a randomized controlled trial. BMC Musculoskelet Disord 17:313
Yi S, Tan J, Chen C, Chen H, Huang W (2014) The use of pneumatic tourniquet in total knee arthroplasty: a meta-analysis. Arch Orthop Trauma Surg 134(10):1469–1476
Zekcer A, Da SR, Filho MC (2011) Anatomical acl reconstruction with double bundle: First 40 cases. Rev Bras Ortop 46(3):262–265
Mori N, Kimura S, Onodera T, Iwasaki N, Nakagawa I, Masuda T (2016) Use of a pneumatic tourniquet in total knee arthroplasty increases the risk of distal deep vein thrombosis: a prospective, randomized study. Knee 23(5):887–889
Chen X, Cao X, Yang C, Guo K, Zhu Q, Zhu J (2016) Effectiveness and safety of Fixed-Dose tranexamic acid in simultaneous bilateral total knee arthroplasty: a randomized Double-Blind controlled trial. J Arthroplasty 31(11):2471–2475
Chimento GF, Huff T, Ochsner JJ, Meyer M, Brandner L, Babin S (2013) An evaluation of the use of topical tranexamic acid in total knee arthroplasty. J Arthroplasty 28(8 Suppl):74–77
Gillette BP, DeSimone LJ, Trousdale RT, Pagnano MW, Sierra RJ (2013) Low risk of thromboembolic complications with tranexamic acid after primary total hip and knee arthroplasty. Clin Orthop Relat Res 471(1):150–154
Shemshaki H, Nourian SM, Nourian N, Dehghani M, Mokhtari M, Mazoochian F (2015) One step closer to sparing total blood loss and transfusion rate in total knee arthroplasty: a meta-analysis of different methods of tranexamic acid administration. Arch Orthop Trauma Surg 135(4):573–588
Georgiadis AG, Muh SJ, Silverton CD, Weir RM, Laker MW (2013) A prospective double-blind placebo controlled trial of topical tranexamic acid in total knee arthroplasty. J Arthroplasty 28(8 Suppl):78–82
Good L, Peterson E, Lisander B (2003) Tranexamic acid decreases external blood loss but not hidden blood loss in total knee replacement. Br J Anaesth 90(5):596–599
Jimenez JJ, Iribarren JL, Brouard M, Hernandez D, Palmero S, Jimenez A, Lorente L, Machado P, Borreguero JM, Raya JM, Martin B, Perez R, Martinez R, Mora ML (2011) Safety and effectiveness of two treatment regimes with tranexamic acid to minimize inflammatory response in elective cardiopulmonary bypass patients: a randomized double-blind, dose-dependent, phase IV clinical trial. J Cardiothorac Surg 6:138
Waddell BS, Zahoor T, Meyer M, Ochsner L, Chimento G (2016) Topical tranexamic acid use in knee periprosthetic joint infection is safe and effective. J Knee Surg 29(5):423–429
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Funding
The study was supported by the Natural Science Foundation of Hubei Province (Grant No. WJ2017Q025).
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Mi, B., Liu, G., Zhou, W. et al. Intra-articular versus intravenous tranexamic acid application in total knee arthroplasty: a meta-analysis of randomized controlled trials. Arch Orthop Trauma Surg 137, 997–1009 (2017). https://doi.org/10.1007/s00402-017-2683-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00402-017-2683-1