Abstract
Introduction
Tranexamic acid (TXA) is becoming widely used in orthopedic surgery to reduce blood loss and transfusion requirements, but consensus is lacking regarding the optimal route and dose of administration. The aim of this study was to compare the efficacy and safety of topical and intravenous routes of TXA with routine hemostasis in patients undergoing primary total knee arthroplasty (TKA).
Materials and methods
We performed a randomized, multicenter, parallel, open-label clinical trial in adult patients undergoing primary TKA. Patients were divided into three groups of 50 patients each: Group 1 received 1 g topical TXA, Group 2 received 2 g intravenous TXA, and Group 3 (control group) had routine hemostasis. The primary outcome was total blood loss. Secondary outcomes were hidden blood loss, blood collected in drains, transfusion rate, number of blood units transfused, adverse events, and mortality.
Results
One hundred and fifty patients were included. Total blood loss was 1021.57 (481.09) mL in Group 1, 817.54 (324.82) mL in Group 2 and 1415.72 (595.11) mL in Group 3 (control group). Differences in total blood loss between the TXA groups and the control group were clinically and statistically significant (p < 0.001). In an exploratory analysis differences between the two TXA groups were not statistically significant (p = 0.073) Seventeen patients were transfused. Transfusion requirements were significantly higher in Group 3 (p = 0.005). No significant differences were found between groups regarding adverse events.
Conclusion
We found that 1 g of topical TXA and 2 g of intravenous TXA were both safe strategies and more effective than routine hemostasis to reduce blood loss and transfusion requirements after primary TKA.
Level of evidence
I.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Blood loss associated with total knee arthroplasty (TKA) may have a negative impact on patient recovery. The application of a tourniquet during the intervention avoids intra-operative blood loss, but once it is released at the end of the procedure, fibrinolytic activity and bleeding increase [1, 2]. Depending on the amount of blood lost, an allogenic blood transfusion may be required. As this procedure increases the risk of postoperative infections, the length of hospital stay and costs [3], it is recommended that patients should have optimal preoperative hemoglobin levels [4]. During surgery and in the postoperative period, pharmacological and non-pharmacological strategies should be applied to reduce bleeding.
One strategy which is now included in many clinical recommendations is the administration of tranexamic acid (TXA), an antifibrinolytic drug that is proving effective in reducing postoperative bleeding and the need for transfusion [5–7]. Several clinical trials have studied the efficacy of intravenous TXA [8–10] in patients undergoing TKA surgery, and a few have studied the efficacy of the topical administration route [11]. However, little is known about the comparative efficacy of both routes and the most appropriate doses.
The present study was designed in 2011, at which time no studies, to our knowledge, had compared topical and intravenous TXA versus routine hemostasis in primary TKA. Several randomized clinical trials [12–18] and a retrospective controlled study [19] have since compared the two routes of administration in TKA surgery. These studies show that TXA is effective in reducing blood loss and the number of transfusions compared with a control group. TXA doses given to these patients vary among studies, but the effectiveness of the topical route appears to be similar to that of the intravenous route [12, 14, 16–18] or even higher [15]. However, there is still no consensus regarding the optimal route and the safest and most effective doses.
The aim of this study was to compare total blood loss reduction and safety of TXA treatment in two groups of patients undergoing primary TKA with patients having only routine hemostasis (a control group). We hypothesized that 1 g of topical TXA or 2 g of intravenous TXA would be more effective and safer than routine hemostasis.
Materials and methods
This study was a multicenter, open-label, three-arm, randomized, parallel-group clinical trial. It was registered at ClinicalTrials.gov (NCT01594671), the protocol number was IIBSP-ATR-2010-23, and the EUDRACT number was 2011-000766-35. The protocol was approved by the local ethics committees at both participating university hospitals (Hospital de la Santa Creu i Sant Pau and Hospital de la Esperanza) and by the Spanish National Agency of Medicines and Medical Devices (April 2011). The study was conducted in accordance with the Declaration of Helsinki and written informed consent was obtained from all participants before surgery.
Patient population
We recruited consecutive patients who were scheduled for elective primary TKA due to osteoarthritis, rheumatoid arthritis or other degenerative knee disorders between February 2012 and October 2012. Inclusion criteria were adult patients aged 18 or older who agreed to participate.
Exclusion criteria were known allergy to TXA, a history of coagulopathy or a thromboembolic event, previous by-pass surgery, use of anticoagulant or contraceptive treatment, cardiovascular prosthesis, and refusal to participate.
Operative and postoperative procedures
Patients were randomized to one of three groups: Group 1, treatment with topical TXA plus routine hemostasis; Group 2, treatment with intravenous TXA plus routine hemostasis, and Group 3, routine hemostasis only.
A table of random numbers was made using the software SPSS/PC Version 21 for Windows (SPSS Inc., Chicago, IL, USA) and stratified by centre. Randomization was assigned on the day of surgery, by phone call to the coordinating center. All clinical investigators were unaware of the allocation sequence of treatments.
Group 1 The topical TXA group received 1 g of TXA in a 10 mL solution. After the prosthesis was inserted and cemented, the entire operative field was thoroughly rinsed and dried meticulously. The TXA was applied by syringe-spray to the following surfaces: the posterior capsule, the surrounding soft tissues, including the muscles and tendons, fatty and subcutaneous tissue, and the exposed surfaces of the femur and tibia. Routine hemostasis was also performed.
Group 2 Besides routine hemostasis, the intravenous TXA group received two intravenous doses of 1 g. The first dose was administered 15–30 min before the pneumatic tourniquet was inflated, and the second dose was given when the tourniquet was removed (60–90 min after the first dose).
Group 3 The control group received routine hemostasis only, consisting of electro-coagulation of all possible bleeding points and vessels.
All surgical interventions were carried out by nine senior orthopedic surgeons, authors of this study, with wide experience in primary TKA. All types of knee prosthesis (Table 2) were cemented. A combined spinal–epidural technique (subarachnoid blockade and epidural catheter placement) was used for anesthesia. During induction of the anesthesia and for the 24 h after surgery, we administered an intravenous prophylactic antibiotic. To prevent intra-operative blood loss, before starting surgery, a pneumatic tourniquet was placed around the upper thigh and inflated to 350 mmHg of pressure. A midline incision was made and a medial parapatellar approach was used. Electro-coagulation was performed during surgery in all patients (routine hemostasis). In all patients, the femoral intramedullary canal was breached. No bone plugs were inserted. A number eight vacuum drain was inserted into the joint space. After application of the bandage, the tourniquet was deflated. The drain was kept closed during the first hour and removed 24 h after surgery. On the day after surgery and for the following 30 days, all patients received low molecular weight heparin to prevent thromboembolic complications.
Both participating hospitals followed the same blood transfusion protocol. Transfusion of red cell concentrate units was indicated when hemoglobin was <8 g/dL, when hemoglobin was <8.5 g/dL in patients with heart disease or older than 70 years, and when hemoglobin was between 8.5 and 9 g/dL in patients with low orthostatic tolerance. The decision to transfuse allogenic blood was made by the anaesthesiologist during surgery and by the ward doctor during the postoperative period.
Postoperative rehabilitation consisting of active and passive movements of the knee joint was started the day after surgery in accordance with the institutional protocols. Weight bearing was allowed after checking the postoperative X-rays. Fifteen days after surgery we assessed the wound and removed the staples. A physiotherapist carried out domiciliary visits three times per week over the first 2 months for all patients. All patients had routine follow-up visits with the surgeon at the outpatient clinic during the study period and all complications were recorded. The total follow-up was 2 months (±15 days).
The primary outcome was total blood loss during the first 24 h after surgery. Total blood loss (mL) was calculated using the equations described by Nadler et al. [20], according to the hemoglobin balance (pre and postoperative, hemoglobin drop), units of blood transfused, weight, height and sex (see Appendix 1 for blood loss calculation method). Total blood loss corresponds to the sum of blood loss collected from drains and the hidden blood loss. Blood loss collected from drains (mL) was recorded on a computer program until drains were removed by nurses 24 h postoperatively as part of routine practice. The nurses were not involved in the study. Hidden blood loss (mL) was calculated as the difference between total blood loss minus blood loss collected from drains. Hidden blood loss corresponds to blood lost during surgery or retained in the wound or adjacent tissue.
Secondary outcomes were blood loss collected from drains, hidden blood loss, the rate of perioperative blood transfusion, the preoperative and postoperative hemoglobin, the number of blood units transfused the rate of surgical infections, the length of hospital stay, the rate of venous thrombosis, and mortality.
One of the study investigators conducted a questionnaire to determine basal characteristics, date of surgery, discharge date, blood hemoglobin concentrations (at baseline and 2, 12, 24, 48, 72 and 120 h after surgery), allogenic blood transfusion, red blood cell units transfused, volume of bleeding in the vacuum drains during the postoperative period, and adverse events.
Adverse events were categorized as mild (not interfering with the patients’ normal activities), moderate (interfering with normal activities), or severe (preventing normal activities). Neither researchers nor patients were blinded to the study interventions.
Statistical analysis
We considered a 200-mL reduction of blood loss in drains in the experimental groups compared to the control group as clinically significant. We assumed a standard deviation of 303.4 mL in blood loss, a 20 % drop-out rate, an alpha risk of 0.05, and a beta risk of 0.2. The number of patients required for each group was 50, or 150 in total. The software used for this calculation was SPSS Sample Power.
Analysis was performed per protocol. For categorical data we calculated frequency count. For quantitative data, when appropriate, we calculated mean and standard deviation (SD), or mean difference and 95 % confidence interval (CI). Pearson’s Chi-square tests were used for categorical data. When the sample size of categorical data was small, we applied Fisher’s exact test.
Blood loss was analyzed using one-way ANOVA. If significant differences were detected, each experimental intervention was compared with the control group, and between them, using Dunnett’s test. Statistical significance was set at p ≤ 0.05. Software used for data analysis was SPSS/PC Version 18.0 for Windows (SPSS Inc., Chicago, IL, USA). Besides, an exploratory analysis in total blood loss reduction between TXA groups was performed.
Results
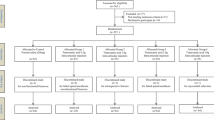
Two hundred and eighty-one patients were screened. Of these, 51 were excluded because they did not meet the study criteria due to their medical condition, 23 declined to participate, and 57 were not proposed to be included. One hundred and fifty patients were randomized and distributed to one of the three treatment groups. Two further patients (1.3 %) were later excluded (see flow diagram Fig. 1).
Patients’ mean age was 72.9 years (SD 7.1; range 49–87 years). There were 48 men (32 %) and 102 women (68 %). There were no differences between treatment groups in the baseline characteristics or co-morbidities (Table 1). Most patients had an American Society of Anesthesiologists score of 2 (Table 1).
There were no significant differences between groups regarding duration of surgery or tourniquet time. The overall mean hemoglobin concentration prior to surgery was 13.55 (1.45) g/dL, without differences between groups (Table 2).
The overall mean pre-transfusional hemoglobin was 8.70 (0.17) g/dL for the topical TXA group and 7.43 (1.3) g/dL for the control group (p = 0.141).
Seventeen (11.3 %) patients needed transfusion, 4 in the topical TXA group, 0 in the intravenous TXA group, and 13 in the control group (p = 0.005). The overall number of blood units transfused was 34: seven in the topical TXA group, 0 in the intravenous TXA group, and 27 in the control group (p = 0.001) (Table 2).
Table 3 shows total blood loss for each group. For the main outcome (total blood loss), we analyzed 47 patients in the topical TXA group, 48 in the intravenous TXA group, and 48 in the control group. Total blood loss was significantly lower in the topical TXA group and in the intravenous TXA group than in the control group (p < 0.001). In the exploratory analysis, there were no statistically significant differences between topical and intravenous TXA groups (p = 0.073).
To assess blood loss outcome through the drain, we analyzed 48 patients in the topical TXA group, 49 in the intravenous TXA group, and 49 in the control group. Blood loss through the drain was significantly lower in the topical TXA group and in the intravenous TXA group than in the control group (p < 0.001) (Table 3). There were no significant differences between topical and intravenous TXA groups (p = 0.318). With respect to hidden blood loss, there were no differences among groups, although this was slightly lower in intravenous TXA group (Table 3).
Adverse events were recorded in 20 patients (Table 4). These were mild in 12 patients, moderate in four, and severe in four. All adverse events were resolved with therapeutic measures.
With respect to the severe adverse events, one patient in the control group presented persistent bleeding due to a popliteal pseudo-aneurysm and developed a prosthetic infection. Both complications were resolved with surgery. A second patient, also in the control group, had severe bleeding from the surgical wound, leading to anemia and secondary hypotension. An allogenic blood transfusion was administered and both events were resolved. Two patients from the intravenous TXA group, needed surgery because one of them had a prosthetic joint infection and the other a knee prosthesis dislocation.
Discussion
In this clinical trial, we found that the administration of 1 g of topical TXA and 2 g of intravenous TXA were both safe and effective treatments in reducing total blood loss and the need for allogenic blood transfusion in primary TKA compared to routine hemostasis.
We decided to use a different dose of TXA for each route of administration [12] as we hypothesized that a small dose could be safer than a higher dose and just as efficient. For the intravenous route, we followed Tanakana et al.’s [21] proposal for two doses each of 1 g. One dose was given preoperatively and the second was administered after tourniquet removal. The efficacy of these intravenous doses was corroborated in a previous randomized clinical trial [22]. For the topical route, we used a single dose of 1 g, hypothesizing that this quantity of TXA could be sufficient and safe. A previous study had shown that topical TXA was effective and safe at a dose of 1.5 g [23], and during the development of our study other doses of topical TXA ranging from 0.5 [21] to 3 g [13, 16, 18, 23, 24–27] proved to be effective and safe.
Both TXA treatment groups showed a reduction in total blood loss compared to routine hemostasis, but total blood loss was smaller (mean difference of 204 mL between TXA groups) when TXA was given by intravenous route. This difference could be explained by the different dose administered (2 g intravenous versus 1 g topical TXA). A recent meta-analysis of topical TXA in primary TKA [11] found that 2 g was more effective than lower doses in terms of transfusion requirements, supporting our suggestion that we may have reduced blood loss even further in the topical group if we had used the same dose as in our intravenous group.
Few clinical trials have focused on clarifying the optimal dose for topical TXA administration in primary TKA. Wong et al. [23] allocated patients to receive 1.5 or 3 g of topical TXA, or placebo. They did not detect any statistical differences in total blood loss between the two TXA groups, but comparison with the placebo group was significant. In terms of allogenic blood transfusion, they did not find any difference between the 1.5 g TXA group (4/31 patients) and the placebo group (5/35 patients). In contrast, when we compared 1 g topical TXA to the control group, we obtained a significant result in favor of TXA. This difference between studies could be due to differences in the study design.
The method of application of topical TXA in TKA varies across clinical trials. Consensus is lacking about the most appropriate method of application. We decided to administer the drug by directly spraying all the structures around the joint and soft tissue, as done in several other studies [13, 18, 27, 28]. Other methods of administration described to date are injecting the drug into the joint after wound closure [12, 29, 30] bathing the joint before wound closure [23, 31, 32] and injecting the drug through the tube drain [27, 33, 34]. It should also be noted that some studies diluted TXA with saline solution for the topical administration, adding from 80 [25] to 100 mL [16, 23, 24, 32]. In our study, we did not add saline solution. By using a total volume of 10 mL of TXA, we avoided drug loss from overflow before wound closure [32]. We also used an intraarticular drain that we kept closed for 1 h after tourniquet removal to optimize the TXA effect.
As TXA reduces anemia and thereby the need for allogenic blood transfusion in patients undergoing TKA, it could be expected that patients receiving this treatment will respond better in the rehabilitation process, present fewer complications in general status, and show a faster functional recovery [34]. As a result, they would require a shorter hospital stay [24, 28]. However, we did not detect any significant differences between groups regarding hospital stay.
The main strength of this study is that it was a multicentre clinical trial that compared routes and also doses of TXA to routine hemostasis in primary TKA. However, the study has several limitations that make it difficult to draw conclusions. Our study design allowed us to compare the treatment groups with routine hemostasis, but only an exploratory analysis can be made between the two TXA treatments groups. A second design limitation is that the study was not blinded to the investigators. However, this did not influence the results because the main outcome was a quantitative value. Another limitation is that 12 % of patients were excluded due to possible TXA-related complications. Consequently, we did not find an increase in complications such as clinical deep vein thrombosis or pulmonary embolism.
Although evidence to date shows that TXA is effective in reducing blood in TKA, further studies will help to determine the optimal route of administration and also the optimal dose. Controlled clinical trials with larger sample sizes are still needed to confirm the safety TXA in primary TKA.
In conclusion, our data indicate that 1 g topical TXA and 2 g intravenous TXA are both safe and effective treatments to reduce blood loss and the need for transfusions after primary TKA compared to routine hemostasis.
References
Fahmy NR, Patel DG (1981) Hemostatic changes and postoperative deep-vein thrombosis associated with use of a pneumatic tourniquet. J Bone Joint Surg Am 63(3):461–465
Petäjä J, Myllynen P, Myllylä G, Vahtera E (1987) Fibrinolysis after application of pneumatic tourniquet. Acta Chir Scand 153(11–12):647–651
Kozek-Langenecker SA, Afshari A, Albaladejo P, Santullano CA, De Robertis E, Filipescu DC, Fries D, Görlinger K, Haas T, Imberger G, Jacob M, Lancé M, Llau J, Mallett S, Meier J, Rahe-Meyer N, Samama CM, Smith A, Solomon C, Van der Linden P, Wikkelsø AJ, Wouters P, Wyffels P (2013) Management of severe perioperative bleeding: guidelines from the European Society of Anaesthesiology. Eur J Anaesthesiol 30(6):270–382
Goodnough LT, Maniatis A, Earnshaw P, Benoni G, Beris P, Bisbe E, Fergusson DA, Gombotz H, Habler O, Monk TG, Ozier Y, Slappendel R, Szpalski M (2011) Detection, evaluation, and management of preoperative anaemia in the elective orthopaedic surgical patient: NATA guidelines. Br J Anaesth 106(1):13–22
Kelley TC, Tucker KK, Adams MJ, Dalury DF (2014) Use of tranexamic acid results in decreased blood loss and decreased transfusions in patients undergoing staged bilateral total knee arthroplasty. Transfusion 54(1):26–30
Henry DA, Carless PA, Moxey AJ, O’Connell D, Stokes BJ, Fergusson DA, Ker K (2011) Anti-fibrinolytic use for minimising perioperative allogeneic blood transfusion. Cochrane Database Syst Rev 19(1):CD001886
Ker K, Beecher D, Roberts I (2013) Topical application of tranexamic acid for the reduction of bleeding. Cochrane Database Syst Rev 23(7):CD010562
Kim TK, Chang CB, Koh IJ (2014) Practical issues for the use of tranexamic acid in total knee arthroplasty: a systematic review. Knee Surg Sports Traumatol Arthrosc 22(8):1849–1858
Yang ZG, Chen WP, Wu LD (2012) Effectiveness and safety of tranexamic acid in reducing blood loss in total knee arthroplasty: a meta-analysis. J Bone Joint Surg Am 94(13):1153–1159
Zhang H, Chen J, Chen F, Que W (2012) The effect of tranexamic acid on blood loss and use of blood products in total knee arthroplasty: a meta-analysis. Knee Surg Sports Traumatol Arthrosc 20(9):1742–1752
Panteli M, Papakostidis C, Dahabreh Z, Giannoudis PV (2013) Topical tranexamic acid in total knee replacement: a systematic review and meta-analysis. Knee 20(5):300–309
Hedge C, Wasnik S, Kulkarni S, Pradhan S, Shetty V (2013) Simultaneous bilateral computer assisted total knee arthroplasty: the effect of intravenous or intraarticular tranexamic acid. J Arthroplasty 28(10):1888–1891
Maniar RN, Kumar G, Singhi T, Nayak RM, Maniar PR (2012) Most effective regimen of tranexamic acid in knee arthroplasty: a prospective randomized controlled study in 240 patients. Clin Orthop Relat Res 470(9):2605–2612
Patel JN, Spanyer JM, Smith LS, Huang J, Yakkanti MR, Malkani AL (2014) Comparison of intravenous versus topical tranexamic acid in total knee arthroplasty: a prospective randomized study. J Arthroplasty 29(8):1080–1083
Seo JG, Moon YW, Park SH, Kim SM, Ko KR (2013) The comparative efficacies of intra-articular and iv tranexamic acid for reducing blood loss during total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc 21(8):1869–1874
Gomez-Barrena E, Ortega-Andreu M, Padilla-Eguiluz NG, Pérez-Chrzanowska H, Figueredo-Zalve R (2014) Topical intra-articular compared with intravenous tranexamic acid to reduce blood loss in primary total knee replacement: a double-blind, randomized, controlled, noninferiority clinical trial. J Bone Joint Surg Am 96(23):1937–1944
Huang Z, Ma J, Shen B, Pei F (2014) Combination of intravenous and topical application of tranexamic acid in primary total knee arthroplasty: a prospective randomized controlled trial. J Arthroplasty 29(12):2342–2346
Soni A, Saini R, Gulati A, Paul R, Bhatty S, Rajoli SR (2014) Comparison between intravenous and intra-articular regimens of tranexamic acid in reducing blood loss during total knee arthroplasty. J Arthroplasty 29(8):1525–1527
Wind TC, Barfield WR, Moskal JT (2013) The effect of tranexamic acid on blood loss and transfusion rate in primary total knee arthroplasty. J Arthroplasty 28(7):1080–1083
Nadler SB, Hidalgo JU, Bloch T (1962) Prediction of blood volume in normal human adults. Surgery 51(2):224–232
Tanaka N, Sakahashi H, Sato E, Hirose K, Ishima T, Ishii S (2001) Timing of the administration of tranexamic acid for maximum reduction in blood loss in arthroplasty of the knee. J Bone Joint Surg Br 83(5):702–705
Aguilera X, Martínez-Zapata MJ, Bosch A, Urrutia G, González JC, Jordán M, Gich I, Maymó RM, Martínez N, Monllau JC, Celaya F, Fernández JA (2013) Efficacy and safety of fibrin glue and tranexamic acid to prevent postoperative blood loss in total knee arthroplasty: a randomized controlled clinical trial. J Bone Joint Surg Am 95(22):2001–2007
Wong J, Abrishami A, El Beheiry H, Mahomed NN, Roderick Davey J, Gandhi R, Syed KA, Muhammad Ovais Hasan S, De Silva Y, Chung F (2010) Topical application of tranexamic acid reduces postoperative blood loss in total knee arthroplasty: a randomized, controlled trial. J Bone Joint Surg Am 92(15):2503–2513
Chimento GF, Huff T, Ochsner JL, Meyer M, Brandner L, Babin S (2013) An evaluation of the use of topical tranexamic acid in total knee arthroplasty. J Artroplasty 28(Suppl 1):74–77
Gilbody J, Dhotar HS, Perruccio AV, Davey JR (2014) Topical tranexamic acid reduces transfusion rates in total hip and knee arthroplasty. J Arthroplasty 29(4):681–684
Konig G, Hamlin BR, Waters JH (2013) Topical tranexamic acid reduces blood loss and transfusion rates in total hip and total knee arthroplasty. J Arthroplasty 28(9):1473–1476
Sarzaeem MM, Razi M, Kazemian G, Moghaddam ME, Rasi AM, Karimi M (2014) Comparing efficacy of three methods of tranexamic acid administration in reducing hemoglobin drop following total knee arthroplasty. J Arthroplasty 29(8):1521–1524
Alshryda S, Mason J, Vaghela M, Sarda P, Nargol A, Maheswaran S, Tulloch C, Anand S, Logishetty R, Stothart B, Hungin AP (2013) Topical (intra-articular) tranexamic acid reduces blood loss and transfusion rates following total knee replacement. A randomized controlled trial (TRANX-K). J Bone Joint Surg Am 95(21):1961–1968
Ishida K, Tsumura N, Kitagawa A, Hamamura S, Fukuda K, Dogaki Y, Kubo S, Matsumoto T, Matsushita T, Chin T, Iguchi T, Kurosaka M, Kuroda R (2011) Intra-articular injection of tranexamic acid reduces not only blood loss but also knee joint swelling after total knee arthroplasty. Int Orthop 35(11):1639–1645
Sa-Ngasoongsong P, Channoom T, Kawinwonggowit V, Woratanarat P, Chanplakorn P, Wibulpolprasert B, Wongsak S, Udomsubpayakul U, Wechmongkolgorn S, Lekpittaya N (2011) Postoperative blood loss reduction in computer-assisted surgery total knee replacement by low dose intra-articular tranexamic acid injection together with 2-h clamp drain: a prospective triple-blinded randomized controlled trial. Orthop Rev (Pavia) 3(2):e1. doi:10.4081/or.2011.e1232
Martin JG, Cassatt KB, Kincaid-Cinnamon KA, Westendorf DS, Garton AS, Lemke JH (2014) Topical administration of tranexamic acid in primary total hip and total knee arthroplasty. J Arthroplasty 29(5):889–894
Mutsuzaki H, Ikeda K (2012) Intra-articular injection of tranexamic acid via a drain plus rain-clamping to reduce blood loss in cementless total knee arthroplasty. J Orthop Surg Res 7:32. doi:10.1186/1749-799X-7-32
Roy SP, Tanki UF, Dutta A, Jain SK, Nagi ON (2012) Efficacy of intra-articular tranexamic acid in blood loss reduction following primary unilateral total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc 20(12):2494–2501
Diamond PT, Conaway MR, Mody SH, Bhirangi K (2006) Influence of haemoglobin levels on inpatient rehabilitation outcomes after total knee arthroplasty. J Arthroplasty 21(5):636–641
Acknowledgments
The authors wish to thank Esther Cánovas Martínez and Mercedes Subiela for data collection, Gemma Horta for logistic support, Sebastíà Videla for assistance with methodology and the revision of the manuscript, the staff at the Orthopedic Surgery Department, the secretary staff of the Iberoamerican Cochrane Centre-Clinical Epidemiology and Public Health Department, and Carolyn Newey for editing the text. The authors would also like to convey thanks to Rottapharm/Madaus for providing the tranexamic acid ampoules. Dr. Xavier Aguilera is a PhD candidate in the Surgery Department, Universitat Autónoma de Barcelona. Spain. This project was funded principally by a grant from the Spanish Ministry of Health and Social Policy to promote independent clinical research 2010 (EC10-73).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Appendix 1
Appendix 1
Calculation of postoperative blood loss on the basis of hemoglobin (Hb) balance according to equations described by Nadler et al. [20].
Hbloss = BV × (Hbi − Hbe) × 0.001 + Hbt.
Blood loss = 1000 × Hbloss/Hbi, where Hbloss is the amount (g) of hemoglobin lost, Hbi is the hemoglobin level (g/L) before surgery, Hbe is either the lowest postoperative recording of the hemoglobin level (g/L) or the hemoglobin level (g/L) recorded right before any transfusion, and Hbt is the total amount (g) of allogeneic or autologous hemoglobin transfused. A unit of banked allogeneic blood is considered to contain 52 g of hemoglobin according to Canadian Blood Services.
Predicted blood volume is estimated for each patient according to Nadler’s method:
PBVmale = (0.3669 × Ht3 (M)) + [0.03219 × Wt (Kg)] + 0.6041.
PBVfemale = (0.3561 × Ht3 (M)) + [0.03308 × Wt (Kg)] + 0.1833, where PBV is predicted blood volume (mL), Ht = height (m), and Wt = weight (kg).
Rights and permissions
About this article
Cite this article
Aguilera, X., Martínez-Zapata, M.J., Hinarejos, P. et al. Topical and intravenous tranexamic acid reduce blood loss compared to routine hemostasis in total knee arthroplasty: a multicenter, randomized, controlled trial. Arch Orthop Trauma Surg 135, 1017–1025 (2015). https://doi.org/10.1007/s00402-015-2232-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00402-015-2232-8